Abstract
Background:
Asthma and COPD are characterized by airway dysfunction and inflammation. Neutrophilic airway inflammation is a common feature of COPD and is recognized in asthma, particularly in severe disease. The T helper (Th) 17 cytokines IL-17A and IL-17F have been implicated in the development of neutrophilic airway inflammation, but their expression in asthma and COPD is uncertain.
Methods:
We assessed IL-17A and IL-17F expression in the bronchial submucosa from 30 subjects with asthma, 10 ex-smokers with mild to moderate COPD, and 27 nonsmoking and 14 smoking control subjects. Sputum IL-17 concentration was measured in 165 subjects with asthma and 27 with COPD.
Results:
The median (interquartile range) IL-17A cells/mm2 submucosa was increased in mild to moderate asthma (2.1 [2.4]) compared with healthy control subjects (0.4 [2.8]) but not in severe asthma (P = .04). In COPD, IL-17A+ cells/mm2 submucosa were increased (0.5 [3.7]) compared with nonsmoking control subjects (0 [0]) but not compared with smoking control subjects (P = .046). IL-17F+ cells/mm2 submucosa were increased in severe asthma (2.7 [3.6]) and mild to moderate asthma (1.6 [1.0]) compared with healthy controls subjects (0.7 [1.4]) (P = .001) but was not increased in subjects with COPD. IL-17A and IL-17F were not associated with increased neutrophilic inflammation, but IL-17F was correlated with the submucosal eosinophil count (rs = 0.5, P = .005). The sputum IL-17 concentration in COPD was increased compared with asthma (2 [0-7] pg/mL vs 0 [0-2] pg/mL, P < .0001) and was correlated with post-bronchodilator FEV1% predicted (r = −0.5, P = .008) and FEV1/FVC (r = −0.4, P = .04).
Conclusions:
Our findings support a potential role for the Th17 cytokines IL-17A and IL-17F in asthma and COPD, but do not demonstrate a relationship with neutrophilic inflammation.
Asthma and COPD are common conditions that account for substantial morbidity and mortality worldwide. Asthma affects 5% to 10% of adults, of whom 10% have severe disease.1,2 Severe asthma represents a disproportionate health-care burden as it leads to debilitating chronic symptoms despite optimal standard asthma treatment and contributes to more than half of the health-care costs attributed to asthma.1-4 COPD is predicted to be the third leading cause of death in 2030.5,6 Both conditions are characterized by airflow obstruction with airway inflammation and remodeling.
COPD is considered a neutrophilic airway disease with increased infiltration of the airway with CD8+ T cells,7 whereas asthma is characterized by Th2 cytokine expression and eosinophilic inflammation.8 However, there is increasing recognition that asthma and COPD are diseases with phenotypic heterogeneity in terms of clinical expression, airway dysfunction, and immunopathology.9,10 Indeed, the application of induced sputum to study large groups of patients with airway disease has suggested that there is considerable overlap between these conditions, with neutrophilic inflammation observed in up to 40% of patients with asthma, particularly in those with severe disease.11 Therefore, there is a pressing need to further understand the potential mechanisms involved in the initiation and persistence of neutrophilic inflammation in airways disease.
A distinct T-cell lineage, called T-helper (Th)17 cells, has been identified and characterized by the production of IL-17A, IL-17F, and IL-22.12 In addition to CD4+ cells, IL-17A and F can be released by neutrophils, eosinophils, CD8+ T cells, basophils, and mast cells.13 Both cytokines can induce the expression of a variety of proinflammatory cytokines and chemokines in epithelial and vascular endothelial cells, fibroblasts, neutrophils, and eosinophils, including IL-6, granulocyte macrophage colony-stimulating factor, CXCL10, and CXCL8.14 The induction of CXCL8, a potent neutrophil chemokine, has implicated these Th17 cytokines in the development of neutrophilic airway inflammation.13,14 In support of this view recent evidence from animal models demonstrates that allergic sensitization through the airway primes strong Th17 responses that promote airway neutrophilia and airway hyperresponsiveness,15 whereas IL-17F-deficient mice have an impaired neutrophilic response to allergen.16 In humans there is emerging evidence to support an increase in IL-17A and IL-17F expression in moderate to severe asthma17,18 and COPD.19 However, whether this expression is associated with granulocytic inflammation in the airway wall or lumen is uncertain.
We hypothesized that IL-17A and IL-17F expression is increased in asthma and COPD and is related to disease severity and the intensity of neutrophilic inflammation. To test our hypothesis we have measured the IL-17A sputum concentration and the number of IL-17A and F+ cells in the bronchial mucosa and assessed the degree of neutrophilic inflammation in the airway in asthma and COPD.
Materials and Methods
Subjects
Subjects were recruited from hospital staff and general respiratory and “Difficult Asthma” clinics at Glenfield Hospital, Leicester; local primary health care; and by local advertising. Asthma was defined according to the current Global Initiative for Asthma (GINA) guidelines.2 Subjects with asthma had typical symptoms and the presence of one or more of the following objective criteria: significant bronchodilator reversibility of FEV1 > 200 mL, a provocation concentration of methacholine causing a 20% fall in FEV1 (PC20) of < 8 mg/mL, or a peak flow amplitude percent mean over 2 weeks of > 20%. Asthma severity was classified using the GINA treatment steps (mild to moderate 1-3, severe 4-5).2 COPD was diagnosed and severity categorized by using Global Initiative for Chronic Obstructive Lung Disease criteria.6 Subjects with COPD who demonstrated partial bronchodilator reversibility were not excluded. Subjects were recruited as three independent cross-sectional groups, to assess IL-17 A, F, and IL-17R expression in proximal airways in asthma (group 1) and COPD (group 2), and sputum IL-17 concentration in asthma and COPD (group 3). Subjects were free from exacerbations for at least 6 weeks. Healthy control subjects had normal spirometry and some smokers with > 10 pack-year history were included to enable comparisons between healthy smokers and subjects with COPD. Sixty-eight of 295 subjects had participated in an earlier report.20 All subjects gave written informed consent with study approval from the Leicestershire ethics committee.
Protocol
Demographics and spirometry were recorded for all subjects. Subjects with asthma and healthy control subjects in group 1 and 2 also underwent methacholine inhalation test using the tidal breathing method21 and allergen skin prick tests for Dermatophagoides pteronyssinus, dog, cat, and grass pollen. Sputum induction was performed in all subjects in groups 1 and 2.22
In group 1, subjects underwent bronchoscopy conducted according to the British Thoracic Society guidelines,23 and biopsies were taken from the right middle and lower lobe carinae. In group 2, proximal airway samples were collected from surgical specimens. All bronchial mucosal specimens were fixed in acetone and embedded in glycomethacrylate as described previously.24
Sputum IL-17 Measurement
Sputum was selected, dispersed using the mucolytic dithiothreitol (DTT), and processed to generate a sputum differential cell count, and cell-free supernatants were stored at -80°C for later analysis as described previously.25Sputum IL-17 was measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems Europe Ltd; Abingdon, England). The lower limit of detection was 15 pg/mL. The IL-17 assay was validated in line with European Respiratory Society recommendations to assess the effect of DTT and the recovery of exogenous spiking with recombinant cytokine.25 IL-17 recovery was not affected by DTT and mean recovery of exogenous spiked IL-17 was 93% (n = 16). IL-17 was also measured in a further independent population of subjects with COPD and asthma using the mesoscale device platform.26
IL-17A and IL-17F Expression in Endobronchial Biopsies
Two-micrometer sections were cut and stained using polyclonal antibodies against IL-17A and IL-17F (R&D Systems Europe Ltd), monoclonal antibodies against eosinophils (major basic protein), neutrophils (neutrophil elastase), and mast cells (tryptase), with appropriate isotype controls mouse IgG1 (Dako UK Ltd; Cambridge, England) and goat immunoglobulins (R&D Systems Europe Ltd). The number of positive nucleated cells was enumerated per mm2 of bronchial submucosa by a blinded observer as described previously.27,28 In a subgroup of six subjects with asthma, IL-17A and IL-17F were colocalized with neutrophils and eosinophils using sequential sections as previously described.27
Statistical Analysis
Statistical analysis was performed using PRISM Version 4 (GraphPad; La Jolla, CA). Parametric data were expressed as mean (SEM), data that had a normal log distribution were log transformed and described as geometric mean (95% CI), and nonparametric data were described as median (interquartile range [IQR]). One-way analysis of variance (Kruskal-Wallis for nonparametric data) was used for across-group comparisons with Tukey and Dunn post hoc tests for between-group comparisons, respectively. Correlations were assessed by Spearman rank (rs) and Pearson (r) correlation coefficients. No corrections were made for multiple comparisons.
Results
IL-17A, IL-17F, and IL-17R Expression in Large Airway Tissue Specimens
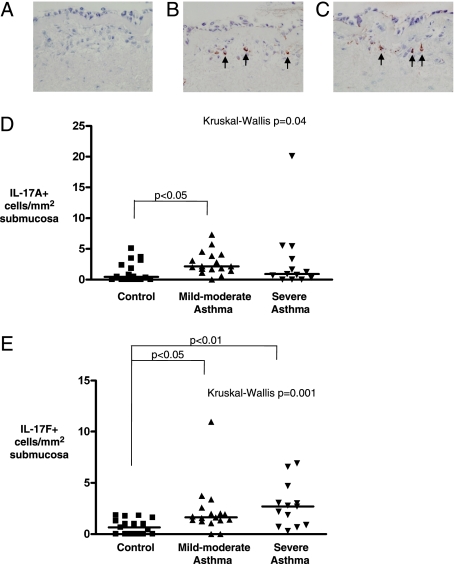
Clinical characteristics of group 1 are as shown in Table 1. Examples of IL-17A+ and IL-17F+ cells in the bronchial submucosa in asthma are as shown in Figures 1A–1C. The median (IQR) IL-17A cells/mm2 submucosa was increased in mild to moderate asthma (2.1 [2.4]) compared with healthy control subjects (0.4 [2.8]) but not compared with asthma (0.9 [4.2]) (P = .04, Kruskal-Wallis; P < .05 mild to moderate asthma vs control subjects) (Fig 1D). The number of IL-17F+ cells/mm2 submucosa was increased in severe asthma (2.7 [3.6]) and mild to moderate asthma (1.6 [1.0]) compared with healthy control subjects (0.7 [1.4]) (P = .001 Kruskal-Wallis; P < .01 severe asthma vs control subjects, and P < .05 mild to moderate vs control subjects) (Fig 1E). The median (IQR) proportion 50% (27%) and 24% (19%) of the IL-17A+ and 44% (40%) and 0 (25%) of IL-17F cells colocalized to neutrophils and eosinophils, respectively.
Table 1.
—Clinical and Sputum Characteristics of Biopsy Group Asthma
| Characteristic | Normal | Mild to Moderate Asthma (GINA 1-3) | Severe Asthma (GINA 4-5) |
| No. | 17 | 17 | 13 |
| Agea | 45 (5) | 49 (4) | 50 (4) |
| Men/women | 7/10 | 8/9 | 6/7 |
| Never smokers/current smokers/ex-smokers | 15/2/0 | 12/5/0 | 8/5/0 |
| Pack-yearsa | 0.4 (0.3) | 3 (1) | 5 (3) |
| Atopy, No. (%) | 6 (35) | 9 (53) | 7 (54) |
| PC20FEV1, mg/mLb | > 16 | 0.33 (0.14-0.78)c | 0.38 (0.1-1.4)c |
| FEV1, % predicteda | 99 (4) | 87 (5) | 80 (7)c |
| Pre-BD FEV1/FVC, %a | 78 (3) | 74 (3) | 74(3) |
| BD response, %a | 1 (1) | 9 (4)c | 13 (6)c |
| Sputum cell counts | |||
| TCCa | 1.9 (0.4) | 2.7 (0.7) | 2.7 (0.6) |
| Eosinophil, %d | 0.3 (0.9) | 0.5 (3.2) | 6.6 (14.6)c |
| Neutrophil, %a | 43 (12) | 54 (6) | 59 (10) |
| Macrophage, %a | 51 (9) | 38 (6) | 25 (6)c |
| Lymphocyte, %a | 1.8 (0.8) | 1.0 (0.2) | 1.5 (0.7) |
| Epithelial cells, %a | 3 (2) | 4 (1) | 7 (3) |
| Cells/mm2 submucosa | |||
| IL-17Ad | 0.4 (2.8) | 2.1 (2.4)c | 0.9 (4.2) |
| IL-17Fd | 0.7 (1.4) | 1.6 (1.0)c | 2.7 (3.6)c |
| Mast cellsd | 24.4 (23) | 28.8 (31.8) | 25.8 (56.5) |
| Neutrophilsd | 8 (13) | 9.3 (12.7) | 8.2 (11.5) |
| Eosinophilsd | 3.0 (9) | 6.2 (18.9) | 10.0 (41.2) |
BD = bronchodilator; GINA = Global Initiative for Asthma; IQR = interquartile range; PC20 = provocation concentration causing a 20% fall in FEV1; SE = standard error. TCC = total cell count.
Mean (SE).
Geometric mean (95% CI).
P < .05 compared with control subjects.
Median (IQR).
Figure 1.
IL-17A and IL-17F expression in the submucosa in asthma. Representative photomicrographs of bronchial biopsy sections from subjects with severe asthma, illustrating isotype control. A, Goat IgG. B, IL-17A+ cells present in the bronchial submucosa. C, IL-17F+ cells in the submucosa (× 400). IL-17A/F+ cells highlighted by arrows. D, The number of IL-17A+ and E, IL-17F+ cells in the bronchial submucosa of healthy control subjects, subjects with mild to moderate asthma (Global Initiative for Asthma [GINA] 1-3), and subjects with severe asthma (GINA 4-5). P values for across-group comparison by Kruskal-Wallis test and Dunn post hoc test for between-group comparisons are as shown.
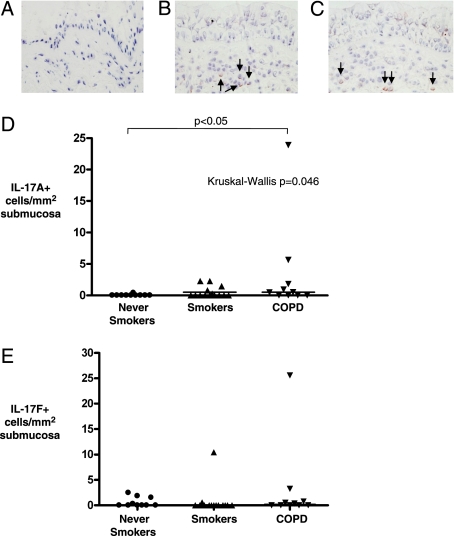
Clinical characteristics of group 2 are as shown in Table 2. All of the COPD subjects were ex-smokers. Examples of IL-17A+ and IL-17F+ cells in the bronchial submucosa in COPD are as shown in Figures 2A–2C. The median (IQR) IL-17A+ cells/mm2 submucosa was increased in COPD (0.5 [3.7]) compared with nonsmoking control subjects (0 [0]), but not compared with smoking control subjects, (P = .046, Kruskal-Wallis; P < .05 COPD vs nonsmoking control subjects) (Fig 2D). There were no differences in the number of IL-17F+ cells/mm2 submucosa in lung resection tissue from subjects with COPD and control subjects with and without a significant smoking history (Fig 2E, Table 2).
Table 2.
—Clinical Characteristics and IL-17A and IL-17F Expression in COPD
| Characteristic | Normal | Smoker | COPD |
| No. | 10 | 14 | 10 |
| Agea | 59 (3) | 53 (5) | 65 (3) |
| Men (women) | 8 (2) | 10 (4) | 7 (3) |
| Never smokers/current smokers/ex-smokers | 8/0/2 | 0/0/14 | 0/0/10 |
| Pack-yearsa | 2 (1) | 28 (5) | 37 (6)b |
| FEV1a | 2.7 (0.2) | 2.6 (0.2) | 1.7 (0.2)b |
| FEV1, % predicteda | 86 (3) | 84 (3) | 62 (3)b |
| Pre-BD FEV1/FVCa | 78 (2) | 78 (1) | 56 (3)b |
| Cells/mm2 submucosa | |||
| IL-17A | 0 (0) | 0 (1.1) | 0.5 (3.7)b |
| IL-17F | 0 (1.7) | 0 (0) | 0.2 (2) |
See Table 1 legend for expansion of the abbreviation.
Mean (SE).
P < .05 compared with nonsmoking control subjects.
Figure 2.
IL-17A and IL-17F expression in the submucosa in COPD. Representative photomicrographs of proximal airway resections from COPD tissue, illustrating isotype control. A, Goat IgG. B, IL-17A+ cells present in the submucosa. C, IL-17F+ cells in the submucosa (× 400). IL-17A/F+ cells highlighted by arrows. D, The number of IL-17A+ and E, IL-17F+ cells in the submucosa of healthy control subjects (never smokers), healthy control subjects (smokers), and subjects with COPD. P values for across-group comparison by Kruskal-Wallis test and Dunn post hoc test for between-group comparisons are as shown.
The number of IL-17A+ cells was correlated with FEV1% predicted (Rs = 0.38; P = .04), and the sputum neutrophil count (Rs = −0.43, P = .03) in asthma but was not associated with the number of neutrophils or eosinophils in the bronchial mucosa (Table 3). The number of IL-17F+ was positively correlated with the number of eosinophils in tissue (rs = 0.50; P = .005) but not significantly correlated with lung function or neutrophil number in tissue or sputum (Table 3).
Table 3.
—Correlations Between IL-17A/F, Granulocytic Inflammation, and Lung Function in Asthma
| Characteristic | IL-17A | IL-17F |
| Neutrophils/mm2 submucosa | rs = −0.05, P = .8 | rs = 0.32, P = .078 |
| Sputum neutrophils (%) | rs = −0.43, P = .03 | rs = −0.15, P = .45 |
| Eosinophils/mm2 submucosa | rs = 0.2, P = .3 | rs = 0.50, P = .005 |
| Sputum eosinophils, % | rs = 0.09, P = .7 | rs = 0.08, P = .7 |
| FEV1, % predicted | rs = 0.38, P = .04 | rs = −0.06, P = .8 |
| FEV1/FVC, % | rs = 0.35, P = .06 | rs = −0.17, P = .39 |
| Methacholine PC20FEV1 | rs = −0.17, P = .5 | rs = −0.25, P = .25 |
rs = Spearman rank correlation coefficient. See Table 1 legend for expansion of the other abbreviation.
Sputum IL-17 Asthma and COPD
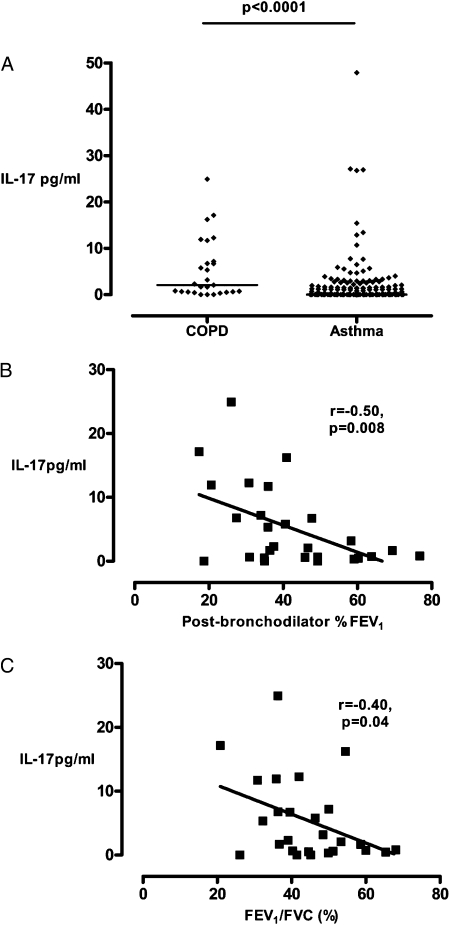
Sputum IL-17 was below the limit of detection in all samples from subjects with asthma (n = 22), subjects with COPD (n = 22), and control subjects (n = 12) measured by ELISA. Using the mesoscale device platform the sputum IL-17 concentration in COPD was increased compared with asthma (2 [0-7] pg/mL vs 0 [0-2] pg/mL, P < .0001) and was correlated with post-bronchodilator FEV1% predicted (r = −0.5, P = .008) and FEV1/FVC (r = −0.4, P = .04) (Figs 3A–3C).The clinical characteristics of these subjects with asthma and COPD are as shown (Table 4).
Figure 3.
Sputum IL-17 in asthma and COPD. A, Sputum IL-17 concentration in asthma and COPD. Horizontal bar represents the median. B, Correlations between sputum IL-17 concentration in subjects with COPD and postbronchodilator FEV1% predicted. C, Correlations between sputum IL-17 concentration in subjects with COPD and FEV1/FVC.
Table 4.
—Clinical Characteristics and IL-17A and IL-17F Sputum Concentration in Asthma and COPD
| Characteristic | COPD (n = 27) | Asthma (n = 165) |
| Agea | 68 (1.6) | 50 (1.1) |
| Men, % | 74 | 41 |
| Never smokers/current smokers/ex-smokers | 0/18/9 | 109/44/12 |
| Pack yearsa | 47 (6) | 15 (3) |
| Post-BD FEV1, La | 1.1 (0.1) | 2.2 (0.1) |
| Post-BD FEV1, % predicteda | 42 (3) | 76 (2) |
| Post-BD FEV1/FVC %a | 44 (2) | 70 (1) |
| Sputum neutrophil count, %a | 65 (5) | 63 (2) |
| Sputum eosinophil count, %b | 1.5 (0.9-2.7) | 2.7 (2.1-3.5) |
| Sputum IL-17, pg/mLc | 2 (0-7) | 0 (0-2) |
See Table 1 legend for expansion of the abbreviation.
Mean (SE).
Geometric mean (95% CI).
Median (IQR).
Discussion
We report here that IL-17A expression in the bronchial submucosa was increased in mild to moderate asthma, and IL-17F was increased in mild to moderate and severe asthma. In contrast, in ex-smoking subjects with mild to moderate COPD the number of IL-17A+ cells in the bronchial submucosa, but not IL-17F, was increased compared with nonsmoking control subjects. In asthma there was a weak relationship between the number of IL-17A+ cells and the FEV1% predicted, and the sputum neutrophil count. There was no association between neutrophilic inflammation and IL-17A or F expression in the bronchial submucosa. However, there was a good correlation between the number of IL-17F+ cells and eosinophils in the bronchial submucosa. In COPD there was a highly significant, albeit small, increase in sputum IL-17 compared with asthma, and this was correlated with airflow obstruction and lung function impairment, but not airway inflammation. Our study therefore supports our hypothesis that IL-17 may play a role in asthma and COPD.
There is an increasing body of evidence supporting a role for Th17 cells in asthma.13,14 Animal models of asthma suggest that Th17 cytokines promote neutrophilic inflammation,15,16 which in concert with Th2 cells is important in the development of airway hyperresponsiveness.17 Previous reports have demonstrated increased IL-17A and IL-17F bronchial submucosa expression in moderate to severe asthma,17,18 which were attenuated by systemic corticosteroids.17 The data for sputum IL-17 is conflicting with one study demonstrating no differences in the sputum IL-17 concentration between asthma and healthy volunteers,29 whereas in another study IL-17 was increased in moderate to severe asthma.30 In the latter study sputum IL-17 was measured before and after inhaled corticosteroids. Importantly, consistent with our findings previous studies have found that the sputum IL-17 concentration in sputum is low and in some studies samples have needed to be concentrated to detect IL-17. We found that IL-17A and IL-F were increased in mild to moderate disease, whereas IL-17F but not IL-17A was increased in severe asthma. Our subjects with severe asthma were treated with high-dose inhaled and/or oral corticosteroids, and therefore this may have attenuated the IL-17A expression in this group. IL-17A and IL-17F expression was not associated with smoking status but importantly this study was not powered to fully explore the effects of smoking in asthma. We report for the first time, to our knowledge, the relationship between IL-17A and F expression and neutrophilic inflammation in tissue and sputum. We found that there was no association between IL-17A and IL-17F expression neutrophilic inflammation in tissue. Intriguingly, there was a weak inverse correlation between submucosal IL-17A expression and the sputum neutrophil count. Corticosteroids attenuate IL-17 expression in tissue17 but do not modulate sputum neutrophil counts.31,32 Sputum IL-17 was also markedly attenuated by inhaled corticosteroids, but the sputum neutrophil count was unchanged, questioning the role of IL-17 in persistent neutrophilic airway inflammation. However, others have demonstrated an association between IL-17 and IL-8 mRNA in sputum cells and the number of sputum neutrophils,33 suggesting that the site of IL-17 expression may be important. Interestingly, we also found a weak relationship between FEV1% predicted and IL-17A expression. Consistent with our finding in a recent comparison between subjects with severe asthma with and without persistent airflow obstruction, IL-17 was among the dominant mediators associated with the absence of persistent airflow obstruction.34 Together these findings do not support a role for IL-17 in the development of fixed airflow obstruction in asthma.
To date there are few data examining the expression of IL-17A or IL-17F in COPD. Neutrophilic inflammation is a common feature of COPD.7,11 Hence, it is predictable that IL-17 may play an important role in this disease. There is a single report of IL-17A and F expression in bronchoscopic biopsies obtained across the spectrum of COPD disease severity compared with smoking and nonsmoking control subjects.15 IL-17A was increased equally across the severity of COPD compared with the nonsmoking control subjects, but was not different from the smoking control subjects. IL-17F was not significantly different across all the disease and control groups. In this study the number of neutrophils in tissue was increased in the COPD groups with a marked increase in severe COPD. There was no relationship between IL-17A and IL-F expression and neutrophilic inflammation. Our findings of increased IL-17A expression in mild to moderate COPD compared with nonsmoking control subjects, but not smoking control subjects, is therefore entirely consistent with these earlier reports. We found that the sputum IL-17 concentration was increased in COPD compared with asthma. However, we were unable to detect IL-17 by ELISA, and with a more sensitive mesoscale device platform a substantial proportion still remained below the limit of detection. The difference between COPD and asthma was highly significant, but small, questioning whether this difference is biologically important. Interestingly, the sputum IL-17 concentration in COPD was related to the degree of airflow limitation and obstruction. Therefore, the potential role of IL-17, particularly in severe disease, and its synergy with the development of airway inflammation in response to cigarette smoke exposure or alternatively through interactions with other cytokines, such as tumor necrosis factor35 or IL-1β,36 needs to be further explored.
Neutrophils were the predominant source of IL-17 as evidenced by colocalization using immunohistochemistry. However, there was no correlation between the number of IL-17+ cells and neutrophils in the bronchial submucosa. In contrast to the established role of IL-17A and IL-17F in the initiation of neutrophilic inflammation, neither has been implicated in the development of eosinophilic inflammation. Indeed, IL-17F transgenic mice develop an airway neutrophilia after allergen challenge, whereas knockout mice have an impaired neutrophilic response and enhanced Th2 cytokine production.16 Therefore, we were surprised by the relationship between the number of IL-17F+ cells and eosinophils in the bronchial submucosal. However, a similar relationship has been described between sputum IL-17 mRNA and IL-5 mRNA,33 possibly suggesting a hitherto undescribed association between eosinophilic airway inflammation and the Th17 axis. Eosinophils express the IL-17 receptors and have enhanced cytokine release after IL-17F activation.37 Thus, the potential interactions between eosinophilic inflammation and up-regulation of the Th17 axis require further study.
Our study has a number of possible criticisms. This is a cross-sectional observational study. Whether IL-17A and IL-17F expression is related to longitudinal clinical outcomes, such as disease progression, lung function decline, and exacerbations, requires further examination. Similarly, we are unable to determine whether differences observed between mild and severe asthma reflect disease severity or are a consequence of differences in treatment. Current literature suggests that IL-17A is sensitive to corticosteroid therapy,17,30 in contrast to neutrophilic inflammation, which is corticosteroid resistant.31,32 Importantly, the number of IL-17A or IL-17F cells was expressed as cells/mm2 of submucosa rather than as a proportion of the total number of cells in the submucosa. Therefore, whether the changes in cell number between groups is a reflection of increased expression or an increase in the total number of cells needs to be examined in future studies. We are confident that our assays to assess IL-17 were robust as recovery was unaffected by the mucolytic DTT and recovery of exogenous spikes of IL-17 to sputum samples was good. However, a number of samples were below the limit of detection; therefore, the sputum data do need to be interpreted cautiously. Furthermore, no corrections for multiple comparisons were made. Therefore, findings with a marginal level of significance also need to be interpreted with caution.
In conclusion, we found that IL-17A expression in bronchial submucosa was increased in mild to moderate asthma and in COPD, although this was not independent of smoking. IL-17F expression was increased in mild to moderate and severe asthma, but not in COPD. The increased IL-17A and IL-17F expression was not associated with increased neutrophilic inflammation. Sputum IL-17 was increased in COPD and was related to airflow obstruction. Our findings therefore do support a potential role for IL-17A and IL-17F in asthma and possibly COPD. Efficacy studies of therapeutic strategies targeted at the IL-17 axis are eagerly awaited that will further define the functional importance of IL-17A and IL-17F in airways disease.
Acknowledgments
Author contributions: Ms Doe: contributed to immunohistochemistry analysis and drafting the original manuscript, and contributed to and approved the final manuscript.
Dr Bafadhel: contributed to supervising clinical characterization, coordinating the sputum sampling and analysis, and performing bronchoscopies, and contributed to and approved the final manuscript.
Dr Siddiqui: contributed to supervising clinical characterization, coordinating the sputum sampling and analysis, and performing bronchoscopies, and contributed to and approved the final manuscript.
Dr Desai: contributed to supervising clinical characterization, coordinating the sputum sampling and analysis, and performing bronchoscopies, and contributed to and approved the final manuscript.
Mr Mistry: contributed to undertaking laboratory characterization of patients and ELISAs, and contributed to and approved the final manuscript.
Dr Rugman: contributed to performing mesoscale measurements and contributed to and approved the final manuscript.
Ms McCormick: contributed to performing mesoscale measurements and contributed to and approved the final manuscript.
Dr Woods: contributed to performing the mesoscale measurements and contributed to and approved the final manuscript.
Dr May: contributed to performing mesoscale measurements and contributed to and approved the final manuscript.
Dr Sleeman: contributed to conception of the project design and contributed to and approved the final manuscript.
Dr Anderson: contributed to conception of the project design and contributed to and approved the final manuscript.
Dr Brightling: contributed to conception of the project design and contributed to and approved the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Brightling has received consultancy fees from MedImmune, AstraZeneca, GlaxoSmithKline, Roche, and Genentech Inc, and research grants from AstraZeneca, MedImmune, and GlaxoSmithKline. Dr Woods owns stock in AstraZeneca Pharmaceuticals. Drs Woods, May, Sleeman, and Anderson are employees of MedImmune. Dr Rugman and Ms McCormick are employees of AstraZeneca. Ms Doe, Drs Bafadhel, Siddiqui, Desai, and Mr Mistry have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Mss Sue Mckenna and Beverley Hargadon for assistance with clinical characterization of the subjects and Mr William Monteiro and Ms Natalie Neale for technical support.
Abbreviations
- DTT
dithiothreitol
- ELISA
enzyme-linked immunosorbent assay
- GINA
Global Initiative for Asthma
- IQR
interquartile range
- PC20
provocation concentration causing a 20% fall in FEV1
- r
Pearson correlation coefficient
- rs
Spearman rank correlation coefficient
- Th
T helper
Ms Doe and Dr Bafadhel contributed equally to this article.
Funding/Support: This study was funded by Asthma UK, MedImmune Ltd, and a Wellcome Senior Clinical Fellowship (C. B.).
References
- 1.British Thoracic Society Scottish Intercollegiate Guidelines Network British Guideline on the Management of Asthma. Thorax. 2008;63(suppl 4):iv1–iv121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma Guidelines 11/06 [Accessed September 12, 2009]. www.ginasthma.com.
- 3.American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162(6):2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 4.Chanez P, Wenzel SE, Anderson GP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119(6):1337–1348. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 5.WHO Statistical Information System World Health Statistics 2008. [Accessed December 19, 2009]. http://www.who.int/whosis/whostat/2008/en/index.html.
- 6.GOLD-the Global Initiative for Chronic Obstructive Lung Disease [Accessed September 12, 2009]. www.goldcopd.com.
- 7.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw AJ, Brightling CE, Green R, Woltmann G, Bradding P, Pavord ID. New insights into the relationship between airway inflammation and asthma. Clin Sci (Lond) 2002;103(2):201–211. doi: 10.1042/cs1030201. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw AJ, Silverman M, Siva R, Pavord ID, Green R. Multi-dimensional phenotyping: towards a new taxonomy for airway disease. Clin Exp Allergy. 2005;35(10):1254–1262. doi: 10.1111/j.1365-2222.2005.02344.x. [DOI] [PubMed] [Google Scholar]
- 10.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brightling CE. Clinical applications of induced sputum. Chest. 2006;129(5):1344–1348. doi: 10.1378/chest.129.5.1344. [DOI] [PubMed] [Google Scholar]
- 12.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123(5):986–994. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123(5):1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RH, Whitehead GS, Nakano H, et al. Allergic sensitisation through the airways primesTh17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(8):720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205(5):1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111(6):1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 18.Al-Ramli W, Préfontaine D, Chouiali F, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Di Stefano A, Caramori G, Gnemmi I, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157(2):316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha S, Doe C, Mistry V, et al. Granulocyte-macrophage colony-stimulating factor expression in induced sputum and bronchial mucosa in asthma and COPD. Thorax. 2009;64(8):671–676. doi: 10.1136/thx.2008.108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 22.Djukanović R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J Suppl. 2002;37:1s–2s. doi: 10.1183/09031936.02.00000102. [DOI] [PubMed] [Google Scholar]
- 23.British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of British Thoracic Society British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56(suppl 1):i1–i21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Britten KM, Howarth PH, Roche WR. Immunohistochemistry on resin sections: a comparison of resin embedding techniques for small mucosal biopsies. Biotech Histochem. 1993;68(5):271–280. doi: 10.3109/10520299309105629. [DOI] [PubMed] [Google Scholar]
- 25.Kelly M, Leigh R, Hargreave FE. Validation of assays for inflammatory mediators in sputum. Eur Respir J. 2000;16(6):1208–1209. doi: 10.1034/j.1399-3003.2000.16f31.x. [DOI] [PubMed] [Google Scholar]
- 26.Bafadhel M, Saha S, Siva R, et al. Sputum IL-5 concentration is associated with a sputum eosinophilia and attenuated by corticosteroid therapy in COPD. Respiration. 2009;78(3):256–262. doi: 10.1159/000221902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery P, Holgate S, Wenzel S. Endobronchial Biopsy Workshop. Methods for the assessment of endobronchial biopsies in clinical research: application to studies of pathogenesis and the effects of treatment. Am J Respir Crit Care Med. 2003;168(6 pt 2):S1–S17. doi: 10.1164/rccm.200202-150WS. [DOI] [PubMed] [Google Scholar]
- 29.Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97(6):726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 30.Zhou QT, Sun YC, Yao WZ. Characteristics of the airway inflammation and the relationship to interleukin-17 in severe asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28(9):630–634. [PubMed] [Google Scholar]
- 31.Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353(9171):2213–2214. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 32.Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60(3):193–198. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullens DMA, Truyen E, Coteur L, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaminska M, Foley S, Maghni K, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J Allergy Clin Immunol. 2009;124(1):45–51. doi: 10.1016/j.jaci.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 35.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179(6):4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 36.Dragon S, Rahman MS, Yang J, Unruh H, Halayko AJ, Gounni AS. IL-17 enhances IL-1beta-mediated CXCL-8 release from human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L1023–L1029. doi: 10.1152/ajplung.00306.2006. [DOI] [PubMed] [Google Scholar]
- 37.Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol. 2008;180(8):5625–5635. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]