Abstract
Background:
The goals of asthma care are reductions in risk and impairment, but achieving these goals requires collaborative work between patients and their clinicians. The purpose of this study was to improve inhaled corticosteroid (ICS) adherence and asthma control by cueing therapeutic communication between patients with asthma and their primary care clinicians.
Methods:
We conducted a prospective, cluster-randomized, controlled effectiveness trial to assess the effect of providing visually standardized, interpreted peak flow graphs (CUE intervention) to patients and their clinicians on ICS adherence and asthma control. Asthma control outcomes were analyzed by season to account for seasonal variations in exacerbation frequency.
Results:
Although mean log-transformed ICS adherence was not significantly different between the two groups, there was a trend toward preserved adherence in the intervention group over time (P = .16). Intervention patients required fewer courses of oral steroids during winter (9% vs 23%, P < .001) and spring (3% and 17%, P < .001) compared with control subjects. Intervention patients also had fewer periods of worsening symptoms (65% vs 89%, P < .001) and fewer urgent care visits (10% vs 23%, P < .001) during winter compared with control subjects. Post hoc analysis showed significant improvement in the intervention group with respect to ICS adherence during winter months (P < .05), the likely explanation for the reduction in prednisone use and symptoms. Day-to-day peak flow variability in the intervention group fell consistently throughout the study from an average of 32% at baseline to 23% at final measurement (P < .001), indicating less airway reactivity over time.
Conclusions:
Our findings provide evidence of the value of peak flow monitoring for patients with asthma during seasons of greatest vulnerability, the cold/flu season. The peak flow information apparently led to improvements in ICS adherence resulting in less need for prednisone rescue and fewer episodes of worsening symptoms.
Trial registry:
ClinicalTrials.gov; No.: NCT00201188; URL: www.clinicaltrials.gov
Asthma is an enormous public health problem in the United States resulting in considerable symptom burden and cost.1 The goal of asthma care is control of the disease; however, control requires collaborative work between patients and their clinicians. The role of the clinician is to provide a treatment plan that includes inhaled medications, recommendations for remediation of relevant environmental exposures, and ongoing assessment of asthma control. The role of the patient is to follow the treatment plan, which includes taking controller medications consistently and correctly, reducing relevant exposures, and self-assessing asthma control. This process depends on effective communication between the patient and clinician. Apter et al2 found poor adherence to be independently associated with poor patient-clinician communication. With average patient adherence to treatment hovering around 50%, it is important to investigate strategies to improve patient-clinician collaboration in asthma care.2
Clinicians can use spirometry, peak flow monitoring (PFM), questionnaires, and regular review of asthma status during clinic visits to assess asthma control. PFM is a tool that requires accurate measurement by patients and consistent review by clinicians. The efficacy of PFM in promoting asthma control has been often studied and long debated.3-6 It has been shown to be efficacious when compared with symptom monitoring alone but inconsistently comparable to planned, regular health-care visits. The variation in efficacy of PFM among studies is believed to be due to inconsistent visual presentation and clinical interpretation of peak flow trends. These challenges are further compounded by the fact that most outpatient asthma care is delivered in primary care settings,7 where clinician self-efficacy for interpreting peak flow trends and using evidence-based strategies to develop an asthma care plan may be limited.7 PFM is only useful to the extent that results are used by patient and/or clinician to quickly identify worsening asthma and implement early interventions to reduce further risk.
The purpose of this study was to improve inhaled corticosteroid (ICS) medication adherence and asthma control by cueing therapeutic communication between patients with asthma and their primary care clinicians. We tested the impact of providing feedback of visually standardized, monthly interpreted graphs of peak flow data to patients and their clinicians (the CUE intervention). The intervention was designed to cue communication about the therapeutic plan with a focus on controlling asthma and improving outcomes.
Materials and Methods
Design
We conducted a prospective, cluster-randomized, controlled effectiveness trial to assess the effect of the CUE intervention on patient ICS medication adherence and asthma control compared with usual care (clinician monitoring). The clinician was the unit of randomization and each clinician’s panel of patients with asthma was balanced for size and block randomized to either the intervention or usual care group. The study was approved by the institutional review board, and all participants provided informed consent.
Sample
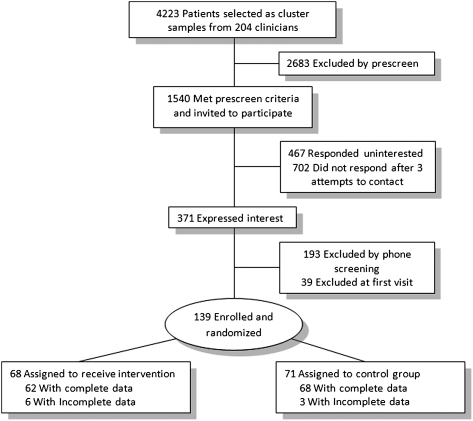
Adults with guidelines-defined persistent asthma,8 who had been prescribed an ICS, were recruited from the general medicine practices of a large academic medical center (Fig 1). Inclusion criteria were history of physician-diagnosed asthma, age between 18 and 72 years, and ≤ 15 pack-year lifetime history of smoking. Exclusion criteria included concurrent lung disease; history of severe psychiatric disease; current smoking of tobacco or marijuana; or use of a dry powder inhaler (DPI) that did not have a dose counter.
Figure 1.
Patient enrollment and assignment.
Protocol
Patients enrolled in the study attended monthly study visits for 1 year, while remaining under the care of their primary care clinicians. The study setting was a clinical laboratory. Patients were informed that the study’s purpose was to examine the effects of two methods of monitoring asthma. Except for the study coordinator and the statistician, all investigators, research staff, and patients were blinded to group assignment.
All participating clinicians received the National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma–Update on Selected Topics 2002.9 At the first study visit all patients were given a brief validated asthma educational session10 with the components recommended in the National Asthma Education and Prevention Program guidelines.11 All patients were given a resource booklet, which included the educational information they had received and a list of clinic telephone numbers to use to contact their clinician, speak to an advice nurse, or schedule an appointment.
Pulmonary function was measured by spirometry12 before and after two puffs of albuterol (180 μg) at the first and last visits. Results of spirometry were shared with the patient and forwarded, with an accompanying interpretation by the study pulmonologist (S. C. L.), to the patient’s clinician. Post-bronchodilator FEV1 was used as a proxy variable for persistent airway obstruction.
Adherence was accessed by electronic medication monitors (DoserCT; NEWMED Corp; Newton, MA) for metered dose inhaler ICS medication or the own dose counter of the inhaler if a DPI was used (Advair Diskus; GlaxoSmithKline; Middlesex, England). Data stored in the electronic medication monitors were concealed from the patients. Patients using the DPI were able to see the number of doses left in the device, but this information was not drawn to the attention of patients. Although “dose dumping” could be easily detected with the electronic medication monitors, detecting this behavior in patients using DPIs was less obvious. Our experience has shown that dose dumping with DPIs typically results in excessive residual medication within the DPI casing. The study coordinator was instructed to carefully inspect the opening of the DPI device at data collection to assess for excess medication residue. Excessive medication residue was detected by the study coordinator on three occasions; in these instances data from the previous month were discarded. Dose count data were collected by the study coordinator; adherence was calculated by a research analyst blind to group assignment. Adherence was calculated as the number of puffs used per month (capped at the prescribed number of doses) divided by the number of puffs prescribed per month. No attempt was made to change the prescribed therapy; no information about medication adherence was given to patients.
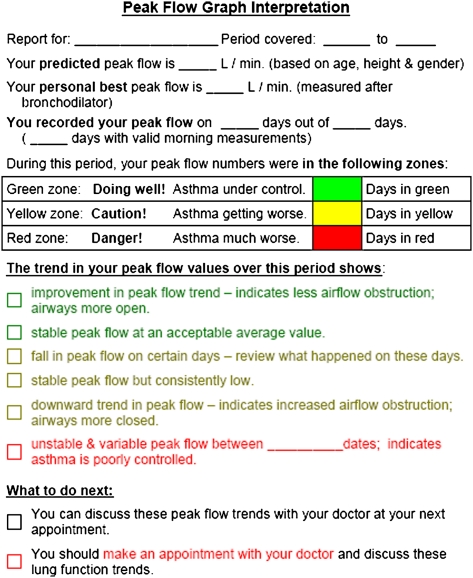
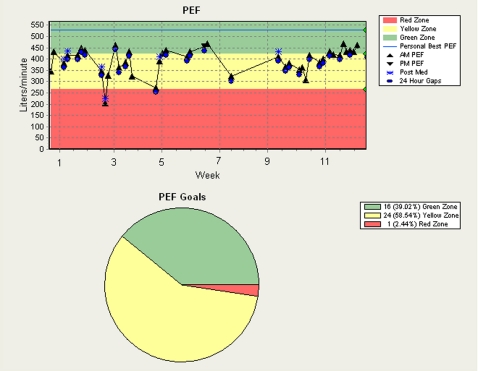
The intervention patients were given an electronic peak flowmeter (AirWatch; iMetrikus, Inc; Carlsbad, CA) and asked to measure their peak flow daily upon waking and before inhaling medication. The best of three measures was captured by the electronic meter and displayed to the patient. The meter was programmed at baseline to display peak flow by green (≥ 80%), yellow (< 80%), or red (< 50%) zones based on the best post-bronchodilator peak flow at baseline. The meter also displayed the previous 7 days of peak flow values within zones as a comparison. At each monthly visit, the study coordinator uploaded the peak flow data to a secure Web site; intervention patients and clinicians were given instructions on how to access these data. The study coordinator printed color graphs of each intervention patient’s monthly trend and trend-to-date, and the summary report of the results at each visit (Fig 2). Copies of the color graphs, including a trend-to-date line chart of peak flow values and a frequency distribution of peak flow values by peak flow zone (Fig 3), and summary reports were given to patients, mailed to clinicians, and placed directly in each patient’s clinic-based medical record after each monthly visit. Day-to-day peak flow variability was measured each month as the variation from the highest peak flow value recorded to the lowest peak flow value recorded in that month ([(highest peak flow-lowest peak flow)/highest peak flow] × 100).13 Patients randomized to the usual care group were not given peak flow meters or provided with feedback on peak flow. Control patients were permitted to use a peak flow meter during the study if so directed by their clinicians.
Figure 2.
Peak flow graph interpretation sheet.
Figure 3.
Sample peak flow trend. PEF = peak expiratory flow.
At each visit, patients completed an asthma status questionnaire, which included questions about asthma-related health-care visits, periods of worsening symptoms, oral steroid use, and missed activities. These data were compared with the patients’ medical records, which were audited biannually by the study investigators. Data were coded according to identified actionable themes, defined as an observation about the patient’s asthma documented in the medical record for which there was a guidelines-based recommendation for follow-up action (eg, “patient peak flow in the yellow zone,” and so forth). Each encounter was documented first by actionable theme and then coded as to whether the clinician documented any change in the treatment plan.
At the final visit all patients completed a nine-item instrument, adapted for adults with permission, which assessed patients’ perception of their clinicians’ communication (score range 10-50),14 and a questionnaire that asked patients to report any behavior changes they made as a result of study participation and to identify which group (experimental monitoring or usual monitoring) they believed they had been in.
Data and Safety Monitoring Considerations
To protect the safety of high-risk study participants, patients with a history of asthma-related intubation and/or baseline FEV1 < 50% predicted were monitored by an independent committee of pulmonologists. Patients monitored by the committee were subject to monthly evaluation by spirometry; if FEV1 was < 50% predicted, the committee sent a letter to the patient’s clinician reporting the patient’s status.
Statistical Analysis
An a priori power analysis showed a sample size of 136 was needed to provide 80% power to detect a 13% change in ICS adherence at α = 0.05 over 1 year of study participation; we enrolled and randomized 139 patients. Intention-to-treat analyses included all participants randomized, 129 with complete data and 10 with incomplete data.
The effect of the intervention on adherence was assessed using linear mixed models analysis (SAS Institute Inc; Cary, NC). This analysis was chosen because it allows for missing data and accounts for all data that each patient provided. Within-intervention group analyses of peak flow data were also analyzed by linear mixed models. Monthly percent adherence was log-transformed to normalize data.15 χ2 Tests were used to detect differences between the two groups for categorical data; continuous data were summarized as the mean ± SD with Student t test used to detect significant differences between groups (SPSS Inc; Chicago, IL). In anticipation of a potential effect of seasons on asthma control a priori, asthma control outcomes were summarized into frequency tables and analyzed by season, to account for annual changes in exacerbation frequency.16 Seasons were defined as Winter (December-February), Spring (March-May), Summer (June-August), and Fall (September-November).
Results
A total of 139 patients were enrolled and randomized; 68 patients, assigned to 22 clinicians, were in the intervention group; 71 patients, assigned to 21 clinicians, were in the control group. There were no significant differences between the groups at baseline with respect to age, sex, race, ethnicity, age of diagnosis, or lung function (Table 1). Randomization was successful; intervention patients were no more likely to correctly guess group assignment than control patients (P = .34).
Table 1.
—Baseline Sample Characteristics
| Variable | Intervention (n = 68) | Control (n = 71) |
| Age, y | 49.7 ± 13.2 | 50.3 ± 11.8 |
| Female sex | 50 (74) | 45 (63) |
| Ethnicity | ||
| Hispanic | 4 (6) | 4 (6) |
| Not Hispanic | 64 (94) | 67 (94) |
| Race | ||
| White | 41 (60) | 42 (59) |
| Black/African American | 14 (21) | 11 (16) |
| Asian | 8 (12) | 13 (18) |
| Pacific Islander | 0 | 1 (1) |
| > 1 race | 5 (7) | 4 (6) |
| Years with asthma | 23.6 ± 18.4 | 23.5 ± 17.9 |
| Prebronchodilator FEV1, % predicted | 82.9 ± 16.5 | 79.7 ± 19.7 |
| Asthma symptoms over past 2 wk | ||
| Asthma symptoms (0: absent, 5: very severe) | 2.17 ± 1.00 | 2.11 ± 1.18 |
| Nighttime awakenings (0: no nights, 4: every night) | 1.25 ± 1.25 | 1.36 ± 1.25 |
| ED use in previous 1 y | 8 (12) | 8 (11) |
| Oral prednisone in previous 1 y | 20 (29) | 22 (31) |
| ICS medication type | ||
| Combination ICS/LABA | 42 (62) | 34 (48) |
| ICS only | 26 (38) | 37 (52) |
| ICS medication dosea | ||
| High | 15 (22) | 14 (20) |
| Medium | 24 (35) | 26 (38) |
| Low | 29 (43) | 29 (42) |
| Insured | 61 (98) | 64 (98) |
| Season of enrollment | ||
| Winter | 15 (22) | 23 (32) |
| Spring | 7 (10) | 8 (11) |
| Summer | 19 (28) | 13 (18) |
| Fall | 27 (40) | 27 (38) |
Values are mean ± SD or No. (%). ICS = inhaled corticosteroids; LABA = long-acting β-agonist.
Dose equivalents for dose categories vary based on medication type; a full list of dose equivalents can be found in Reference 3.
Medication Adherence
There was no significant change in log-transformed ICS adherence in either group during the study, although there appeared to be a trend toward preserved adherence in the intervention group over time (P = .16), whereas adherence in the control group appeared to wane over time. This observation prompted a post hoc analysis of the contribution of season to log-transformed ICS adherence. The group-by-season interaction showed a statistically significant improvement in ICS adherence favoring the intervention group during winter (P = .045) and a similar trend in spring (P = .12) when compared with summer as the reference period. To further explore changes in adherence post hoc, we examined daily paired peak flow and adherence data in 26 patients using metered dose inhalers equipped with the electronic medication monitor. An inverse relationship between peak flow and adherence was found in about half of the patients. When daily peak flow was dichotomized to ≥ 80% predicted vs < 80% predicted, again about half of the patients showed the pattern of increasing adherence on the days when peak flow was low.
Intervention Effect on Indicators of Asthma Control
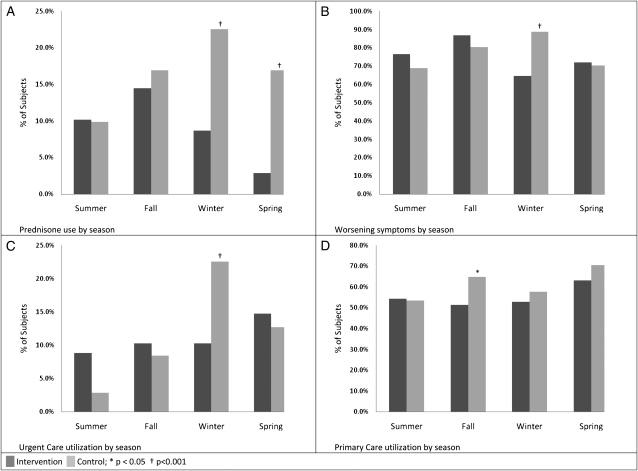
Significantly fewer intervention patients experienced exacerbations requiring oral steroids compared with control subjects (Fig 4A) during the winter (9% vs 23%, P < .001) and spring (3% vs 17%, P < .001) (Table 2). There were significantly fewer intervention patients reporting periods of worsening symptoms compared with control subjects in the winter (65% vs 89%, P < .001) (Fig 4B). Significantly fewer intervention patients required urgent care visits during the Winter compared with control subjects (10% vs 23%, P < .001) (Fig 4C). Intervention patients had fewer primary care visits in the fall when compared with the control subjects (58% vs 65%, P = .02).
Figure 4.
Asthma control outcomes by season. A, Prednisone use. B, Worsening symptoms. C, Urgent care utilization. D, Primary care utilization.
Table 2.
—Asthma Control Outcomes by Season
| Outcome | Intervention, No. (%) (n = 68) | Control, No. (%) (n = 71) | P Value |
| Prednisone | |||
| Summer | 7 (10) | 7 (10) | .70 |
| Fall | 10 (15) | 12 (17) | .52 |
| Winter | 6 (9) | 16 (23) | < .001 |
| Spring | 2 (3) | 12 (17) | < .001 |
| Worsening symptoms | |||
| Summer | 52 (77) | 49 (69) | .12 |
| Fall | 59 (87) | 57 (80) | .09 |
| Winter | 44 (65) | 63 (89) | < .001 |
| Spring | 49 (72) | 50 (70) | .63 |
| Urgent care | |||
| Summer | 6 (9) | 2 (3) | .06 |
| Fall | 7 (10) | 6 (9) | .52 |
| Winter | 7 (10) | 16 (23) | < .001 |
| Spring | 10 (15) | 9 (13) | .54 |
| Primary care | |||
| Summer | 37 (54) | 38 (54) | .69 |
| Fall | 35 (52) | 46 (65) | .02 |
| Winter | 36 (53) | 41 (58) | .37 |
| Spring | 63 (43) | 50 (70) | .18 |
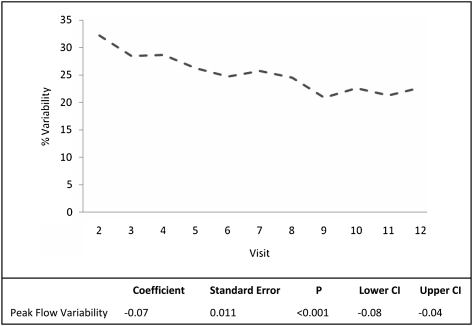
There was no significant change in post-bronchodilator FEV1% predicted within either group from baseline to end of study and no differences between the groups at either baseline or at end of study, controlling for baseline. Day-to-day peak flow variability in the intervention group fell consistently throughout the study from an average of 32% at baseline to 23% at final measurement (P < .001) (Fig 5).
Figure 5.
Intervention group peak flow variability.
Communication
There were no significant differences between intervention (30.7 ± 6.4) and control (30.6 ± 3.7) patients with respect to perception of their clinician’s communication (P = .95), nor were there any differences between intervention (35.6 ± 2.7) and control (37.1 ± 4.1) clinicians with respect to their own perceived communication (P = .18).
Audit Results
Seven common actionable themes emerged from audits of medical records (need for asthma education, abnormal peak flow, comorbid disease management, asthma exacerbation, well-controlled asthma, poorly controlled asthma, and need for smoking cessation counseling). Action by clinicians was taken on 52% of statements in the intervention group and 55% in the control group. There were no differences between the groups with respect to documented changes in the patient’s asthma plan (P = .51).
Additionally, there were no differences between groups with respect to likelihood of being on a dose-appropriate controller therapy based on severity at baseline (P = .78) nor were there differences between groups with respect to having their controller medication adjusted during the course of the study (P = .31). There was no evidence that any of the patients in either group had received a written asthma action plan from their treating clinicians.
Changes in Self-Management Behavior
Consistent with the design of the intervention, a significantly greater proportion of intervention patients reported regular PFM as an improved self-management behavior when compared with control subjects (31% vs 3%, P < .001) (Table 3). A greater proportion of intervention patients also reported increased awareness of asthma status as an improved self-management behavior when compared with control patients (24% vs 10%, P < .001).
Table 3.
—Self-Reported Improvements in Self-Management Behavior
| Reported Improvement | Intervention, No. (%) (n = 62) | Control, No. (%) (n = 68) | P Value |
| Increased awareness of asthma | 15 (24) | 7 (10) | < .001 |
| Inhaler technique | 22 (35) | 32 (47) | .06 |
| Regular PFM | 19 (31) | 2 (3) | < .001 |
| Improved perceived adherence | 7 (11) | 10 (15) | .30 |
| Took steps to control environment | 7 (11) | 5 (7) | .18 |
| PFM = peak flow monitoring. | |||
Discussion
The results of our analysis do not support the original hypothesis that interpreted peak flow information would improve ICS adherence by cueing therapeutic communication between patients and their clinicians. Instead it appears that adherence was maintained in the intervention group and declined in the control group independent of clinician communication. The CUE intervention had a positive effect on asthma health outcomes and a protective effect during periods of seasonal vulnerability, especially winter and spring. It has been previously reported that adverse asthma outcomes in middle age and elderly patient cohorts reach peak levels in the winter season,17 driven largely by viral exacerbations. Similarly, the control group in this study, with a mean age of 50 years, experienced a marked increase in periods of worsening symptoms, oral steroid use, and urgent care use in the winter season. However, this characteristic spike in seasonal worsening of asthma was not seen in the intervention group, suggesting a protective effect of the CUE intervention. Additionally, the overall decline in peak flow variability seen in the intervention group provides objective evidence of a general trend toward improved asthma control. Variability in peak flow measured at the same time each day is believed to indicate airway irritability; reduction in this variability indicates declining airway reactivity. The improvement in asthma control for the intervention group can be explained by increased adherence to ICS medication during winter months. Others have described a relationship between decreased seasonal adherence and subsequent increases in asthma morbidity.18 Worsening peak flow values and trends during the winter season may have prompted brief improvements in adherence to ICS medication. This hypothesis of intermittent improvements in adherence is consistent with the improved day-to-day peak flow variability seen in the intervention group. In addition, intervention patients may have made behavioral adjustments to asthma self-management (eg, prophylactic allergy medications, trigger avoidance, and so forth), in response to worsening peak flow, that were not captured by our methods of assessment. Additionally, interactions between patients and their clinicians may have resulted in advice and/or treatment of comorbid allergic rhinitis or gastric esophageal reflux that was not detected by our chart audits. Control of these comorbid diseases could decrease vulnerability to asthma exacerbations.
Assessment of the quality of communication did not differ between groups, but our questionnaires asked about general and not specific communication. Adherence to ICS medication was preserved but not improved in the intervention group. A plausible explanation for the lack of detectable improvement in adherence is that the study cohort had relatively mild asthma with FEV1 approximately 80% to 83% predicted; it is plausible that patients with relatively mild disease may have been able to achieve symptom control with only 60% adherence. Given that a high percentage of patients received oral prednisone in the year preceding enrollment in our study, it was concerning that none of the patients had received a written asthma action plan from their treating clinicians. Written action plans to manage worsening asthma are strongly recommended by the National Heart, Lung and Blood Institute Expert Panel Report-3 asthma guidelines based on research evidence of their value in reducing ED visits and hospitalizations.3
Limitations
A limitation of this study was the inability to assess the effect of the intervention on patient-initiated self-care activities. In addition to medication adherence, there are a number of asthma-related self-care interventions that can improve outcomes.3 However, it has been noted in previous research that the efficacy of these self-initiated changes in behavioral and cognitive processes is highly variable from patient to patient, making it challenging to assess using a randomized clinical trial design.19 Although it is evident that the CUE intervention made significant impacts on select asthma control outcomes during cold and flu season, it is not possible to determine the exact mechanisms of the intervention.
Another limitation of the study was that it was conducted in an academic medical center where clinicians are present for only one to two half-day clinics per week, limiting their ability to conduct rapid follow-up with patients. Follow-up visits are typically conducted by different clinicians. Despite this lack of continuity, any clinician who saw the patient had access to the printed, interpreted peak flow graphs filed in the patient’s medical record. These clinics do not use electronic medical records, and documentation is scant. Frequently the written note provided no clues as to clinician-patient discussions, assessments, or interventions.
Additionally, data and safety monitoring could have altered outcomes for patients (n = 9; four intervention, five control) who were subjected to additional monitoring by the review committee. The additional spirometry reports may have influenced the care these patients received from their clinicians.
Conclusions
The study findings provide evidence of the value of PFM coupled with providing patients and clinicians with visually standardized, interpreted monthly peak flow reports. The National Heart, Lung and Blood Institute Asthma Guidelines recommend PFM for selected patients with moderate or severe persistent asthma, those whose asthma is not controlled, and those who are adjusting to new therapy. Our findings suggest that interpreted PFM may be beneficial to people with asthma during the seasons of greatest vulnerability, the cold and flu season. However, further research is needed to elucidate the specific mechanisms of this intervention as they relate to improved outcomes.
Acknowledgments
Author contributions: Dr Janson: contributed in her role as principal investigator, supervising all aspects of the research, including trial design, direction of research staff, medical chart audit, and oversight of analysis and manuscript preparation.
Ms McGrath: contributed to data analysis and manuscript preparation.
Mr Covington: contributed to trial coordination and manuscript review.
Dr Baron: contributed to facilitating patient and clinician recruitment from primary care practices and manuscript review.
Dr Lazarus: contributed to trial design, interpretation of spirometry, medical chart audit, and manuscript review.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- DPI
dry powder inhaler
- ICS
inhaled corticosteroid
- PFM
peak flow monitoring
Footnotes
Funding/Support: This work was supported by the National Institutes of Health [R01HL073098].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Kamble S, Bharmal M. Incremental direct expenditure of treating asthma in the United States. J Asthma. 2009;46(1):73–80. doi: 10.1080/02770900802503107. [DOI] [PubMed] [Google Scholar]
- 2.Apter AJ, Reisine ST, Affleck G, Barrows E, ZuWallack RL. Adherence with twice-daily dosing of inhaled steroids. Socioeconomic and health-belief differences. Am J Respir Crit Care Med. 1998;157(6 pt 1):1810–1817. doi: 10.1164/ajrccm.157.6.9712007. [DOI] [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5) suppl:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 4.López-Viña A, del Castillo-Arévalo E. Influence of peak expiratory flow monitoring on an asthma self-management education programme. Respir Med. 2000;94(8):760–766. doi: 10.1053/rmed.2000.0815. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD, Cahill P, Willet K, Burton P. Accuracy of mini peak flow meters in indicating changes in lung function in children with asthma. BMJ. 1994;308(6928):572–574. doi: 10.1136/bmj.308.6928.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner MO, Taylor D, Bennett R, Fitzgerald JM. A randomized trial comparing peak expiratory flow and symptom self-management plans for patients with asthma attending a primary care clinic. Am J Respir Crit Care Med. 1998;157(2):540–546. doi: 10.1164/ajrccm.157.2.9703060. [DOI] [PubMed] [Google Scholar]
- 7.Reddel HK. Peak flow monitoring in clinical practice and clinical asthma trials. Curr Opin Pulm Med. 2006;12(1):75–81. doi: 10.1097/01.mcp.0000198065.65704.08. [DOI] [PubMed] [Google Scholar]
- 8.New NHLBI guidelines for the diagnosis and management of asthma. National Heart, Lung and Blood Institute. Lippincott Health Promot Lett. 1997;2(7):1. 8-9. [PubMed] [Google Scholar]
- 9.National Asthma Education and Prevention Program Expert Panel Report: Guidelines for the diagnosis and management of asthma-update on selected topics 2002. J Allergy Clin Immunol. 2002;110:S141–S219. [PubMed] [Google Scholar]
- 10.Janson SL, McGrath KW, Covington JK, Cheng SC, Boushey HA. Individualized asthma self-management improves medication adherence and markers of asthma control. J Allergy Clin Immunol. 2009;123(4):840–846. doi: 10.1016/j.jaci.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cressy DS, DeBoisblanc BP. National Institutes of Health Diagnosis and management of asthma: a summary of the National Asthma Education and Prevention Program guidelines. J La State Med Soc. 1998;150(12):611–617. [PubMed] [Google Scholar]
- 12.Gardner RM, Hankinson JL. Standardization of spirometry—1987 ATS update (American Thoracic Society) J Occup Med. 1988;30(3):272–273. [PubMed] [Google Scholar]
- 13.Porsbjerg C, Brannan JD, Anderson SD, Backer V. Relationship between airway responsiveness to mannitol and to methacholine and markers of airway inflammation, peak flow variability and quality of life in asthma patients. Clin Exp Allergy. 2008;38(1):43–50. doi: 10.1111/j.1365-2222.2007.02878.x. [DOI] [PubMed] [Google Scholar]
- 14.Clark NM, Gong M, Schork MA, et al. Long-term effects of asthma education for physicians on patient satisfaction and use of health services. Eur Respir J. 2000;16(1):15–21. doi: 10.1034/j.1399-3003.2000.16a04.x. [DOI] [PubMed] [Google Scholar]
- 15.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180(9):817–822. doi: 10.1164/rccm.200902-0166OC. [DOI] [PubMed] [Google Scholar]
- 16.Burt CW, Knapp DE. Ambulatory care visits for asthma: United States, 1993-94. Adv Data. 1996;(277):1–19. [PubMed] [Google Scholar]
- 17.Fleming DM, Cross KW, Sunderland R, Ross AM. Comparison of the seasonal patterns of asthma identified in general practitioner episodes, hospital admissions, and deaths. Thorax. 2000;55(8):662–665. doi: 10.1136/thorax.55.8.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spahn J, Sheth K, Yeh WS, Stempel DA, Stanford RH. Dispensing of fluticasone propionate/salmeterol combination in the summer and asthma-related outcomes in the fall. J Allergy Clin Immunol. 2009;124(6):1197–1203. doi: 10.1016/j.jaci.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Creer TL. Behavioral and cognitive processes in the self-management of asthma. J Asthma. 2008;45(2):81–94. doi: 10.1080/02770900701247236. [DOI] [PubMed] [Google Scholar]