Abstract
Background:
On September 11, 2001, the World Trade Center (WTC) collapse caused massive air pollution, producing variable amounts of lung function reduction in the New York City Fire Department (FDNY) rescue workforce. α1-Antitrypsin (AAT) deficiency is a risk factor for obstructive airway disease.
Methods:
This prospective, longitudinal cohort study of the first 4 years post-September 11, 2001, investigated the influence of AAT deficiency on adjusted longitudinal spirometric change (FEV1) in 90 FDNY rescue workers with WTC exposure. Workers with protease inhibitor (Pi) Z heterozygosity were considered moderately AAT deficient. PiS homozygosity or PiS heterozygosity without concomitant PiZ heterozygosity was considered mild deficiency, and PiM homozygosity was considered normal. Alternately, workers had low AAT levels if serum AAT was ≤ 20 μmol/L.
Results:
In addition to normal aging-related decline (37 mL/y), significant FEV1 decline accelerations developed with increasing AAT deficiency severity (110 mL/y for moderate and 32 mL/y for mild) or with low AAT serum levels (49 mL/y). Spirometric rates pre-September 11, 2001, did not show accelerations with AAT deficiency. Among workers with low AAT levels, cough persisted in a significant number of participants at 4 years post-September 11, 2001.
Conclusions:
FDNY rescue workers with AAT deficiency had significant spirometric decline accelerations and persistent airway symptoms during the first 4 years after WTC exposure, representing a novel gene-by-environment interaction. Clinically meaningful decline acceleration occurred even with the mild serum AAT level reductions associated with PiS heterozygosity (without concomitant PiZ heterozygosity).
The New York City World Trade Center (WTC) collapse on September 11, 2001, produced significant exposures to respirable particulates and combustion by-products.1 In rescue and recovery workers2-10 and in residents,11-13 new respiratory symptoms have emerged. Rescue workers (firefighters and emergency medical services [EMS] personnel) from the New York City Fire Department (FDNY) received intensive postexposure follow-up as part of the FDNY WTC Medical Monitoring Program (MMP). In a longitudinal spirometric study of 12,079 FDNY workers, adjusted average pulmonary function (FEV1 and FVC) was substantially reduced during the first year post-September 11, 2001, with an exposure-response gradient.3,4 Resolution of symptoms and airflow obstruction has been variable,14-16 pointing toward an individual predisposition for the persistence of airway inflammation.
α1-Antitrypsin (AAT) is a key inflammatory regulator in the human airway.17,18 It belongs to the serine protease superfamily, members of which act as proteinase regulators, especially in inflammatory pathways.19,20 Persons with ATT deficiency are predisposed to chronic airflow obstruction20-22 and hyperreactivity.23,24 Tests for AAT serum levels and deficiency protein phenotypes are well validated, and a clinical diagnosis of AAT deficiency requires both low serum concentrations and a deficient AAT phenotype (performed with protease inhibitor [Pi] typing).25,26 Given the variability of airflow obstruction and hyperreactivity in FDNY workers with WTC exposure and the known link between AAT deficiency and airway disease, we analyzed the influence of AAT expression on longitudinal spirometric change in 90 FDNY workers with WTC exposure over the first 4 years post-September 11, 2001.
Materials and Methods
Study Design and Timeline
This prospective cohort study compared longitudinal spirometric decline during the first 4 years post-September 11, 2001, among three AAT phenotype combinations in FDNY workers with high and moderate WTC exposure. Spirometry was obtained at 1 to 3 and 6 months, and 1, 2, and 4 years post-September 11, 2001. AAT testing was offered only at 4 years post-September 11, 2001.
WTC Exposure Groups
Exposure intensity was self-reported (FDNY-WTC-MMP questionnaire, confirmatory interviews). Exposure intensity was categorized according to workers’ first WTC site arrival time: high intensity if arrival preceded the collapse of either the North or the South Tower (morning of September 11, 2001), intermediate intensity if arrival followed the collapse and occurred on September 11, 2001, and low intensity if arrival occurred after September 12, 2001.
Enrollment and Exclusion Criteria
Study enrollment took place 1 and 3 months post-September 11, 2001, during the FDNY-WTC-MMP examination (October 2001-December 2002). None of the 1,546 eligible subjects (hereafter referred to as the source population) were on medical leave. One month post-September 11, 2001, every second worker with high or moderate exposure registering for the FDNY-WTC-MMP who met study eligibility criteria was approached for enrollment. Three months post-September 11, 2001, because of the strain on resources from larger numbers of workers registering for the MMP at that time, every 20th worker with high or moderate exposure who met study eligibility criteria was approached for enrollment. Exclusion criteria were current smoking, allergies, FEV1 < 65% predicted, or low-intensity WTC exposure.
Follow-up Visits
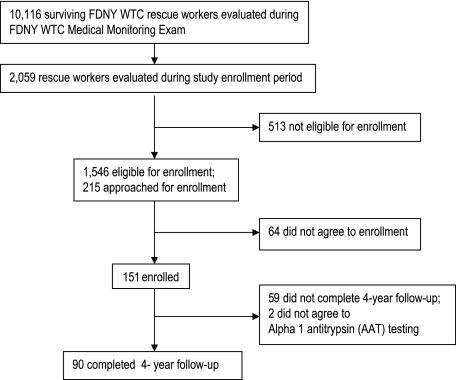
Study participation was voluntary. Each study visit required informed consent approved by the Montefiore Medical Center institutional review board (protocol # 01-12-299). Longitudinal participation is shown in Figure 1 and Table 1. At the final follow-up 4 years post-September 11, 2001, no participant reported new or recurrent tobacco use, and two refused AAT testing. Thus, the final cohort comprised 90 FDNY workers with WTC exposure (60% retention). All follow-ups included spirometry and a self-administered questionnaire assessing respiratory symptoms. All symptoms were recorded prior to disclosing AAT status.
Figure 1.
Source population, study cohort, and study time course. Recruitment from the source population, numbers for follow-up testing at each time point, and workers who consented to AAT testing are shown. AAT testing was offered only at the final follow-up visit. AAT = α1-antitrypsin; FDNY = New York City Fire Department; WTC = World Trade Center.
Table 1.
—Enrollment and Follow-up Data of Study Cohort
| Enrollment |
Follow-up |
||||
| AAT Deficiency Category | 1-3 mo Post-September 11, 2001 | 6 mo Post-September 11, 2001 | 1 y Post-September 11, 2001 | 2 y Post-September 11, 2001 | 4 y Post-September 11, 2001 |
| Moderate | 4 | 4 | 2 | 4 | 4 |
| Mild | 7 | 6 | 6 | 5 | 7 |
| Normal AAT phenotype | 79 | 65 | 65 | 52 | 79 |
| Total No. tested each time period | 90 | 75 | 73 | 61 | 90 |
AAT = α1-antitrypsin.
Spirometry
Spirometry was performed according to American Thoracic Society guidelines.27 Spirometers were calibrated daily, and testing was performed while seated and wearing noseclips and with up to eight forced expiratory maneuvers per session to maximize quality. To allow calculation of separate spirometric rates for time periods pre- and post-September 11, 2001, as well as to allow for more precise modeling for post-September 11, 2001, spirometric measurements obtained from the FDNY-WTC-MMP pre-September 11, 2001 (Portascreen; S&M Instruments; Doylestown, PA) and post-September 11, 2001 (EasyOne; NDD Medical Technologies; Andover, MA) were included with those obtained on the study dates (KoKo Spirometers; PDS Instrumentation; Louisville, CO). Each spirogram was reviewed by a board-certified pulmonologist blinded to patient identifier, exposure status, AAT phenotype, and serum level to determine that adherence to strict quality assurance guidelines was met.28 Spirograms were accepted if they (1) did not show artifacts of cough or glottis closure during the first second of exhalation, early termination, variable effort, leak, and obstructed mouthpiece; (2) had good starts with back-extrapolated volume not exceeding 5% of FVC or 150 mL (whichever was larger); and (3) had satisfactory exhalation length (at least 6 s or a plateau in the volume-time curve). Spirometric measurements were considered reproducible if the best and second-best FVC or FEV1 measurements were within 200 mL of each other. The largest FVC and FEV1 from among all acceptable spirograms were selected for electronic archiving. Pre-September 11, 2001, 142 spirograms were accepted for inclusion in the database; the median number per study participant was two (range, 0-3), and 77 (86%) participants had at least one spirogram. Post-September 11, 2001, 221 spirograms from the FDNY-WTC-MMP and 299 from the study visits were accepted for inclusion for a total of 520 spirograms; the median number per study participant was six (range, 2-8). Nine spirometric measurements were rejected.
AAT Testing
On the final visit, participants were phlebotomized before spirometry. Seventeen milliliters of unheparinized venous blood were coagulated for 20 min and then centrifuged for 6 min at 3,300 rpm at room temperature. Serum was stored at -80°C. Pi phenotyping used high-resolution gel isoelectric focusing (pH 4-5).29 Serum AAT concentrations were determined using rate-limited nephelometry (purified AAT standard).30
AAT Deficiency Categories
Two different methods were used to categorize AAT deficiency severity. The main categorization used AAT Pi phenotype combinations; the alternate categorization used serum AAT level. For the main categorization, workers with PiZ heterozygosity were considered moderately deficient; workers with PiS homozygosity or PiS heterozygosity without concomitant PiZ heterozygosity were considered mildly deficient; and workers with PiM homozygosity were considered normal. For the alternate categorization, a serum ATT level ≤ 20 μmol/L was considered low.31
Demographic, Clinical, and Spirometric Comparisons—Univariate Analyses
FDNY work assignment on September 11, 2001 (firefighter vs EMS worker), FDNY tenure, age, race, height, sex, and smoking status were extracted from the FDNY-WTC-MMP database. Sex, race, ex-smoking status, work assignment, WTC exposure intensity, and upper- or lower-respiratory symptoms were compared at enrollment among the following groups: source population and study cohort, AAT phenotype combination categories, and low vs normal serum AAT categories (χ2, Fisher exact test).
Four years post-September 11, 2001, upper- or lower-respiratory symptoms were compared among AAT phenotype combinations and between low vs normal serum AAT levels (χ2, Fisher exact test). Symptom persistence from enrollment to final visit was explored within each AAT phenotype combination and within low vs normal serum levels (McNemar test). Percentages of AAT deficiency phenotypes were compared between low vs normal serum levels (Fisher exact test). Mean serum AAT levels were compared among AAT phenotype combinations (Mann-Whitney U, Kruskal-Wallis test). Spirometric measurements before September 11, 2001, at enrollment, and at 4 years post-September 11, 2001, as well as AAT levels, age, and FDNY tenure were compared between the same groups detailed previously (t test, one-way analysis of variance, Mann-Whitney U, Kruskal-Wallis test).
Clinical and Spirometric Comparisons—Multivariate Analyses
Indicators for clinical symptoms 1 to 3 months post-September 11, 2001, were compared between the source population and study cohort, adjusting for the following factors: age, FDNY tenure, WTC exposure intensity, work assignment, and ex-smoker percentage). Indicators for clinical symptoms 4 years post-September 11, 2001, were compared among AAT phenotype combinations and between workers with low vs normal serum AAT levels adjusted for the same factors using logistic regression. Spirometric measurements 1 to 3 months post-September 11, 2001 (adjusted for the same factors plus sex and height) were compared using linear regression between the source population and study cohort, among the different AAT phenotype combinations, and between workers with low vs normal serum AAT levels.
Spirometric Decline Rates—Mixed Linear Random Effects Models
Using mixed linear random effects modeling, we analyzed differences in average spirometric change rates (FVC or FEV1) during 3 years pre-September 11, 2001, and during 4 years post-September 11, 2001, and whether AAT deficiency combinations influenced spirometric change rates during 4 years post-September 11, 2001.32-34 Separate models were run for FEV1 and FVC as dependent variables. Workers contributed two to 10 observations. The primary predictor of interest was the interaction between spirometric change rate during the 4 years post-September 11, 2001, and AAT deficiency combinations (based on either phenotype combinations or serum levels). AAT deficiency severity was modeled both as nominal predictor and as ordinal predictor (to test for linear trend) in separate models. Separate spirometric change rates and separate interaction terms between spirometric change and AAT categories were included for 3 years pre-September 11, 2001, and for 4 years post-September 11, 2001. In addition, models allowed a spirometry decrement post-September 11, 2001, because this was previously observed in longitudinal spirometric analysis of this workforce.3 Additional predictors were included as the following confounders: age, sex, height, race, smoking status, work assignment (firefighter, EMS worker), FDNY tenure, WTC exposure intensity, and interaction between AAT deficiency and history of tobacco use. All predictors were fixed effects. A random intercept was used to reflect across-subject heterogeneity and correlation induced by having repeated same-subject observations. To eliminate nonlinear confounding because of the known interaction of smoking with AAT deficiency,35 we modeled spirometric change rates both in the study cohort that included ex- and never smokers (n = 90) and in the subcohort of never smokers (n = 75). SPSS version 12.0 (SPSS Inc; Chicago, IL) was used for all analyses.
Results
Study Cohort and Source Population
The study cohort consisted of 90 source population members (FDNY workers with high to moderate WTC exposure, no allergies, ex- or never-smoking status, and an FEV1 ≥ 65% predicted as measured during FDNY-WTC-MMP 1-3 months post-September 11, 2001) who agreed to participate in and completed this longitudinal 4-year study (Fig 1, Table 1). Demographic and symptom information of the source population and study cohort are shown in Table 2. No significant difference in sex, age, and ex-smoker percentage between study cohort and source population was found. The study cohort included significantly more workers with high WTC exposure and, to a lesser extent, significantly more nonwhite and EMS workers. Compared with the source population, study participants at enrollment (1-3 months post-September 11, 2001) were more symptomatic, with increased prevalence of nocturnal respiratory symptoms and nasal drip and congestion.
Table 2.
—Demographic and Clinical Characteristics of Study Cohort and Source Population at Enrollment
| Characteristic | Source Populationa | Study Cohort |
| Total No. | 1,546 | 90 |
| Demographic characteristics | ||
| Male sex | 99 | 98 |
| White race | 93b | 86 |
| Age, y | 39.9 ± 7.6 | 40.7 ± 7.1 |
| Ex-smoker | 15 | 16 |
| EMS work assignment on September 11, 2001 | 5d | 11 |
| High WTC exposure intensityc | 23e | 66 |
| FDNY tenure length on September 11, 2001, y | 12.2 ± 8.2 | 12.4 ± 8.2 |
| Clinical characteristics | ||
| Upper-respiratory symptoms | ||
| Nasal drip and congestion | 44b | 56 |
| Sore or hoarse throat | 60 | 71 |
| Lower-respiratory symptoms | ||
| Daily cough | 56 | 62 |
| Wheezing | 21 | 25 |
| Chest tightness or pain | 25b | 37 |
| Dyspnea | 27b | 41 |
| Nocturnal respiratory symptoms interfering with sleep | 27f | 41 |
Data are presented as % or mean ± SD, unless otherwise indicated. EMS = emergency medical services; FDNY = New York City Fire Department; WTC = World Trade Center.
Includes those workers who enrolled at study initiation 1 to 3 months post-September 11, 2001, but did not participate in follow-up testing 4 years post-September 11, 2001, and those workers who did not consent to AAT testing during the follow-up visit 4 years post-September 11, 2001.
P < .005, χ. Difference in symptoms was no longer significant after adjustment for age, length of FDNY tenure, WTC exposure intensity, work assignment, and percentage of ex-smokers.
Arrived at WTC site morning of September 11, 2001.
P < .05, Fisher exact test.
P < .001, χ2.
P < .005, χ2. Difference in symptoms remained significant after adjustment for age, length of FDNY tenure, WTC exposure intensity, work assignment, and percentage of ex-smokers.
Source population and study cohort spirometric measurements are shown in Table 3. Pre-September 11, 2001, study participants had significantly lower mean spirometric measurements than the source population, but these differences were not significant when normalized as percent-predicted values. At enrollment 1 to 3 months post-September 11, 2001, lower spirometric measurements in study participants was consistent with the larger number of workers with high WTC exposure in the study cohort than in the source population.3,4
Table 3.
—Spirometric Characteristics of Study Cohort and Source Population Prior to and During Enrollment
| Characteristic | Source Populationa | Study Cohort |
| Prior to September 11, 2001b | ||
| FEV1, L | 4.37 ± 0.71c | 4.19 ± 0.68 |
| Percent predicted | 103 ± 14 | 101 ± 15 |
| FVC, L | 5.16 ± 0.85c | 4.91 ± 0.82 |
| Percent predicted | 100 ± 14 | 98 ± 14 |
| 1-3 mo post-September 11, 2001d | ||
| FEV1, L | 4.08 ± 0.71e | 3.90 ± 0.64 |
| Percent predicted | 96 ± 15 | 94 ± 15 |
| FVC, L | 4.86 ± 0.84e | 4.64 ± 0.83 |
| Percent predicted | 94 ± 14 | 92 ± 15 |
Data are presented as mean ± SD. See Table 2 legend for expansion of abbreviations.
Includes those workers who enrolled at study initiation 1 to 3 months post-September 11, 2001, but who did not participate in follow-up testing 4 years post-September 11, 2001, and those workers who did not consent to AAT testing during the follow-up visit 4 years post-September 11, 2001.
Last spirometric measurement prior to September 11, 2001, obtained during routine occupational health surveillance at FDNY (available for 83% of source population and 86% of study cohort).
P < .05, t test for independent samples.
Obtained during enrollment 1 to 3 months post-September 11, 2001.
P < .05, t test for independent samples. These spirometric differences were no longer statistically significant after adjusting for differences in WTC exposure intensity between study cohort and source population.
AAT Phenotype Distributions and AAT Deficiency Combinations
For analysis, workers were grouped according to AAT phenotype combination deficiency severity as follows: four workers with PiZ heterozygosity were considered moderately deficient (two with M1Z, one with M3Z, and one with SZ), seven workers with PiS homozygosity or PiS heterozygosity without concomitant PiZ heterozygosity were considered mildly deficient (three with M1S, two with M2S, one with M3S, and one with SS), and 79 workers with PiM homozygosity were considered normal (38 with M1M1, 28 with M1M2, 11 with M1M3, and two with M3M3). Significant differences in mean serum AAT levels were observed among the three AAT deficiency combinations (Table 4). Alternatively, 13 workers were categorized as having low AAT serum levels (≤ 20 μmol/L) (Table 5), and significant differences in percentages of AAT deficiency combinations were observed between workers with low vs normal serum AAT levels.
Table 4.
—Clinical and Biochemical Characteristics of Study Cohort by AAT Deficiency Category
| AAT Phenotype Combination |
|||
| Characteristic | Moderate Deficiency | Mild Deficiency | Normal |
| Total Number | 4 | 7 | 79 |
| AAT characteristics | |||
| AAT phenotype combinations | PiMZ, PiSZ | PiMS, PiSS | PiMM |
| Serum AAT level, μmol/L | 14.9 ± 3.2a | 18.4 ± 2.6 | 23.9 ± 3.0 |
| Demographic characteristics | |||
| Male sex | 100 | 100 | 98 |
| White race | 100 | 71 | 86 |
| Age, y | 46.1 ± 6.1 | 38.7 ± 4.4 | 40.2 ± 7.3 |
| Ex-smokers | 25 | 14 | 25 |
| EMS work assignment on September 11, 2001 | 0 | 29 | 10 |
| High WTC exposure intensityb | 75 | 71 | 65 |
| FDNY tenure length on September 11, 2001, y | 20.0 ± 6.4c | 11.5 ± 4.9 | 10.9 ± 8.4 |
Data are presented as mean ± SD or %, unless otherwise indicated. Pi = protease inhibitor. See Table 1 and 2 legends for expansion of abbreviations.
P < .001, Kruskal-Wallis test.
Arrived at WTC site morning of September 11, 2001.
P < .05, comparing workers with and without mildly AAT deficiency to workers with moderately AAT deficiency, t test.
Table 5.
—AAT Values and AAT Phenotype Combinations of Study Cohort by AAT Deficiency Category and AAT Serum Level
| AAT Serum Level, μmol/L |
||
| AAT Deficiency Category | Normal | Low |
| Normal | 24.55 ± 2.81 | 18.60 ± 1.01 |
| Phenotype combination | 75 PiMM | 4 PiMM |
| Mild deficiency | 21.70 ± 0.28 | 17.34 ± 1.85 |
| Phenotype combination | 2 PiMS | 4 PiMS, 1 PiSS |
| Moderate deficiency | … | 14.18 ± 3.25ab |
| Phenotype combination | … | 3 PiMZ, 1 PiSZ |
Data are presented as mean ± SD, unless otherwise indicated. See Table 1 and 4 legends for expansion of abbreviations.
P < .001 comparing mean serum AAT levels among AAT phenotype categories, Kruskal-Wallis test.
P < .001 comparing AAT deficiency phenotype combinations among workers with low vs normal AAT serum levels, Mann-Whitney U.
Symptoms by AAT Combination
There were no significant differences in sex, age, race, ex-smoking status, work assignment, WTC exposure intensity, and pre-September 11, 2001, FEV1 or FVC values among the three AAT phenotype combinations (Table 4) or between workers with low vs normal serum AAT levels. Among workers with low serum AAT levels, cough persisted in a significant number of individuals from 1 to 3 months to 4 years post-September 11, 2001.
Spirometric Decline Rates by AAT Phenotype Combination Category
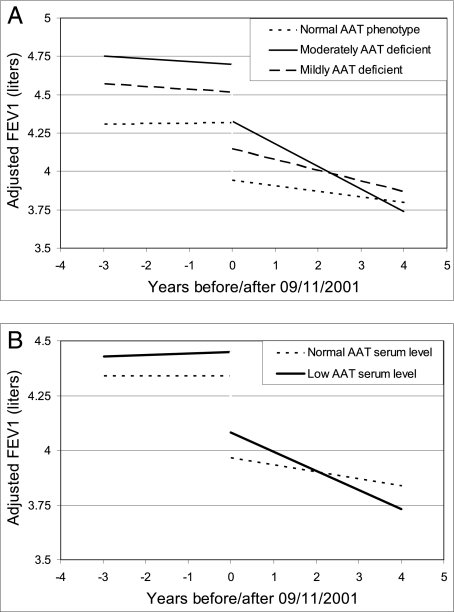
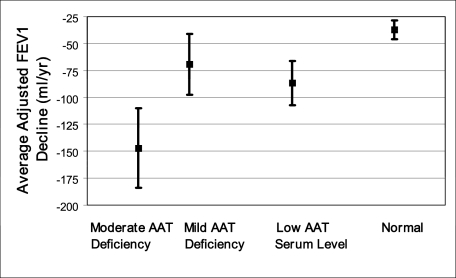
We compared adjusted FEV1 decline rates during the 4 years post-September 11, 2001, among AAT phenotype combinations and between workers with low vs normal serum AAT levels. Significant accelerations in FEV1 decline were evident with increasing AAT deficiency severity (Fig 2A), or with decreasing AAT serum levels (Fig 2B). These declining rate accelerations occurred even though models allowed for a spirometric decrement immediately post-September 11, 2001. The magnitude of this immediate decrement (370 mL) equaled 10 times the cohort’s yearly adjusted longitudinal aging-related decline rate of 37 mL/year. After accounting for both aging-related and immediate post-September 11, 2001, decrement, workers with moderate AAT-deficiency had an additional 110-mL/y FEV1 decline rate acceleration, whereas workers with mild AAT deficiency had an additional 32-mL/y FEV1 decline rate acceleration during the 4 years post-September 11, 2001. The magnitude of AAT deficiency-related adjusted FEV1 decline rate acceleration thus equaled nearly triple the cohort’s yearly adjusted aging-related decline rate for workers with moderate AAT deficiency and almost equaled the yearly adjusted aging-related decline rate for those with mild AAT deficiency (Fig 3). This finding was true regardless of whether smokers were included or whether the single individual with a PiSZ phenotype combination was included. Furthermore, after accounting for aging-related decline and the 370-mL immediate decrement, workers with low AAT serum levels had an additional 49-mL/y decline rate acceleration compared with those with normal levels during the 4 years post-September 11, 2001. For workers with low serum AAT levels, the magnitude of adjusted AAT deficiency-related decline rate acceleration thus exceeded the cohort’s yearly adjusted aging-related decline rate (Fig 3). This finding was true regardless of whether ex-smokers were included. Similar results were obtained for adjusted FVC decline rate accelerations (data not shown). When adjusted spirometric decline rates pre-September 11, 2001, were compared with rates post-September 11, 2001, no decline rate acceleration attributable to AAT deficiency was observed for the 3-year period preceding the WTC exposure (Fig 2).
Figure 2.
A, Time course of average adjusted FEV1 pre- and post-September 11, 2001, for the three AAT deficiency phenotype combinations. B, Time course of average adjusted FEV1 pre- and post-September 11, 2001, for low vs normal AAT serum levels. Significant acceleration in average adjusted spirometric declines according to AAT phenotype combination (110-mL/y FEV1 for FDNY workers with moderate AAT deficiency, 32-mL/y FEV1 for workers with mild AAT deficiency; P for trend, .003) occurred during the 4 years post-September 11, 2001, but not during the 3 years pre-September 11, 2001. Spirometric measurements for a white male never-smoking FDNY firefighter with high WTC-exposure of mean age and height and with median length of FDNY tenure are depicted. A, Workers with protease inhibitor (Pi) Z heterozygosity were categorized as moderately AAT deficient (n = 4), those with PiS homozygosity or PiS heterozygosity without concomitant PiZ heterozygosity were categorized as mildly AAT deficient (n = 7), and those with PiM homozygosity were categorized as normal (n = 79). B, Workers with serum AAT levels ≤ 20 μmol/L were categorized as having low levels (n = 13), and those with serum AAT levels > 20 μmol/L were categorized as having normal levels (n = 77). Spirometric decline rates were adjusted for sex, race, age, height, ex-smoking status, work assignment on September 11, 2001, length of FDNY tenure, WTC exposure intensity, and the interaction of smoking with AAT deficiency. The statistical models allowed for a decrement in spirometric measurements post-September 11 because this had previously been observed in the FDNY workforce.3 See Figure 1 legend for expansion of other abbreviations.
Figure 3.
Magnitude of average adjusted FEV1 decline rates post-September 11, 2001, for the two AAT deficiency combinations and for low serum AAT levels. Normal aging-related decline rates for the cohort provide a clinically meaningful comparison. Decline rate magnitudes due to AAT deficiency and aging and SEs are shown. AAT-related accelerations in decline rate equaled nearly triple the cohort’s adjusted aging-related decline rate for FDNY workers with moderate AAT deficiency and almost equaled the cohort’s adjusted aging-related decline rate for workers with mild AAT deficiency (P = .011), with a statistically significant trend for decline rate acceleration by AAT phenotype combination deficiency category (P = .003). In addition, AAT-related accelerations in decline rate for low AAT serum levels exceeded the cohort’s yearly adjusted aging-related decline rate (P = .027). The rightmost data point represents the FEV1 decline rate due to aging alone, which study participants with normal AAT phenotypes experienced because they did not experience any additional decline rate acceleration due to AAT deficiency. Decline rates for a white male never-smoking FDNY firefighter with high WTC exposure of mean age and height and with median FDNY tenure length are depicted. Moderate AAT deficiency was defined as PiZ heterozygosity (n = 4), and mild AAT deficiency was defined as PiS homozygosity or PiS heterozygosity without concomitant PiZ heterozygosity (n = 7). Low serum AAT level was defined as ≤ 20 μmol/L. Decline rates were adjusted for sex, race, age, height, ex-smoking status, work assignment on September 11, 2001, length of FDNY tenure, WTC exposure intensity, and the interaction of smoking with AAT deficiency. See Figure 1 and 2 legends for expansion of abbreviations.
Discussion
In this prospective longitudinal cohort study, we showed that FDNY rescue workers with AAT deficiency developed significant spirometric decline accelerations during the first 4 years post-September 11, 2001, even though such deficiency did not affect spirometric declines pre-September 11, 2001. A gene-by-environment interaction exists when disease risk among individuals with both genotype and environmental exposure is greater than predicted from either genotype or exposure alone.36 Accelerated lung function decline developed in workers with AAT deficiency after the intense inflammatory stimulus of WTC inhalation injury,37-39 representing a novel gene-by-environment interaction. Clinically meaningful and statistically significant lung function loss developed even with only the mild serum AAT level reduction associated with PiS heterozygosity without concomitant PiZ heterozygosity.
It is now well accepted that respirable pollutants after the WTC attack caused inhalation injury.2-14 Biochemical,38,39 physiologic,2-10,12 and clinical2-16,40,41 correlates of airway inflammation due to this exposure have been described in multiple cohorts, including FDNY rescue workers.7 Subacutely, during study initiation 1 to 3 months post-September 11, 2001, irritative respiratory symptoms and physiologic correlates (eg, decreased spirometric measurements) were associated with WTC exposure intensity.2-5 This association was no longer detectable at final follow-up 4 years post-September 11, 2001. Instead, AAT deficiency severity emerged as a determinant of both persistent symptoms and spirometric decline acceleration, highlighting its role in lung injury and repair. Although AAT deficiency has repeatedly been implicated in the development of chronic airflow obstruction,42,43 this study revealed development of AAT deficiency-related spirometric decline acceleration during a much shorter period (ie, 4 years), thus highlighting how quickly AAT deficiency can produce clinical disease and airflow obstruction. WTC-derived airborne pollution was a complex mixture of particulates and chemicals.1,7 To date, severe deteriorations in pulmonary function are well described for persons with AAT deficiency following bacterial infections44,45 but have not been described following exposures to particulates, chemicals, or mixtures. In addition, no such gene-by-environment interactions for pulmonary disease have been previously described for mild to moderate AAT deficiency due to PiS heterozygosity without concomitant PiZ heterozygosity. This interesting, novel finding might be due to the strength of the inhalational inflammatory stimulus sustained by FDNY rescue workers at the WTC site.
It is important to note our investigation’s limitations. First and most importantly, sample size was moderate, but this moderate-sized study cohort represented the FDNY source population quite well in key demographic and spirometric aspects. Second, FDNY rescuers sustained extremely high-intensity exposures, which might be qualitatively different compared with other rescue and recovery workers or for residents. For these reasons, caution is prudent when extrapolating our current findings. Third, the missing AAT characterization of the initially enrolled subjects who did not participate in the 4-year follow-up examination has the potential to bias our results. Specifically, study results would falsely favor an association between AAT deficiency and accelerated FEV1 decline if a disproportionate number of subjects with PiM homozygosity did not participate in the follow-up and at the same time did have accelerated airflow obstruction. The fact that the prevalence of deficiency phenotype carriers in our current cohort (12%) is close to the prevalence of deficiency phenotype carriers in the general North American population (9%)46 suggests that our results were likely not substantially affected by incomplete follow-up.
We partitioned participants into three AAT phenotype combinations, considering those with PiZ heterozygosity as moderately deficient, those with PiS homozygosity or with PiS heterozygosity without concomitant PiZ heterozygosity as mildly deficient, and those with PiM homozygosity as normal. Statistically significant differences in mean serum AAT levels among the three phenotype combinations supported this categorization. With this categorization, we demonstrated significant, clinically meaningful spirometric decline rate accelerations, even for mildly abnormal PiS carriers—to our knowledge, a unique finding in the literature.
Magnitude of AAT deficiency-related spirometric decline rate acceleration was both clinically and statistically significant, equaling almost triple this cohort’s aging-related spirometric decline rate for workers with moderate AAT-deficiency, despite allowing for a one-time decrement in spirometric measurements post-September 11, 2001. When we reported this one-time spirometric decrement 1 year post-September 11, 2001,3 we speculated whether the acute inflammatory response and pulmonary function decrement would be transient and reversible. However, in our current study, which includes spirometric measurements obtained as long as 4 years post-September 11, 2001, we still observed a decrement of almost equal magnitude as that observed during the first year post-September 11, 2001 (370-mL FEV1 decline). This one-time decrement persisted in addition to the AAT-related decline rate acceleration post-September 11, 2001, and persistence of this decrement has been reported in the entire FDNY workforce.4 These findings argue strongly against a transient, reversible WTC-related loss of pulmonary function.
In conclusion, we demonstrated significant associations between spirometric decline rate acceleration and AAT deficiency severity in the FDNY workforce during the first 4 years after WTC-related inhalation injury. These decline rate accelerations represent a novel gene-by-environment interaction, are both clinically and statistically significant, and occur even in workers with PiS heterozygosity who had only mild reductions in serum AAT levels. This finding of accelerated pulmonary function decline despite modest sample size, milder degrees of AAT deficiency, and only 4 years of follow-up allows for inferences about the key antiinflammatory role of AAT in the lower airways and about the strength of the WTC-related inhalation injury in FDNY workers.
Acknowledgments
Author contributions: Dr Banauch: contributed to the study design; data acquisition, analysis, and interpretation; drafting and revision of the manuscript; and preparation of the final version of the manuscript.
Dr Brantly: contributed to the data acquisition, laboratory measurements, and revision of the manuscript.
Dr Izbicki: contributed to the data acquisition, revision of the manuscript, and preparation of the final version of the manuscript.
Dr Hall: contributed to the data analysis and interpretation, revision of the manuscript, and preparation of the final version of the manuscript.
Dr Shanske: contributed to the study design, revision of the manuscript, and preparation of the final version of the manuscript.
Dr Chavko: contributed to the data acquisition and revision of the manuscript.
Dr Santhyadka: contributed to the data acquisition and revision of the manuscript.
Lt Christodoulou: contributed to the data acquisition and revision of the manuscript.
Dr Weiden: contributed to the revision of the manuscript.
Dr Prezant: contributed to the study design, drafting and revision of the manuscript, and preparation of the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This study was performed at the Montefiore Medical Center and New York City Fire Department (Bureau of Health Services).
Abbreviations
- AAT
α1-antitrypsin
- EMS
emergency medical services
- FDNY
New York City Fire Department
- MMP
Medical Monitoring Program
- Pi
protease inhibitor
- WTC
World Trade Center
Footnotes
Funding/Support: Dr Banauch received funding from the National Center for Research Resources (5K12RR017672). Dr Brantly received support from the Alpha-1 Foundation. Dr Izbicki was supported by the Jesselon Einstein-Shaare Zedeck Fellowship Program. Dr. Hall received salary support from the National Institute of Occupational Safety and Health through the New York City Fire Department and Montefiore Medical Center. Dr Weiden received funding from the National Institutes of Health [Grants M01 00096, K23HL084191, K24A1080298, and R01HL057879]. Dr Prezant (principal investigator) received funding from the Centers for Disease Control and Prevention [U1Q/CCU221158] and the National Institute of Occupational Safety and Health [U10-OH008243 and U10-OH008242].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.McGee JK, Chen LC, Cohen MD, et al. Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ Health Perspect. 2003;111(7):972–980. doi: 10.1289/ehp.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prezant DJ, Weiden M, Banauch GI, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347(11):806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 3.Banauch GI, Hall C, Weiden M, et al. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. Am J Respir Crit Care Med. 2006;174(3):312–319. doi: 10.1164/rccm.200511-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldrich TK, Gustave J, Hall CB, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362(14):1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banauch GI, Alleyne D, Sanchez R, et al. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am J Respir Crit Care Med. 2003;168(1):54–62. doi: 10.1164/rccm.200211-1329OC. [DOI] [PubMed] [Google Scholar]
- 6.Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 suppl):S102–S106. doi: 10.1097/01.ccm.0000151138.10586.3a. [DOI] [PubMed] [Google Scholar]
- 7.Banauch GI, Dhala A, Prezant DJ. Pulmonary disease in rescue workers at the World Trade Center site. Curr Opin Pulm Med. 2005;11(2):160–168. doi: 10.1097/01.mcp.0000151716.96241.0a. [DOI] [PubMed] [Google Scholar]
- 8.Herbert R, Moline J, Skloot G, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect. 2006;114(12):1853–1858. doi: 10.1289/ehp.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skloot GS, Schechter CB, Herbert R, et al. Longitudinal assessment of spirometry in the World Trade Center medical monitoring program. Chest. 2009;135(2):492–498. doi: 10.1378/chest.08-1391. [DOI] [PubMed] [Google Scholar]
- 10.Salzman SH, Moosavy FM, Miskoff JA, Friedmann P, Fried G, Rosen MJ. Early respiratory abnormalities in emergency services police officers at the World Trade Center site. J Occup Environ Med. 2004;46(2):113–122. doi: 10.1097/01.jom.0000111612.68916.d0. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center—Manhattan, New York, 2001. MMWR Morb Mortal Wkly Rep. 2002;51(35):781–784. [PubMed] [Google Scholar]
- 12.Reibman J, Lin S, Hwang SA, et al. The World Trade Center residents’ respiratory health study: new-onset respiratory symptoms and pulmonary function. Environ Health Perspect. 2005;113(4):406–411. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szema AM, Khedkar M, Maloney PF, et al. Clinical deterioration in pediatric asthmatic patients after September 11, 2001. J Allergy Clin Immunol. 2004;113(3):420–426. doi: 10.1016/j.jaci.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Kelly KJ, Niles J, McLaughlin MT, et al. World Trade Center Health Impacts on FDNY Rescue Workers: a Six Year Assessment: September 2001 to September 2007. New York, NY: Fire Department of the City of New York; 2007. [Accessed October 10, 2007]. http://www.nyc.gov/html/om/pdf/2007/wtc_health_impacts_on_fdny_rescue_workers_sept_2007.pdf. [Google Scholar]
- 15.Weiden MD, Ferrier N, Nolan A, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137(3):566–574. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauer MP, Herdt-Losavio ML, Carlson GA. Asthma and lower respiratory symptoms in New York State employees who responded to the World Trade Center disaster. Int Arch Occup Environ Health. 2010;83(1):21–27. doi: 10.1007/s00420-009-0474-x. [DOI] [PubMed] [Google Scholar]
- 17.Hill AT, Bayley DL, Campbell EJ, Hill SL, Stockley RA. Airways inflammation in chronic bronchitis: the effects of smoking and alpha1-antitrypsin deficiency. Eur Respir J. 2000;15(5):886–890. doi: 10.1034/j.1399-3003.2000.15e12.x. [DOI] [PubMed] [Google Scholar]
- 18.Woolhouse IS, Bayley DL, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of alpha(1)-antitrypsin deficiency and the role of leukotriene B(4) and interleukin 8. Thorax. 2002;57(8):709–714. doi: 10.1136/thorax.57.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowther DC, Belorgey D, Miranda E, Kinghorn KJ, Sharp LK, Lomas DA. Practical genetics: alpha-1-antitrypsin deficiency and the serpinopathies. Eur J Hum Genet. 2004;12(3):167–172. doi: 10.1038/sj.ejhg.5201127. [DOI] [PubMed] [Google Scholar]
- 20.Lomas DA, Parker B, Parker B. Francis lectureship Antitrypsin deficiency, the serpinopathies, and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(6):499–501. doi: 10.1513/pats.200603-069MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eden E, Hammel J, Rouhani FN, et al. Asthma features in severe alpha1-antitrypsin deficiency: experience of the National Heart, Lung, and Blood Institute Registry. Chest. 2003;123(3):765–771. doi: 10.1378/chest.123.3.765. [DOI] [PubMed] [Google Scholar]
- 22.The Alpha-1-Antitrypsin Deficiency Registry Study Group Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med. 1998;158(1):49–59. doi: 10.1164/ajrccm.158.1.9712017. [DOI] [PubMed] [Google Scholar]
- 23.Sigsgaard T, Brandslund I, Omland O, et al. S and Z alpha1-antitrypsin alleles are risk factors for bronchial hyperresponsiveness in young farmers: an example of gene/environment interaction. Eur Respir J. 2000;16(1):50–55. doi: 10.1034/j.1399-3003.2000.16a09.x. [DOI] [PubMed] [Google Scholar]
- 24.von Ehrenstein OS, Maier EM, Weiland SK, et al. Alpha1 antitrypsin and the prevalence and severity of asthma. Arch Dis Child. 2004;89(3):230–231. doi: 10.1136/adc.2002.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snyder MR, Katzmann JA, Butz ML, et al. Diagnosis of alpha-1-antitrypsin deficiency: an algorithm of quantification, genotyping, and phenotyping. Clin Chem. 2006;52(12):2236–2242. doi: 10.1373/clinchem.2006.072991. [DOI] [PubMed] [Google Scholar]
- 26.Ferrarotti I, Scabini R, Campo I, et al. Laboratory diagnosis of alpha1-antitrypsin deficiency. Transl Res. 2007;150(5):267–274. doi: 10.1016/j.trsl.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 29.Brantly M. Laboratory diagnosis of α-1-antitrypsin deficiency. In: Crystal R, editor. Alpha-1-Antitrypsin Deficiency. New York, NY: Marcel Dekker; 1995. pp. 45–60. [Google Scholar]
- 30.Brantly ML, Wittes JT, Vogelmeier CF, Hubbard RC, Fells GA, Crystal RG. Use of a highly purified alpha 1-antitrypsin standard to establish ranges for the common normal and deficient alpha 1-antitrypsin phenotypes. Chest. 1991;100(3):703–708. doi: 10.1378/chest.100.3.703. [DOI] [PubMed] [Google Scholar]
- 31.Senn O, Russi EW, Imboden M, Probst-Hensch NM. Alpha1-Antitrypsin deficiency and lung disease: risk modification by occupational and environmental inhalants. Eur Respir J. 2005;26(5):909–917. doi: 10.1183/09031936.05.00021605. [DOI] [PubMed] [Google Scholar]
- 32.Lebowitz MD. Age, period, and cohort effects. Influences on differences between cross-sectional and longitudinal pulmonary function results. Am J Respir Crit Care Med. 1996;154(6 pt 2):S273–S277. doi: 10.1164/ajrccm/154.6_Pt_2.S273. [DOI] [PubMed] [Google Scholar]
- 33.Schouten JP, Tager IB. Interpretation of longitudinal studies. An overview. Am J Respir Crit Care Med. 1996;154(6 pt 2):S278–S284. doi: 10.1164/ajrccm/154.6_Pt_2.S278. [DOI] [PubMed] [Google Scholar]
- 34.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 35.Eriksson S, Lindell SE, Wiberg R. Effects of smoking and intermediate alpha 1-antitrypsin deficiency (PiMZ) on lung function. Eur J Respir Dis. 1985;67(4):279–285. [PubMed] [Google Scholar]
- 36.Clayton D, McKeigue PM. Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet. 2001;358(9290):1356–1360. doi: 10.1016/S0140-6736(01)06418-2. [DOI] [PubMed] [Google Scholar]
- 37.Fireman EM, Lerman Y, Ganor E, et al. Induced sputum assessment in New York City firefighters exposed to World Trade Center dust. Environ Health Perspect. 2004;112(15):1564–1569. doi: 10.1289/ehp.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gavett SH, Haykal-Coates N, Highfill JW, et al. World Trade Center fine particulate matter causes respiratory tract hyperresponsiveness in mice. Environ Health Perspect. 2003;111(7):981–991. doi: 10.1289/ehp.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Payne JP, Kemp SJ, Dewar A, et al. Effects of airborne World Trade Center dust on cytokine release by primary human lung cells in vitro. J Occup Environ Med. 2004;46(5):420–427. doi: 10.1097/01.jom.0000126021.25149.64. [DOI] [PubMed] [Google Scholar]
- 40.Mauer MP, Cummings KR, Hoen R. Long-term respiratory symptoms in World Trade Center responders. Occup Med (Lond) 2010;60(2):145–151. doi: 10.1093/occmed/kqp176. [DOI] [PubMed] [Google Scholar]
- 41.Mauer MP, Cummings KR. Impulse oscillometry and respiratory symptoms in World Trade Center responders, 6 years post-9/11. Lung. 2010;188(2):107–113. doi: 10.1007/s00408-009-9206-y. [DOI] [PubMed] [Google Scholar]
- 42.Mayer AS, Stoller JK, Bucher Bartelson B, James Ruttenber A, Sandhaus RA, Newman LS. Occupational exposure risks in individuals with PI*Z α(1)-antitrypsin deficiency. Am J Respir Crit Care Med. 2000;162(2 pt 1):553–558. doi: 10.1164/ajrccm.162.2.9907117. [DOI] [PubMed] [Google Scholar]
- 43.Piitulainen E, Tornling G, Eriksson S. Effect of age and occupational exposure to airway irritants on lung function in non-smoking individuals with alpha 1-antitrypsin deficiency (PiZZ) Thorax. 1997;52(3):244–248. doi: 10.1136/thx.52.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ) Am J Respir Crit Care Med. 1999;160(6):1968–1975. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]
- 45.Gourley MF, Gourley GR, Gilbert EF, Odell GB. Alpha 1-antitrypsin deficiency and the PiMS phenotype: case report and literature review. J Pediatr Gastroenterol Nutr. 1989;8(1):116–121. doi: 10.1097/00005176-198901000-00021. [DOI] [PubMed] [Google Scholar]
- 46.de Serres FJ. Alpha-1 antitrypsin deficiency is not a rare disease but a disease that is rarely diagnosed. Environ Health Perspect. 2003;111(16):1851–1854. doi: 10.1289/ehp.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]