Abstract
Background:
Asthma is a major public health problem that affects millions of children worldwide, and exacerbations account for most of its morbidity and costs. Primary-care providers lack efficient tools to identify children at high risk for exacerbations. We aimed to construct a clinical score to help providers to identify such children.
Methods:
Our main outcome was severe asthma exacerbation, which was defined as any hospitalization, urgent visit, or systemic steroid course for asthma in the previous year, in children. A clinical score, consisting of a checklist questionnaire made up of 17 yes-no questions regarding asthma symptoms, use of medications and health-care services, and history, was built and validated in a cross-sectional study of Costa Rican children with asthma. It was then evaluated using data from the Childhood Asthma Management Program (CAMP), a longitudinal trial cohort of North American children.
Results:
Compared with children at average risk for an exacerbation in the Costa Rican validation set, the odds of an exacerbation among children in the low-risk (OR, 0.2; 95% CI, 0.1-0.4) and high-risk (OR, 5.4; 95% CI, 1.5-19.2) score categories were significantly reduced and increased, respectively. In CAMP, the hazard ratios for an exacerbation after 1-year follow-up in the low-risk and high-risk groups were 0.6 (95% CI, 0.5-0.7) and 1.9 (95% CI, 1.4-2.4), respectively, with similar results at 2 years.
Conclusions:
The proposed Asthma Exacerbation Clinical Score is simple to use and effective at identifying children at high and low risk for asthma exacerbations. The tool can easily be used in primary-care settings.
Disease exacerbations account for the majority of asthma-related costs. Among children living in the United States, > 4 million asthma exacerbations occur each year, resulting in approximately 700,000 ED visits, 205,000 hospital admissions, and 200 deaths per year.1,2 Without including prescriptions, costs related to pediatric asthma exacerbations accounted for $9.8 billion (63.2%) of the estimated $15.5 billion total asthma costs in the United States in 2002.3 According to guidelines for the diagnosis and management of asthma from the National Heart, Lung and Blood Institute (NHLBI), two or more exacerbations requiring oral steroids per year place children in the persistent category, represent suboptimal control, and increase morbidity and mortality.4
The prevalence of pediatric asthma exacerbations varies widely by ethnicity. Among Hispanic children living in the United States, the prevalence ranges from 2.9% in Mexicans to 11.8% in Puerto Ricans, and the rate of ED visits is 14.1% for Hispanics vs 10.8% for non-Hispanic whites.2 The prevalence of childhood asthma in Costa Rica, a Hispanic American country, is among the highest in the world.5 During phase 3 of the International Study for Asthma and Allergies in Childhood (ISAAC) study, the reported current prevalence of asthma was 34.8% for 6- to 7-year-olds and 25.5% for 13- to 14-year-olds; with 9.7% and 6.7%, respectively, reporting more than four exacerbations a year. Little is known, however, about risk factors for asthma exacerbations in childhood in Hispanics, such as Costa Ricans.
In this study, we first identified risk factors for asthma exacerbations in Costa Rican children. Given the paucity of clinically relevant tools to predict asthma exacerbations, we then developed and validated a predictive score for asthma exacerbations that we further validated in a cohort of North American children with asthma.
Materials and Methods
Study Population
A detailed description of the study methods is provided in the e-Appendix 1 and e-Table 1. Children who participated in this study were index cases for a family-based study of the genetics of asthma in Costa Rica. Subject recruitment and study procedures have been described in detail elsewhere.6,7 The study was approved by the Institutional Review Boards of the Hospital Nacional de Niños (San José, Costa Rica) and Brigham and Women’s Hospital (Boston, MA).
Questionnaires and Laboratory Testing
Parents of the study participants completed slightly modified versions of the Collaborative Study on the Genetics of Asthma8 and the ISAAC questionnaires.9 Spirometry was conducted with a Survey Tach Spirometer (Warren E. Collins; Braintree, MA) following American Thoracic Society (ATS) recommendations.10 On a subsequent visit, children with an FEV1 ≥ 65% predicted underwent methacholine challenge using a modified version of the Chatham protocol11; the test was terminated if the FEV1 declined by ≥ 20% from the best FEV1 after inhalation of saline solution. Allergy skin testing was performed using the ISAAC protocol.12 Antigens tested for were dust mite (Dermatophagoides pteronyssinus), cockroach (Blatella germanica and Periplaneta americana), cat dander, dog dander, mixed grass pollen, mixed tree pollen, and Alternaria tenuis.
Validation Population
Data for the external validation sample were taken from the Childhood Asthma Management Program (CAMP), a double-blind, placebo-controlled study of the long-term effects of budesonide and nedocromil on lung growth in children. Details on the design and methods of CAMP have been published elsewhere.13,14 Children with asthma aged 5 to 12 years were assigned to one of three treatment arms (budesonide, nedocromil, or placebo) and followed for 4 years. The protocol for CAMP was approved by the institutional review boards of each of the participating clinical sites and by the CAMP Data Coordinating Center in Baltimore, Maryland.
Statistical Analysis
Based on recommendations from the Expert Panel Report 3 (EPR-3) from the NHLBI for the diagnosis and management of asthma,4 we defined a severe exacerbation as any hospitalization or two or more ED or urgent care visits. Scheduled physician visits were not considered. This definition (EPR-3 outcome) was our main outcome for the exploratory phase of the present study and for the construction of the clinical score. To further assess our clinical score, we used a secondary definition of a severe asthma exacerbation (any hospitalization, ED or urgent care visit, or systemic steroid burst for asthma in the previous year) that was recently proposed by the ATS/European Respiratory Society (ERS) for clinical trials.15
The database for Costa Rica was randomly split in two sets: one for the exploratory analysis (n = 465) and one for validation of the clinical score (n = 150). These numbers were calculated to achieve > 90% power to detect a 20% difference in the outcome. Univariate analyses were conducted to identify variables to be included in the multivariate analysis. We then used principal component analysis (PCA) to identify clusters of variables and build a clinical score to stratify subjects by risk. Finally, we evaluated the performance of this score in the Costa Rican validation set and in CAMP.
Univariate analyses were conducted using Fisher exact tests for categorical variables and two-tailed t tests for categorical and continuous variables. Stepwise logistic regression was then used; initial models included variables associated with an exacerbation at P < .20 and potential confounders. All final models included age, sex, and parental education level as well as variables that were significant at P < .05 or led to a ≥ 10% change in risk measures.
PCA with varimax rotation was used to explore the relationships among significant variables. PCA is a statistical technique that condenses the information from a large set of variables into a reduced number of factors that can explain the common variance of the sample. Factors with an eigenvalue > 1 were used to build the score. For ease of use, only questionnaire variables were included in the final score, which was then evaluated in the internal validation set in Costa Rica.
To assess the performance of the clinical score in a different population, we applied it to CAMP using both our main and our secondary outcomes. The score was applied at baseline (before randomization) and evaluated by Cox survival analysis adjusted for age, sex, race, and treatment arm. We limited our analysis of CAMP to the first 2 years because prediction beyond that extended past the age range of the Costa Rican children and because most exacerbations occurred in the first year of the CAMP. All analyses were performed with SAS, version 9.1, statistical software (SAS Institute; Cary, NC).
Results
Costa Rican Cohort
A total of 615 children with asthma enrolled in the study had information on exacerbations. There were no significant differences between the exploratory and validation data sets (e-Table 2). The baseline characteristics of the 465 children in the exploratory data set are shown in Table 1. The age of participants ranged from 6 to 14 years. Physician-diagnosed hay fever, parental history of asthma and hay fever, and skin test reactivity (STR) to one or more allergens were all common; mean total IgE level was elevated. Of the 465 participants, 367 (80%) used short-acting β2-agonists, 27 (6%) used xanthines, and 178 (38%) used antiinflammatory medications (inhaled steroids [n = 157], leukotriene inhibitors [n = 11], or both [n = 21]) in the previous year.
Table 1.
—Characteristics of Study Participants in Costa Rica Exploratory Set and Childhood Asthma Management Program
| Costa Rica |
CAMP |
|||
| Characteristic | Yes | No | Yes | No |
| Severe asthma exacerbation, No. | 324 | 141 | 579 | 462 |
| Female sex | 40.7 | 38.3 | 43.2 | 39.0 |
| Physician-diagnosed hay fever | 28.1 | 25.5 | 75.3 | 67.9 |
| Physician-diagnosed eczema | 5.9 | 3.6 | n/a | n/a |
| Bronchiolitis or pneumonia as infant | 34.0a | 22.0 | n/a | n/a |
| Maternal historyb | ||||
| Asthma | 32.2 | 24.8 | 20.5 | 21.9 |
| Hay fever | 26.5 | 34.8 | 40.0 | 38.5 |
| Eczema | 3.7a | 9.2 | … | … |
| Paternal historyb | ||||
| Asthma | 24.0 | 20.9 | 25.4 | 26.1 |
| Hay fever | 27.1a | 17.1 | 48.5 | 47.4 |
| Eczema | 5.0 | 1.4 | … | … |
| Symptoms/health-care utilization last year | ||||
| Symptoms present ≥ 3 mo | 70.1c | 36.9 | 100 | 100 |
| Shortness of breath with activity | 82.7a | 69.5 | 79.0 | 80.5 |
| Physician visits (four or more)d | 57.7c | 8.5 | 93.3 | 90.8 |
| Admitted to ICU (ever) | 1.5 | 2.1 | 5.2 | 2.9 |
| Medication use in the last year | ||||
| Short-acting β2-agonists | 85.8c | 63.1 | 95.4 | 93.7 |
| Inhaled steroids | 40.4a | 25.5 | 47.5a | 32.9 |
| Leukotriene inhibitors | 4.6 | 4.3 | n/a | n/a |
| Bronchodilator responsiveness | 16.2c | 8.2 | 42.2 | 35.9 |
| Smoke exposure, current | 28.8 | 24.8 | 42.7 | 42.2 |
| Positive IgE test for dust mite (Dermatophagoides pteronyssinus) | 79.0a | 68.1 | n/a | n/a |
| Positive IgE test for cockroach (Blatella germanica) | 44.1a | 34.0 | n/a | n/a |
| Positive IgE test for Ascarislumbricoides | 42.6a | 31.9 | n/a | n/a |
| Positive skin test for at least one allergen | 89.5a | 81.6 | n/a | n/a |
| Age, y | 8.4c (7.4-9.9) | 9.7 (8.1-10.9) | 8.1c (6.6-9.6) | 9.3 (7.6-11.0) |
| Height, in. | 50.8c (48.1-53.9) | 52.2 (49.6-55.1) | 50.7c (47.4-54.7) | 53.3 (49.0-57.5) |
| BMI (percentile), kg/m2 | 61 (29-89) | 64 (36-92) | 64a (36-86) | 69 (43-91) |
| No. oral steroid courses last year (range) | 2c (2-2) | 1 (0-1) | 0c (0-5) | 0 (0-4) |
| FEV1 prebronchodilator, L | 1.6c (1.3-1.9) | 1.8 (1.5-2.1) | 1.5c (1.2-1.8) | 1.7 (1.4-2.0) |
| FEV1/FVC prebronchodilator, % (range) | 82.7a (77.5-87.2) | 84.9 (80.1-87.3) | 80.2 (74.1-85.7) | 80.4 (75.1-85.3) |
| FEV1/FVC postbronchodilator, % change (range) | 3.4a (0.8-6.1) | 2.7 (0.4-4.9) | 5.8 (3.5-8.7) | 5.3 (2.9-7.9) |
| Eosinophils, cells/mm3e | 580c (320-870) | 430 (240-630) | 410 (200-700) | 393 (200-600) |
| Total IgE, IU/mLe | 444 (150-1029) | 411 (87-832) | 463 (164-1388) | 429 (176-1145) |
Data are presented as % or median (interquartile range), unless otherwise indicated. CAMP = Childhood Asthma Management Program; n/a = not available.
P < .05.
In CAMP, maternal and parental history of eczema and hay fever was assessed as one question.
P < .001.
Nonacute (scheduled) visits.
Analyzed as log10.
Based on the EPR-3 and ATS/ERS definitions, 324 (69.7%) and 383 (82.4%) children had an exacerbation in the previous year, respectively. A total of 27 (5.8%) children were hospitalized in the previous year.
Predictors of Exacerbations in Costa Rica
The results of the univariate analyses of asthma exacerbations are shown in Table 1. Variables significantly associated with asthma exacerbations were age, height, bronchiolitis or pneumonia during infancy, maternal eczema, paternal history of hay fever, asthma symptoms ≥ 3 months/year, dyspnea when walking uphill, more than four scheduled physician visits for asthma in the previous year, and use of certain medications (short-acting β2-agonists, antiinflammatory medications, and one or more courses of oral steroids) in the prior year. Markers of lung function and allergy that showed significant associations were baseline FEV1 and FEV1/FVC ratio; change in FEV1/FVC ratio after use of a bronchodilator; airway responsiveness to methacholine challenge (as log-transformed dose-response slope); eosinophil count; positive IgE test for dust mite, cockroach, and Ascaris lumbricoides; and STR to one or more allergens.
The results of the multivariate analysis of asthma exacerbations are shown in Table 2. Children with an exacerbation were more likely to have paternal history of hay fever, four or more scheduled physician visits, a higher number of oral steroid bursts in the previous year, and asthma symptoms for ≥ 3 months/year than children without an exacerbation. Additionally, total IgE level and eosinophil count were associated with increased odds of exacerbations. (Inclusion of both variables in the same model was not possible because of high colinearity.)
Table 2.
—Risk Factors for Asthma Exacerbations in Costa Rica Exploratory Set
| Risk Factor | Unadjusted | Model 1 | Model 2 |
| Four or more physician visitsa for asthma in previous year | 14.7 (7.8-27.6)b | 6.8 (3.3-13.9)b | 6.4 (3.1-13.1)b |
| Number of oral steroid courses in the previous year | 6.0 (4.0-9.0)b | 4.1 (2.6-6.5)b | 4.1 (2.6-6.5)b |
| Paternal history of hay fever | 1.8 (1.1-3.0)c | 1.9 (1.02-3.7)c | 1.9 (1.02-3.7)c |
| Symptoms for ≥ 3 mo/y | 4.0 (2.6-6.1)b | 1.9 (1.1-3.3)c | 1.9 (1.1-3.2)c |
| Total serum IgE level, IU/mLd | 1.3 (0.98-1.8) | 1.5 (1.03-2.3)c | … |
| Eosinophil count, cells/mm3d | 2.8 (1.6-5.0)b | … | 2.7 (1.2-5.7)c |
| FEV1 % change postbronchodilatore | 1.03 (1.01-1.1)c | ns | ns |
| Antiinflammatory medicationse | 1.9 (1.3-3.0)b | ns | ns |
| C statistic | … | 0.87 | 0.88 |
| Goodness of fit | … | 0.92 | 0.84 |
Data are presented as OR (95% CI), unless otherwise indicated. ns = not significant. Each model included all variables listed in the respective column; both models also was adjusted for age, sex, and parental education level.
Nonacute visits.
P < .001.
P < .05.
Analyzed as log10.
Not statistically significant but included as confounders.
Clinical Score in the Exploratory Data Set
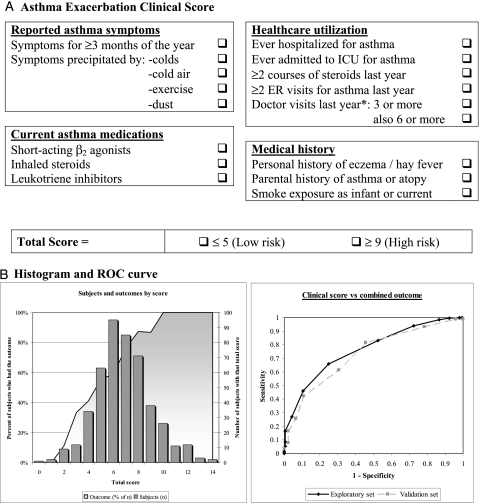
Factor analysis identified 10 clusters of variables in the exploratory set in Costa Rica (e-Figure 1, e-Table 3) corresponding to six categories: asthma symptoms, health-care utilization, medical history, asthma medications, laboratory tests, and airway responsiveness. A scoring questionnaire was built using these categories, excluding laboratory tests and airway responsiveness for ease of use. The resulting score with 17 total points was grouped in four categories (Fig 1). In our exploratory set, the mean ± SD total score was 6.85 ± 2.25 (range, 0-14), and the median score was 7 (interquartile range, 5-8). We classified patients into three groups: low risk (score, ≤ 5), intermediate or average risk (score, 6-8), and high risk (score, ≥ 9).
Figure 1.
A, Clinical score instructions: One point is assigned for each question answered as yes. The score is calculated by adding all points (total score range, 0-17). For “Doctor visits last year,” one point is assigned for ≥ 3 visits, and one more point (two total) if the patient also had ≥ 6 visits. B, Histogram of total clinical score with superimposed proportion of children who had the Expert Panel Report 3 (EPR-3) outcome (theoretical range, 0-17; actual range, 0-14; mean ± SD, 6.85 ± 2.5; median, 7; interquartile range, 5-8). ROC curve for the total clinical score and the EPR-3 outcome (area under the curve, 0.78 for the exploratory set and 0.76 for the validation set [dashed line]). ROC = receiver operating characteristic.
The multivariate analysis in the exploratory set showed that each 1-point increment in the score resulted in a 1.75-fold increment in the odds of an exacerbation (95% CI, 1.5-2.0; P < .0001). The coefficient of determination for this model was R2 = 0.31, and the C statistic (equivalent to the receiver operating characteristic area under the curve [AUC]) was 0.79. Based on our main outcome, children in the low-risk group had markedly reduced odds for an asthma exacerbation, whereas those in the high-risk group had greatly increased odds for an exacerbation (Table 3). Of the 93 children in the high-risk group, 87 had an exacerbation (predictive value [PV], 93.6%), with a false-positive rate (FPR) (1-specificity) of 4.2%. Of the 121 children in the low-risk group, 67 did not have an exacerbation (PV, 55.4%; FPR, 16.7%). Of interest, there was only one hospitalization for asthma among children at low risk (PV for no hospitalizations, 99.2%). Similar results were obtained for our secondary outcome (Table 4).
Table 3.
—Asthma Exacerbations (Expert Panel Report 3 Definition) by Score Category
| Data Set | Low Risk (≤ 5) | Average Risk (6-8) | High Risk (≥ 9) |
| Exploratory set (n = 465), No. | 121 | 251 | 93 |
| Subjects with outcome, No. (%)a | 54 (44.6) | 183 (72.9) | 87 (93.6) |
| OR (95% CI)b | 0.2 (0.1-0.3)c | … | 8.3 (3.5-19.5)c |
| PV, %d | 55.4 | … | 93.6 |
| FPR, %e | 16.7 | … | 4.3 |
| Validation set (n = 150), No. | 44 | 76 | 30 |
| Subjects with outcome, No. (%)a | 19 (43.2) | 58 (76.3) | 27 (90.0) |
| OR (95% CI)b | 0.2 (0.1-0.4)c | … | 5.4 (1.5-19.2)f |
| PV, %d | 56.8 | … | 90 |
| FPR, %e | 18.3 | … | 6.5 |
| CAMP 1 y (n = 1,041), No.g | 371 | 530 | 140 |
| Subjects with outcome, No. (%)a | 92 (24.8) | 185 (34.9) | 67 (47.9) |
| HR (95% CI)b | 0.6 (0.5-0.7)f | … | 1.9 (1.4-2.4)c |
| PV, %d | 75.2 | … | 47.9 |
| FPR, %e | 26.7 | … | 10.5 |
| CAMP 2 y (n = 1,041), No.h | 371 | 530 | 140 |
| Subjects with outcome, No. (%)a | 145 (39.1) | 262 (49.4) | 95 (67.9) |
| HR (95% CI)b | 0.6 (0.5-0.8)f | … | 1.9 (1.5-2.4)c |
| PV, %d | 60.9 | … | 67.9 |
| FPR, %e | 28.9 | … | 8.3 |
FPR = false-positive rate; HR = hazard ratio; PV = predictive value. See Table 1 legend for expansion of other abbreviation.
Percentage of subjects in the same score category.
Adjusted for age, sex, and parental education in Costa Rica and for age, sex, race, and treatment group in CAMP.
P < .001.
PV for no exacerbation in the low-risk group and for exacerbation in the high-risk group.
FPR = 1-specificity.
P < .05.
From start of cohort through 1-y follow-up.
From start of cohort through 2-y follow-up.
Table 4.
—Asthma Exacerbations (American Thoracic Society/European Respiratory Society Definition) by Score Category
| Data Set | Low Risk (≤ 5) | Average Risk (6-8) | High Risk (≥ 9) |
| Exploratory set (n = 465), No. | 121 | 251 | 93 |
| Subjects with outcome, No. (%)a | 77 (63.6) | 216 (86.1) | 90 (96.8) |
| OR (95% CI)b | 0.2 (0.1-0.4)c | … | 7.9 (2.4-25.7)c |
| PV, %d | 36.4 | … | 96.8 |
| FPR, %e | 20.1 | … | 3.7 |
| Validation set (n = 150), No. | 44 | 76 | 30 |
| Subjects with outcome, No. (%)a | 26 (59.1) | 66 (86.4) | 29 (96.7) |
| OR (95% CI)b | 0.2 (0.1-0.4)c | … | 9.7 (1.3-75.2)f |
| PV, %d | 40.9 | … | 96.7 |
| FPR, %e | 21.5 | … | 3.4 |
| CAMP 1 y (n = 1,041), No.g | 371 | 530 | 140 |
| Subjects with outcome, No. (%)a | 181 (48.8) | 301 (56.8) | 97 (69.3) |
| HR (95% CI)b | 0.7 (0.6-0.8)c | … | 1.6 (1.2-1.9)c |
| PV, %d | 51.2 | … | 69.3 |
| FPR, %e | 31.3 | … | 9.3 |
| CAMP 2 y (n = 1041), No.h | 371 | 530 | 140 |
| Subjects with outcome, No. (%)a | 220 (59.3) | 358 (67.6) | 109 (77.9) |
| HR (95% CI)b | 0.7 (0.6-0.8)c | … | 1.5 (1.2-1.8)c |
| PV, %d | 40.7 | … | 77.9 |
| FPR, %e | 32.0 | … | 8.8 |
Percentage of subjects in the same score category.
Adjusted for age, sex, and parental education in Costa Rica and for age, gender, race, and treatment group in CAMP.
P < .001.
Predictive value for no exacerbation in the low-risk group and for exacerbation in the high-risk group.
FPR = 1-specificity.
P < .05.
From start of cohort through 1-y follow-up.
From start of cohort through 2-y follow-up.
Clinical Score in the Validation Set
The results of the multivariate analysis were similar to those in the exploratory set: each 1-point increment in the clinical score was associated with a 1.6-fold increment in the odds of an exacerbation (95% CI, 1.3-2.0; P < .0001); the AUC was 0.75, and the odds ratio for an exacerbation in both the low-risk and high-risk groups were similar to those in the exploratory set. Of 30 children in the high-risk group, all but three had an asthma exacerbation (PV, 90%; FPR, 14.3%). Among the 44 children in the low-risk group, 25 did not have an asthma exacerbation (PV, 56.8%; FPR, 19.3%). Only two children at low risk were hospitalized (PV, 95.4%). Similar results were obtained for our secondary outcome (Table 4).
Validation in CAMP
The characteristics of the CAMP cohort are shown in Table 1. The Costa Rican and CAMP cohorts were similar with regard to age, sex, lung function, and total IgE level. Severe exacerbations at 1 and 2 years of follow-up were less frequent in CAMP than in Costa Rica (main outcome, 33% vs 48%, respectively; secondary outcome, 56% vs 66%, respectively). The clinical score in CAMP had similar characteristics to that in Costa Rica (mean, 6.35 ± 1.9; range, 1-13; median, 6; interquartile range, 5-8).
At 1 and 2 years of follow-up, each 1-point increment in the score was associated with a ∼ 25% increased risk of exacerbations (95% CI, 1.2-1.4; P < .0001). The AUC was 0.69 at 1 year and 0.66 at 2 years. Compared with children in the average-risk group, those in the low-risk group had a 40% reduction in the risk of exacerbations at both 1 and 2 years of follow-up, and those in the high-risk group had a 90% increase in their risk at 1 and 2 years of follow-up (Table 3). The PV for the high-risk group was 48% for year 1 and 68% for year 2, with an FPR of 10.5% and 8.3%, respectively. The PV for the low-risk group was 75% for year 1 and 61% for year 2, with an FPR of 26.7% and 28.9%, respectively. Similar to our findings in Costa Rica, the PV of the low-risk group was higher for hospitalizations (∼ 94%). Similar results were found for our secondary outcome (Table 4).
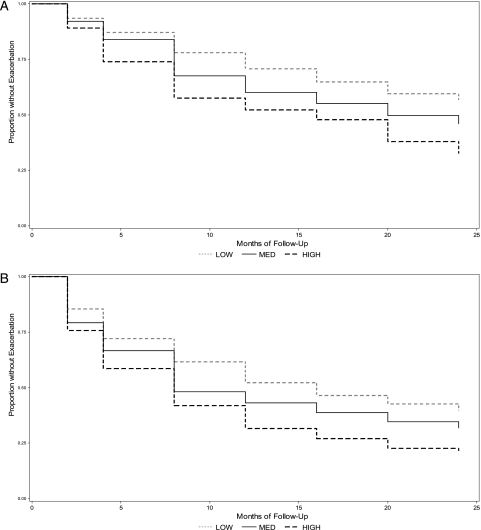
Figure 2 and Table 5 show the Kaplan-Meier survival curves and hazard ratios for exacerbations for each of the score categories. There was a statistically significant difference among the three groups for both our main and secondary outcomes starting at the 4-month visit, which remained through to the end of our follow-up period.
Figure 2.
Kaplan-Meier curves of time to first exacerbation during 24 months of follow-up in the Childhood Asthma Management Program by score category. A, EPR-3. B, American Thoracic Society/European Respiratory Society. See Figure 1 legend for expansion of the abbreviation.
Table 5.
—Hazard Ratios of Time to First Exacerbation During 24 Months of Follow-up in the CAMP
| 1-Year Follow-up |
2-Year Follow-up |
||||
| Category | No. | Events, %a | HR (95% CI)b | Events, %a | HR (95% CI)b |
| EPR-3 definition | |||||
| Low risk | 371 | 24.8 | 0.6 (0.5-0.7)c | 39.1 | 0.6 (0.5-0.8)c |
| High risk | 140 | 47.9 | 1.9 (1.4-2.4)d | 67.9 | 1.9 (1.5-2.4)d |
| ATS/ERS definition | |||||
| Low risk | 341 | 48.8 | 0.7 (0.6-0.8)d | 59.3 | 0.7 (0.6-0.9)d |
| High risk | 140 | 69.3 | 1.6 (1.3-2.0)d | 77.9 | 1.5 (1.2-1.9)d |
ATS = American Thoracic Society; EPR-3 = Expert Panel Report 3; ERS = European Respiratory Society; See Table 1 and 3 legends for expansion of other abbreviations.
Percentage of subjects in each category that had asthma exacerbations during the follow-up period.
HR adjusted for age, sex, race, and treatment group.
P < .05.
P < .001.
Discussion
To our knowledge, this study is the first to examine risk factors for asthma exacerbations in a well-characterized cohort of Hispanic children. We constructed a checklist-type clinical score to identify Costa Rican children at low and high risk for asthma exacerbations. This score performed well in the exploratory and validation sets, indicating good reproducibility. In Costa Rica, compared with children at average risk for exacerbations, children at low risk had 0.2 times lower odds for exacerbations, and children at high risk had five to eight times higher odds for exacerbations. When applied to a cohort of North American children with asthma participating in a clinical trial (CAMP), the magnitude of the effect was attenuated, but the results were consistent: Compared with children at average risk for exacerbations, children with a low score had a 40% lower risk, and children with a high score had a 90% higher risk .
The PVs allow us to predict how many of the children in a category will have the outcome. The PV of the score’s high-risk group was 97% in Costa Rica and 69% to 78% in CAMP, meaning that between 70% and 90% of children classified as high risk will indeed have a severe exacerbation. The usefulness of having a low-risk group was less clear cut, with the PV being 36% to 40% in Costa Rica and 51% to 61% in CAMP. The differences in PVs are expected because of the lower prevalence of exacerbations in CAMP. Importantly, the low-risk group had a very high PV for no hospitalizations in both cohorts (94%-99%). To make our results comparable for future studies, we included a second definition of an asthma exacerbation based on ATS/ERS definitions. This outcome was more prevalent in our cohorts (leading to variation in the PVs), but the overall results and risk ratios were similar and remained significant.
Several differences existed between the study cohorts. Costa Rica was a cross-sectional study, whereas CAMP was a prospective clinical trial. Participation in a clinical trial has been shown to increase adherence and improve outcomes. Whereas CAMP included children with mild to moderate persistent asthma, the Costa Rican cohort had a broader range of severity. Differences also existed between the cohorts with regard to medical history (eg, family history of atopy) and health-care utilization (eg, unscheduled visits and hospitalizations). Despite these differences, we were able to predict the risk of asthma exacerbations in CAMP after 1 and 2 years of follow-up.
Identification of risk factors for asthma morbidity (from genetics, to in utero factors, to environmental exposures16-24) has had limited success.19,25 Although two computer-based models had good predictive performances for asthma morbidity (AUC, 0.70-0.78),16,18 they required a detailed clinical record integrated with medication refill information. We obtained results similar to those of more complex models (training set AUC, 0.76; validation phases AUC, 0.65-0.73) using variables attainable in a physician’s office. The performance of our score compares well with those used for other diseases.26-28 For example, acute physiology and chronic health evaluation scores have an AUC of 0.66 to 0.79 when assessing prognosis in cirrhosis and severe sepsis.26,27
Factor analysis and PCA inspect multiple variables and look at interrelations and clustering to identify the underlying structure of a large set of variables.29,30 Rather than evaluating variables in relationship to an outcome (as in regression analysis), factor analysis and PCA describe how variables are related to one another. Each resulting factor comprises several variables that may reflect a unique disease aspect. By using different factors, we aimed to cover multiple disease aspects. Although a few studies have used factor analysis and PCA to identify components of asthma morbidity and care,31-33 none have evaluated such components in relation to clinical outcomes.29 To our knowledge, this study is the first to build a clinical score based on factor analysis and PCA and to evaluate its performance and predictive utility both in a validation subset and in an independent cohort. Although exclusion of laboratory and pulmonary function data eliminated some of the identified clusters, we were interested in developing a predictive score for use in primary-care settings in different countries.
There are several limitations to our study. First, the predictive score was built in a cross-sectional study. Without a prospective timeline, it is difficult to assess whether the risk factors really antedated the outcomes or whether they were related, and this can lead to significant difficulties in the interpretation of the results. However, we were able to evaluate outcomes over 2 years of follow-up by applying the score to CAMP, where the score’s predictive utility remained significant over time. Second, there was an appreciable reduction in risk ratios and PVs in CAMP compared with Costa Rica, which likely reflects the different characteristics described previously. Costa Rica had a higher prevalence of exacerbations and a broader spectrum of disease severity, and importantly, the score was originally designed using this cohort. Third, the PCA technique shows correlations among the variables introduced, but it does not indicate whether using more variables would improve the results. Finally, the goodness of fit for Costa Rica and the first year of CAMP were high, indicating a very good calibration of the score, but it was lower in year 2 (e-Table 4), which may imply that the instrument is not as accurate that far out.
Developing an effective predictive score for clinical practice is potentially important for identifying patients at high or low risk for asthma exacerbations and would allow providers to improve management, better allocate treatment, and make referrals when necessary. The practical usefulness of such a score, on the other hand, depends on its ease of use and interpretability. Including more variables and components may yield a more accurate tool, but the trade-off would be to produce an instrument that is too lengthy and, thus, too cumbersome for clinical practice.
In summary, we present a clinical score that is effective at identifying groups of children with asthma at high risk for exacerbations. The score is designed as a checklist questionnaire and excludes laboratory data in order to be easily used in primary-care settings, particularly in areas with few resources or where access to subspecialty care is difficult. Ideally, this tool should be prospectively assessed in a cohort in this type of population. If validated, a next step would be to evaluate whether it is useful in guiding or adjusting therapy. To ensure generalizability, we recommend using the ATS/ERS definitions for mild, moderate, and severe asthma exacerbations in future prospective studies assessing this score.
Supplementary Material
Acknowledgments
Author contributions: Dr Forno: contributed to the data analysis and was primarily responsible for writing the manuscript.
Dr Fuhlbrigge: contributed to the data analysis and the drafting of the manuscript and review of its final version.
Dr Soto-Quirós: contributed to the data collection and the drafting of the manuscript and review of its final version.
Dr Avila: contributed to the data collection and the drafting of the manuscript and review of its final version.
Dr Raby: contributed to the data analysis and the drafting of the manuscript and review of its final version.
Dr Brehm: contributed to the data analysis and the drafting of the manuscript and review of its final version.
Ms Sylvia: contributed to the data collection and the drafting of the manuscript and review of its final version.
Dr Weiss: contributed to obtaining funding, the study design, data analysis, and the drafting of the manuscript and review of its final version.
Dr Celedón: contributed to obtaining funding, the study design, data collection, data analysis, and the drafting of the manuscript and review of its final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Fuhlbrigge received research funding from the National Institutes of Health and unrestricted research support from GlaxoSmithKline (GSK), Merck, and Boehringer Ingelheim; served as a consultant to GSK and Merck for the design and analysis of epidemiologic studies; and served on an advisory board and has been a member of a speakers bureau for GSK and Merck. Dr Soto-Quirós received lecture fees from AstraZeneca, GSK, and Merck Sharp & Dohme; Dr Avila received lecture fees from AstraZeneca and Merck Sharp & Dohme; Dr Raby received lecture fees from Novartis Pharmaceuticals; and Dr Weiss received consulting fees from Genentech. Drs Forno, Brehm, and Celedón and Ms Sylvia have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: All work on data collected for the Genetics of Asthma in Costa Rica was conducted at the Channing Laboratory of the Brigham and Women’s Hospital under appropriate policies and human subject protections.
Other contributions: We thank all the families for their invaluable participation in the Genetics of Asthma in Costa Rica and the Childhood Asthma Management Program (CAMP) studies. We acknowledge the CAMP investigators and research team for their help in data collection.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/138/5/1156/suppl/DC1.
Abbreviations
- ATS
American Thoracic Society
- AUC
area under the curve
- CAMP
Childhood Asthma Management Program
- EPR-3
Expert Panel Report 3
- ERS
European Respiratory Society
- ISAAC
International Study for Asthma and Allergies in Childhood
- NHLBI
National Heart, Lung, and Blood Institute
- PCA
principal component analysis
- PV
predictive value
- STR
skin test reactivity
Footnotes
Funding/Support: The Genetics of Asthma in Costa Rica study is supported by National Institutes of Health [Grants HL04370 and HL66289]. The Childhood Asthma Management Program is supported by National Heart, Lung, and Blood Institute [Grants NO1-HR-16044, NO1-HR-16045, NO1-HR-16046, NO1-HR-16047, NO1-HR-16048, NO1-HR-16049, NO1-HR-16050, NO1-HR-16051, and NO1-HR-16052] and General Clinical Research Center [Grants M01RR00051, M01RR0099718-24, M01RR02719-14] and the National Center for Research Resources [Grant RR00036]. This work was conducted at the Channing Laboratory of Brigham and Women’s Hospital.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Asher MI, Montefort S, Björkstén B, et al. ISAAC Phase Three Study Group Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Moorman JE, Rudd RA, Johnson CA, et al. Centers for Disease Control and Prevention (CDC) National Surveillance for Asthma—United States, 1980–2004. MMWR Surveill Summ. 2007;56(8):1–54. [PubMed] [Google Scholar]
- 3.Dilley JA, Pizacani BP, Macdonald SM, Bardin J. The Burden of Asthma in Washington State. Olympia, WA: State Department of Health; 2005. pp. 345–201. DOH publication. [Google Scholar]
- 4.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Pearce N, Aït-Khaled N, Beasley R, et al. ISAAC Phase Three Study Group Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62(9):758–765. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunninghake GM, Soto-Quiros ME, Avila L, et al. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol. 2007;119(3):654–661. doi: 10.1016/j.jaci.2006.12.609. [DOI] [PubMed] [Google Scholar]
- 7.Ly NP, Soto-Quirós ME, Avila L, et al. Paternal asthma, mold exposure, and increased airway responsiveness among children with asthma in Costa Rica. Chest. 2008;133(1):107–114. doi: 10.1378/chest.07-2130. [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal MN, Banks-Schlegel S, Bleecker ER, Marsh DG, Ober C. Collaborative studies on the genetics of asthma–National Heart, Lung and Blood Institute. Clin Exp Allergy. 1995;25(suppl 2):29–32. doi: 10.1111/j.1365-2222.1995.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 9.ISAAC Phase II modules of the International Study of Asthma and Allergies in Childhood. http://www.uni-ulm.de/med/med-epidemiologie/forschung-in-der-epidemiologie/isaac.html. Modified March 6, 2009. Accessed May 14, 2010.
- 10.American Thoracic Society Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 11.Chatham M, Bleecker ER, Smith PL, Rosenthal RR, Mason P, Norman PS. A comparison of histamine, methacholine, and exercise airway reactivity in normal and asthmatic subjects. Am Rev Respir Dis. 1982;126(2):235–240. doi: 10.1164/arrd.1982.126.2.235. [DOI] [PubMed] [Google Scholar]
- 12.Weiland SK, Björkstén B, Brunekreef B, Cookson WO, von Mutius E, Strachan DP. International Study of Asthma and Allergies in Childhood Phase II Study Group Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): rationale and methods. Eur Respir J. 2004;24(3):406–412. doi: 10.1183/09031936.04.00090303. [DOI] [PubMed] [Google Scholar]
- 13.The Childhood Asthma Management Program Research Group Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343(15):1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 14.Childhood Asthma Management Program Research Group The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Control Clin Trials. 1999;20(1):91–120. [PubMed] [Google Scholar]
- 15.Reddel HK, Taylor DR, Bateman ED, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 16.Schatz M, Cook EF, Joshua A, Petitti D. Risk factors for asthma hospitalizations in a managed care organization: development of a clinical prediction rule. Am J Manag Care. 2003;9(8):538–547. [PubMed] [Google Scholar]
- 17.Celedón JC, Soto-Quiros ME, Silverman EK, Hanson L, Weiss ST. Risk factors for childhood asthma in Costa Rica. Chest. 2001;120(3):785–790. doi: 10.1378/chest.120.3.785. [DOI] [PubMed] [Google Scholar]
- 18.Lieu TA, Quesenberry CP, Sorel ME, Mendoza GR, Leong AB. Computer-based models to identify high-risk children with asthma. Am J Respir Crit Care Med. 1998;157(4 pt 1):1173–1180. doi: 10.1164/ajrccm.157.4.9708124. [DOI] [PubMed] [Google Scholar]
- 19.Berger WE, Legorreta AP, Blaiss MS, et al. The utility of the Health Plan Employer Data and Information Set (HEDIS) asthma measure to predict asthma-related outcomes. Ann Allergy Asthma Immunol. 2004;93(6):538–545. doi: 10.1016/S1081-1206(10)61260-4. [DOI] [PubMed] [Google Scholar]
- 20.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 21.Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Vermeire PA, Van Bever HP. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy. 2000;30(11):1547–1553. doi: 10.1046/j.1365-2222.2000.00939.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20(6):682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 23.Carroll KN, Hartert TV. The impact of respiratory viral infection on wheezing illnesses and asthma exacerbations. Immunol Allergy Clin North Am. 2008;28(3):539–561. doi: 10.1016/j.iac.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110(1):83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Taylor BW. The identification of high risk asthmatic children using the emergency department asthma visit count. J Emerg Med. 1999;17(6):953–956. doi: 10.1016/s0736-4679(99)00122-5. [DOI] [PubMed] [Google Scholar]
- 26.Cholongitas E, Senzolo M, Patch D, Shaw S, Hui C, Burroughs AK. Review article: scoring systems for assessing prognosis in critically ill adult cirrhotics. Aliment Pharmacol Ther. 2006;24(3):453–464. doi: 10.1111/j.1365-2036.2006.02998.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnston JA. Determinants of mortality in patients with severe sepsis. Med Decis Making. 2005;25(4):374–386. doi: 10.1177/0272989X05278933. [DOI] [PubMed] [Google Scholar]
- 28.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 29.Riekert KA, Eakin M. Factor analysis: a primer for asthma researchers. J Allergy Clin Immunol. 2008;121(5):1181–1183. doi: 10.1016/j.jaci.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. Stanford, CA: Springer; 2001. [Google Scholar]
- 31.Leung TF, Wong GW, Ko FW, Lam CW, Fok TF. Clinical and atopic parameters and airway inflammatory markers in childhood asthma: a factor analysis. Thorax. 2005;60(10):822–826. doi: 10.1136/thx.2004.039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt EW, Cook EF, Covar RA, Spahn J, Fuhlbrigge AL. Identifying the components of asthma health status in children with mild to moderate asthma. J Allergy Clin Immunol. 2008;121(5):1175–1180. doi: 10.1016/j.jaci.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juniper EF, Wisniewski ME, Cox FM, Emmett AH, Nielsen KE, O’Byrne PM. Relationship between quality of life and clinical status in asthma: a factor analysis. Eur Respir J. 2004;23(2):287–291. doi: 10.1183/09031936.04.00064204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.