SUMMARY
Is oxytocin the hormone of happiness? Probably not. However, this small nine amino acid peptide is involved in a wide variety of physiological and pathological functions such as sexual activity, penile erection, ejaculation, pregnancy, uterus contraction, milk ejection, maternal behavior, osteoporosis, diabetes, cancer, social bonding, and stress, which makes oxytocin and its receptor potential candidates as targets for drug therapy. In this review, we address the issues of drug design and specificity and focus our discussion on recent findings on oxytocin and its heterotrimeric G protein‐coupled receptor OTR. In this regard, we will highlight the following topics: (i) the role of oxytocin in behavior and affectivity, (ii) the relationship between oxytocin and stress with emphasis on the hypothalamo–pituitary–adrenal axis, (iii) the involvement of oxytocin in pain regulation and nociception, (iv) the specific action mechanisms of oxytocin on intracellular Ca2+ in the hypothalamo neurohypophysial system (HNS) cell bodies, (v) newly generated transgenic rats tagged by a visible fluorescent protein to study the physiology of vasopressin and oxytocin, and (vi) the action of the neurohypophysial hormone outside the central nervous system, including the myometrium, heart and peripheral nervous system. As a short nine amino acid peptide, closely related to its partner peptide vasopressin, oxytocin appears to be ideal for the design of agonists and antagonists of its receptor. In addition, not only the hormone itself and its binding to OTR, but also its synthesis, storage and release can be endogenously and exogenously regulated to counteract pathophysiological states. Understanding the fundamental physiopharmacology of the effects of oxytocin is an important and necessary approach for developing a potential pharmacotherapy.
Keywords: ACTH, Analgesics, Behavior, c‐fos‐mRFP1 fusion gene, CNS, Development, Drug design, eGFP, eCFP, Glial cells, Heart, HPA, Hypothalamus, Neurohypophysis, Neuropeptides, PNS, Receptors, Stress, Transgenic rat model, Vasopressin
Introduction
Oxytocin, a nine amino acid CNS neuropeptide, was discovered by Sir Henry Dale in 1906 when he found that extracts from the human posterior pituitary gland contracted the uterus of a pregnant cat; it was also Sir Henry Dale who coined the name oxytocin from the Greek words ωκνξ, τoκoxξ, meaning “swift birth.” Oxytocin was the first peptide hormone to be sequenced and synthesized by Vincent du Vigneaud in 1953; for this achievement he was awarded the Nobel Prize in 1955 [1].
Regulation of Oxytocin Release
Oxytocin is produced in the supraoptic and paraventricular nuclei of the hypothalamus and is mainly released (by exocytosis) from the neurohypophysis and nerve terminals in response to multiple physiological stimuli (see Refs. 2, 3, 4, 5, 6). The somatodendritic release of vasopressin and oxytocin has been widely demonstrated [7]; the release of oxytocin increases during the milk‐ejection reflex [8]. The mechanisms underlying the autoregulation of oxytocin and vasopressin neurones by the peptide that they themselves synthesize and the involvement of autoreceptors [9], Ca2+ channels [10], intracellular Ca2+ signals [11], and intracellular Ca2+ stores have also been clearly described [12, 13].
In oxytocin cells, oxytocin binds to specific oxytocin receptors (OTR), which trigger an increase in [Ca2+]i. This Ca2+ response is selectively blocked by the oxytocin receptor antagonist [d(CH2)5,Tyr(Me)2,Orn8]‐vasotocin but is not affected by selective antagonists of AVP receptors, suggesting the specific action of oxytocin. The [Ca2+]i increase induced by oxytocin results mainly from a mobilization of Ca2+ from thapsigargin‐sensitive intracellular Ca2+ stores, and from an effect of OTR on Ca2+ influx similarly to the V1a receptors (12). In contrast, the vasopressin‐induced [Ca2+]: increase can predominantly result from an influx of Ca2+ through voltage‐dependent Ca2+ channels in some cell types [10], together with a Ca2+ release from internal thapsigargin‐sensitive stores via the activation of inositol‐trisphosphate receptors (InsP3R), since the AVP receptor subtype V1a is always directly coupled to the phospholipase C (PLC) signaling pathway. It should be noted, however, that in the supraoptic vasopressin neurones, though the vasopressin V1a receptors have been shown to be simultaneously linked to the adenylyl cyclase (AC) pathway [11, 12, 14], may reflect indirect regulations possibly via the presence of Ca2+‐sensitive AC in this tissue, up to now, no direct coupling between V1a and AC had been described.
It is important to indicate that the concentrations of oxytocin, vasopressin, and their respective agonists and antagonists used in our previous studies for both measuring intracellular Ca2+ signals in isolated SON neurones and oxytocin/vasopressin release from the isolated supraoptic nuclei in vitro, ranged between 1 nM and 1 μM [9]. The threshold concentration necessary to induce a modification of the firing rate of oxytocin neurones is between 1 and 10 nM oxytocin [15]. The selectivity for OTR is still an open debate. The selectivity of most of the peptide agonists and antagonists described later in this communication is mainly based on in vivo assays and not directly on receptor assays. Moreover, these peptides have never been tested for their affinities on V1b receptor. Thus, it is advisable to be quite cautious on the question of their selectivity. Of interest, in a recent review, Chini et al. have clearly stated that establishing the affinity and efficacy of selective agonists and antagonists for vasopressin and oxytocin receptors is a complex task and depends on all the vasopressin/oxytocin receptor subtypes within the species under investigation [16].
In this particular physiologically relevant model, that is, the SON neurones, no detailed receptor pharmacology was performed. Noteworthy that the activation of oxytocin receptors by oxytocin and the messengers involved in the signaling cascade are more straightforward than those described for vasopressin (see review by Dayanithi et al. [12]). There is still debate regarding the appropriate concentrations of specific agonists and antagonists to use in examining the physiology of SON neurones.
Another aspect that deserves attention is the regulation of oxytocin and vasopressin release by neurosteroids. In this study, experiments were performed to look at the [Ca2+]i profiles and peptide release from both supraoptic nuclei and their axon terminals in different age groups, ranging from young animals to fully grown adults [17]. The results showed that at the level of SONs, the oxytocin release induced by neurosteroids involves a mechanism that partly depends on the presence of GABA (depolarizing in young rats) and that the effect of the neuroactive steroid allopregnanolone upon oxytocin release changes with age, as the action of GABAA receptors changes from excitation to inhibition of oxytocin neurones [17].
Recently we have highlighted the importance of the relationship between neurosteroids, oxytocin and the role of calcium ions [18, 19]. Further studies have demonstrated that glial coverage of neurones and of their synapses is modified in response to stimulation. During stimulation, intersynaptic crosstalk is enhanced when astrocytes withdraw their processes. Therefore, astrocytes are critically important for the regulation of communications between neighbouring synapses and extrasynaptic transmission. Under conditions of increased oxytocin secretion, for exmaple, during lactation or osmotic stimulation, astroglial coverage of supraoptic nucleus neurones in the hypothalamus is significantly diminished [20, 21]. Because glial cells represent a physical barrier to diffusion, they have been shown to influence extrasynaptic (or volume) transmission [22,23]. Reduction of the astrocytic coverage of SON neurones dramatically increases the extracellular diffusion of the primary neurotransmitter glutamate and, as a consequence, increases the glutamate‐induced heterosynaptic depression of GABAergic transmission [24]. The changes in diffusion properties and in glutamate spillover that are associated with anatomical remodeling are thus likely to improve neurohypophyseal hormone release in response to suckling or dehydration [25]. The question arises to what extent does oxytocin control the astroglial remodeling.
Localization of Oxytocin Receptors within the Brain
The central actions of oxytocin are mediated via oxytocin receptors (OTRs) distributed widely in the brain in a remarkably species‐specific fashion. Areas containing OTRs include, but are not restricted to, the ventromedial nucleus of the hypothalamus, the amygdala, the lateral septum, the bed nucleus of the stria terminalis, the anterior olfactory nucleus, the preoptic and ventral tegmental areas, and the hippocampus [26, 27]. Oxytocin binding sites in the medial preoptic and ventral tegmental areas are up‐regulated during pregnancy and cooperation and are thought to be involved in the mediation of maternal behavior (for a recent review, see Ref. 28).
In 1998, Nancy L Ostrowski showed that OTR mRNAs are distributed widely in the forebrain, including the limbic system and the hypothalamus, as well as in the brain stem [29]. This author divided the brain regions expressing OTR mRNA as follows: (i) regions involved in steroid‐sensitive reproductive behaviors (hypothalamic ventromedial nucleus: VMH, paraventricular nucleus: PVN), (ii) regions involved in maternal behaviors (PVN, substantia nigra, ventral tegmental area), (iii) region involved in learning and memory (hippocampus) and (iv) regions involved in reinforcement (substantia nigra, ventral tegmental area, lateral septum, caudate putamen, amygdaloid nuclei, olfactory tubercle and cingulate, perirhinal, and frontal cortices).
Another study employed functional magnetic resonance imaging (fMRI) to detect the locations of oxytocin‐responding brain regions (not necessarily equivalent to OTR locations, because some of these areas could be target sites of oxytocin‐stimulated neurones having transmitters other than oxytocin) [26]. In this report the authors tried to visualize oxytocin‐responding regions following the central injection of oxytocin into lactating rats (compared with noninjected control animals). They also performed several behavioral tests: the freezing (fear‐associated immobility) time in response to odor stimulation with 2,3,5‐trimethyl‐3‐thiazoline (or TMT, a chemical extracted from fox feces) was significantly shortened by oxytocin injection, while the grooming time was significantly prolonged by oxytocin. No difference was detected in other tests. These results showed that oxytocin effectively reduced the anxiety caused by a fear stimulus. The outcomes of fMRI mapping were as follows: the authors illustrated the brain regions that had an increased blood oxygenation level dependent (BOLD) response as red to yellow and those that had decreased BOLD response as blue to purple. Oxytocin affected both positive and negative BOLD responses across the olfactory and forebrain nuclei (larger increases: the anterior cingulate, the bed nucleus of the stria terminalis, and the perirhinal area; larger decreases: the mammillary bodies, the secondary motor cortex, the gustatory cortex, the prelimbic prefrontal cortex, the orbital cortex, and the anterior olfactory nucleus).
A different approach to the assessment of OTR localization utilizes immunocytochemistry. Adan et al. found immunoreactivity for rat OTR in the pituitary and mammary glands, the uterus and in the brain (the ventromedial hypothalamus, the bed nucleus of the stria terminalis, the ventral pallidum, the PVN, the dorsal part of the supraoptic nucleus) [30]. Surprisingly no immunoreactivity could be seen in the ventral hippocampus or the central nucleus of the amygdala, although autoradiography showed oxytocin binding sites in these two areas of the brain. The authors interpreted the data as being due to the presence of different OTR subtypes, which was not, however, demonstrated directly. Moreover, to date, no reliable antibodies raised against OTR are available, which prevents any good immunocytochemical localization studies. Therefore, only histo‐autoradiography gives consistent results.
A more precise study of oxytocin binding using histo‐autoradiography, especially regarding the rat amygdala, was undertaken by Veinante and Freund‐Mercier in 1997 [31]. For a complete overview on OTR with a special emphasis on OTR localization, the readers are advised to refer to the outstanding review of Gimpl and Fahrenholz [32].
The Role of Oxytocin in Social Behavior and Affectivity
Social bonding is essential to species survival since it favors reproduction, protection against predators and environmental changes, and brain development [33]. Exclusion from the group results in individual physical and mental disorders and leads ultimately to death, both in animal models and in primitive human tribes [34]. Moreover, behavioral pathologies characterized by neurochemical pathway defects, such as autism and depression, lead to social isolation. Thus, it is of particular importance to understand the molecular mechanisms that sustain the establishment and modulation of relationships between individuals, especially in the context of treatments and drug therapies for patients.
Although a wide range of specific neurotransmitters (dopamine, endorphins) is involved in the processes of synaptic activity formation and regulation that occur especially during cognition phenomena and the onset of other behavioral patterns, oxytocin and its receptors appear to hold the leading position among the candidates for the substance of “happiness.” If not “happiness,” at least it seems to be an important brain compound in building trust, which is necessary in developing emotional relationships, a process also referred to as social bonding. There is a discharge of the neuropeptide oxytocin during parturition, milk ejection, and orgasm, indicating that it could play a major role in the social bonding of animals and humans as well. A recent study demonstrated that a nasal spray of oxytocin raised the trust (in a stranger) of people playing a money game [35]. Furthermore, the spray was said to reduce the activities of the amygdala and caudate nucleus, regions of the brain implicated in emotions, fear conditioning and social cognition on the one hand, and learning, memory and feedback processing on the other hand [36, 37]. Nevertheless, the role of oxytocin in ordinary relationships and real‐life circumstances is still unclear. However, such findings could bring some hope in the treatment of social disorders such as phobia and autism [38].
Recently, Israel and collaborators demonstrated a correlation between oxytocin receptor gene polymorphisms and individual differences in prosocial behavior [39]. This finding highlights the fact that any kind of oxytocin therapy should be addressed individually, which might make the design of drugs much more complex.
Another demonstration of the direct role of the oxytocin receptor in the socialization of normal patients was provided by Lucht et al., though with a reduced sample size. They could associate oxytocin receptor haplotypes with affect regulation, social interaction (social and emotional loneliness) and cognition (intelligence) [40].
Furthermore, oxytocin and its receptors are involved in a plethora of social and affective, physiological and pathophysiological behaviors, ranging from attachment security, mating [41], paternal behavior and motherhood [33] to autism [42, 43, 44] and obsessive–compulsive disorder [45]. In addition, the neuropeptide also modulates learning, memory and intelligence as shown in animal and human investigations [46, 47]. In mice, an anxiolytic effect of oxytocin via the direct activation of the oxytocin receptors has been demonstrated [48]. Noteworthy is the link made by a recent study between oxytocin administration and injury healing. Vitalo et al. provide evidence that injections of the hormone oxytocin (as well as nest making activity) had a positive influence on wound healing in isolated reared rats [49]. They believe that exogenous oxytocin and an enriched environment (i.e., the possibility to build nests) play a role in the modulation of the hypothalamic–pituitary axis (HPA) responsible for stress responses. Thus, the effects of the neuropeptide go beyond its interaction with its receptors in the CNS and can also indirectly control phenomena occurring at the periphery.
Therefore, the potential of oxytocin for drug targeting is immense and brings some hope for alleviating serious social disorders, but the issue appears obviously extremely complex to tackle since the specificity of action might be quite difficult to control [50].
Effect of Oxytocin on the Regulation of the Hypothalamo–Pituitary–Adrenal (HPA) axis
Several studies have demonstrated that, under physiological conditions, the secretion of adrenocorticotropic hormone (ACTH) by the anterior pituitary corticotrophs is controlled by hypothalamic hormones such as corticotrophin‐releasing hormone (CRH) and vasopressin (see reviews in Refs. 51, 52) and by oxytocin under stress conditions [53]. In addition to CRH and vasopressin, the oxytocin‐induced ACTH secretion is suppressed by adrenal glucocorticoid hormones, and hence oxytocin is considered to be a hypophysiotrophic hormone. In the early 1990s, Dayanithi's group first demonstrated that oxytocin alone at physiological concentrations (already at 1 nM) under normal conditions, applied in a pulsatile fashion, induces the release of ACTH in freshly isolated rat anterior pituitary cells [54]. It should be noted that the oxytocin level can be markedly elevated in hypophysial portal plasma after pituitary stalk damage in the rat (see Ref. 55). The ACTH response due to oxytocin can be variable, that is, oxytocin exerts a synergistic or additive effect on ACTH secretion when applied with CRH or vasopressin, respectively [54]. Oxytocin‐dependent stimulation of ACTH release is mediated by an increase in [Ca2+]i, which occurs in the absence of external Ca2+[54, 56] by mobilizing Ca2+ from InsP3‐sensitive intracellular Ca2+ stores [57]. Furthermore, this ACTH release induced by oxytocin is blocked by glucocorticoids (corticosterone or RU 28362, a selective glucocorticoid receptor agonist) without interfering with [Ca2+]i transients [57]. Subsequently in the literature, no further emphasis was placed on better understanding the effect of oxytocin on ACTH secretion.
In recent times, however, many studies have demonstrated that, in addition to its role in reproduction and during pregnancy and lactation, oxytocin has central actions in moderating behavioral responses to various stressors and the activity of the HPA axis. For example, in adult animals, endogenous brain oxytocin enhances only the long‐lasting response of the HPA axis to stress [58], and various developmental consequences of oxytocin have been reported [59]. Subsequently, it was demonstrated that chronic intracerebroventricular oxytocin administration attenuates pathologically high anxiety in selectively bred Wistar rats [60]. Another group has presented evidence to support the concept that the hypothalamic–neurohypophysial system (HNS) might directly affect the activity of the HPA axis, by emphasizing its possible impact on some aspects of behavioral regulation and psychopathology [61]. In a different experimental approach, using oxytocin knockout mice, results has been published suggesting that oxytocin pathways play a role in attenuating the HPA axis’ response to psychogenic stress in female mice [62]. It was also reported that the ability of central oxytocin to inhibit the HPA axis’ activity depends on the levels of oestradiol, suggesting a direct interaction between them [63]. Hence, oxytocin seems to attenuate stress‐induced HPA activity and anxiety behaviors. Central oxytocin attenuates both the stress‐induced neuroendocrine and the molecular responses of the HPA axis, and the oxytocin‐sensitive forebrain stress circuit comprises the dorsal hippocampus, the ventrolateral septum and the PVN [64]. In contrast, it should be noted that the stress‐induced responses can be improved by oxytocin, by reducing ACTH and cortisol secretion, thus representing a potential therapeutic pathway in postpartum pathologies such as depression [65]. These authors therefore reinforce the notion that oxytocin, at the moment of initiating breastfeeding, acts not only on the physiological condition, but also on the psychic condition of the mother.
Is There Oxytocin Signaling in the Sensory System?
Returning to the nervous system, we shall now focus on the sensory system with particular emphasis on the dorsal root ganglia (DRG) neurones. In 1986, Kai‐Kai et al. identified immunoreactivity for vasopressin and oxytocin in neurones of the rat DRG [66]. Shortly thereafter, the accumulation of inositol phosphates following the application of vasopressin and oxytocin at concentrations within the micromolar range in the rat DRG was described [67]. This increase in inositol phosphate production was mediated through the V1 vasopressin receptor subtype. These reports demonstrated that: (i) both peptides of the HNS, vasopressin and oxytocin, are present in the PNS and specifically in DRG neurones, (ii) the neurohypophysial hormones are involved in the regulation of the inositol phosphate pathway through Gq protein coupled receptors, suggesting the existence of vasopressin receptors in ganglia cells and the possibility for the peptides to induce the production of inositol trisphosphate (InsP3) responsible for the activation of Ca2+ release channels localized on the membrane of the endoplasmic reticulum. Preliminary results from our group showed [Ca2+]i increases in response to oxytocin application in neuronal cells from the DRG. In 2002, Qing Yang et al. demonstrated that oxytocin had an inhibitory effect on ATP‐mediated currents in rat DRG neurones [68]. The mechanism of this inhibition was based on the oxytocin receptor leading to the activation of protein kinase A and the elevation of intracellular Ca2+, thus corroborating our data. While these studies clearly describe the action of oxytocin on DRGs, it is still difficult to relate a definite function of oxytocin to DRG cells. Whether oxytocin is excitatory or inhibitory probably depends on complex signaling pathways involved in the response to various stimuli. However, since oxytocin seems to be involved in antinociceptive effects in the spinal cord, as will be discussed below, understanding the physiological role of oxytocin in DRG neurones remains an important challenge.
Antinociceptive Functions of Oxytocin
It has long been known that neurones in the hypothalamic paraventricular nucleus (PVN) project not only to the posterior pituitary gland, but also to other brain areas and the spinal cord (e.g., see review in Ref. 69). The antinociceptive effect of OT was hinted at by the early demonstration of oxytocin‐immunoreactive fibers in the dorsal horn of the spinal cord [70, 71, 72]. Several initial behavioral investigations speculated on the possibility of intrathecally administered oxytocin exerting antinociceptive effects in a dose‐dependent manner [71, 73, 74]. The antinociceptive effect of oxytocin injected intrathecally was also reported in humans, where oxytocin was shown to relieve low back pain [75]. Oxytocin was also shown to exhibit antinociceptive effects in a model of experimental neuropathy developed following a spinal nerve ligation in rats [76]. It was further shown that oxytocin injected intraperitoneally into rats caused antinociceptive effects, which were reversed by an oxytocin antagonist (1‐deamino‐2‐D‐Tyr‐(OEt)‐4‐Thr‐8‐Orn‐oxytocin), but not by naloxone [77].
The release of oxytocin from synaptosomes prepared from the spinal cord was observed in response to 50 mM K+, and this release was inhibited by naloxone [78, 79]. The inhibitory effect of naloxone was mimicked by dynorphin but not by [D‐Ala2,N‐Me‐Phe4,Gly‐ol]‐enkephalin, suggesting that oxytocin release is under inhibitory control by opioids, and the major receptor involved in the inhibitory control is the kappa opioid receptor [79].
The antinociceptive effect of oxytocin was further studied in electrophysiological experiments. Spinal cord neurones, mainly located in the intermediolateral cell column (IML) and in the intermediomedial gray matter (IMM), responded to the application of oxytocin with either activation (48%) or inhibition (52%) [80]. The authors interpreted this complex response to oxytocin as the activation of inhibitory interneurones acting on second order projecting cells to modulate afferent tactile and nociceptive information.
A more precise analysis was made by comparing the latency after electrical stimulation of the dorsal root to distinguish the nature of the recorded neurones [81]. The analysis provided evidence that the signals carried by A‐δ and C fibers were selectively blocked by oxytocin and also by electrical stimulation of the PVN. The experiments further showed that the suppression of A‐δ and C fiber responses by PVN electrical stimulation was blocked by a selective oxytocin receptor antagonist (d(CH2)5[Tyr(Me)2,Thr4,Tyr‐(NH2)9]OVT) [82]. The same group reported that electrical stimulation of the PVN prolonged leg withdrawal latencies and caused an increase in oxytocin concentration in the spinal cord, indicating that the oxytocin system arising from the PVN participates in endogenous analgesia [83]. The authors also demonstrated that both PVN stimulation and intrathecal oxytocin administration prevented LTP (long‐term potentiation) in the dorsal horn, which is thought to be the central sensitization mechanism by which acute pain can turn into chronic pain [84]. Another group showed, using isolated spinal cord preparations, that not only oxytocin, but also corticotrophin releasing factor (CRF) and DAMGO (mu opioid receptor agonist) can increase the pain threshold [85].
The complex synaptic circuit involved in oxytocin‐mediated antinociception has been revealed by measurements of synaptic currents in dorsal horn neurones [86]. Electrical stimulation of the PVN or oxytocin application caused the activation of presynaptic oxytocin receptors at the terminals of glutamatergic interneurones, which, in turn, activated local GABA neurones to suppress the action potential firing of the laminar II dorsal horn neurones induced by inputs from A‐δ/C fibers.
Transgenic Animals Tagged by a Visible Fluorescent Protein to Study the Physiology of Vasopressin and Oxytocin
Recently, transgenic techniques have been developed and applied to the study of neurohypophyseal hormones [87, 88]. In particular, it is important and useful to identify oxytocin‐ and vasopressin‐secreting neurones tagged by fluorescent proteins such as green fluorescent protein (GFP).
GFP was originally identified from a bioluminescent jellyfish (Aequorea Victoria) [89], and cloned GFP has been modified to produce enhanced fluorescence in a variety of colors [90]. The first application of this transgenic strategy to neurohypophyseal hormones was performed in mice by Dr. Young and his colleagues.
The enhanced GFP (eGFP) was selectively expressed in oxytocin‐secreting neurones in the SON and PVN of the hypothalamus and nerve terminals in the posterior pituitary in oxytocin‐eGFP transgenic mice [91, 92]. A vasopressin‐eGFP fusion gene [93] and its mRNA were selectively expressed in the vasopressin‐secreting neurones in the hypothalamus and the posterior pituitary of a transgenic rat [93, 94] and successfully used in other studies [95, 96].
Furthermore, to visualize the oxytocin‐producing neurones in the hypothalamus and their terminals in the posterior pituitary, another transgenic rat was generated using an oxytocin‐enhanced cyan fluorescent protein (eCFP) fusion gene designed from a mouse construct (provided by Scott Young 3rd, USA). In situ hybridization revealed that the oxytocin‐eCFP fusion gene was expressed in the SON and the PVN of these rats [97]. The fluorescence emanating from eCFP was observed only in the SON, PVN, the internal layer of the median eminence, the neurohypophysis and isolated nerve terminals. In freshly dissociated SON neurones or nerve terminals (for review see Ref. 12) the fluorescence could be visualized up to 6 h (no attempt was made to keep them for longer durations) after isolation, and these neurones and terminals could be used to measure the [Ca2+]i transients upon depolarization with high K+ or stimulation with glutamate, ATP, caffeine and ryanodine, suggesting the survival of these tissues (unpublished results by G. Dayanithi). Details of the gene constructs of the different fluorescent fusion proteins are illustrated in Figure 1.
Figure 1.

Constructs of the fluorescent protein fusion genes used for transgenic animal models. (A) Structure of the arginine vasopressin (AVP)‐enhanced green fluorescent protein (eGFP) transgene. In the AVP‐eGFP transgene, the eGFP coding region is inserted at the frame in the middle of exon III. “Copyright 2005, The Endocrine Society” modified and reproduced with permission from Ref. [94]. (B) Structure of the c‐fos‐monomeric red fluorescent protein 1 (mRFP1) transgene. In the c‐fos‐mRFP1 transgene, the mRFP1 coding region is inserted at the frame at the end of exon IV followed by the stop codon. “Copyright 2009, The Endocrine Society” reproduced with permission from Ref. [98]. (C) Structure of the oxytocin‐enhanced cyan fluorescent protein (eCFP) transgene. In the oxytocin‐eCFP transgene, the eCFP coding region is inserted at the frame in the middle of exon III, after the oxytocin and the bulk of the neurophysin coding regions. “Copyright 2010, Society for Endocrinology” modified and reproduced with permission from Ref. [97].
Immunocytochemistry for oxytocin and vasopressin revealed that the eCFP fluorescence co‐localizes with oxytocin‐immunofluorescence, but not with vasopressin‐immunofluorescence, in the SON and the PVN. Moreover, the physiological responses to osmotic stimulation have been well demonstrated [97].
These vasopressin‐eGFP, double vasopressin‐eGFP and c‐fos‐mRFP1 [98], and oxytocin‐eCFP transgenic rats provide for unequivocal identification of vasopressin and oxytocin neurones and their terminals using fluorescent microscopy in in vitro preparations. Thus, these successful animal models are very useful for electrophysiological studies such as whole cell‐patch clamp recordings and imaging techniques such as intracellular Ca2+ concentration measurements, leading to major advances in the study of the physiology of vasopressin and oxytocin neurones and certainly of other cell/neuronal types.
Pharmacology of Oxytocin Agonists and Antagonists
In addition to fundamental insights into the role of oxytocin in the CNS [99], an increasing number of studies performed recently has shown the importance of oxytocin and its involvement, directly or indirectly, in several pathophysiological disorders in the nervous system and other organs. For example, oxytocin has been broadly discussed under the following titles: “oxytocin and addiction”[100]; “oxytocin increases trust in humans”[35]; “oxytocin increases generosity in humans”[101]; “search for autism treatments turns to ‘trust hormone’”[102]; “being human: love: neuroscience reveals all”[103]; “oxytocin: the great facilitator of life”[104]. Oxytocin, therefore, has become an interesting tool, especially through the design of oxytocin agonists and antagonists, and a potential candidate for drug research and therapeutics in humans [50].
In this section, we would like to address the issue of drug design based on oxytocin and oxytocin receptors in the CNS.
Drug Forms: Synthetic Nonapeptides
First of all, we shall consider the neurohormone itself and its peptide sequence. Though the amino acid sequence is rather short, its first complete synthesis more than 55 years ago was a struggle of many years because of difficulties, in the first place, in elucidating the structure, in degradation and in obtaining a sufficient amount of purified material [1, 105]. Oxytocin is a peptide of only nine amino acids: the sequence is Cysteine–Tyrosine–Isoleucine–Glutamine–Asparagine–Cysteine–Proline–Leucine–Glycineamide (CYIQNCPLG‐NH2). The Cysteine residues form a sulfur bridge. At this stage, it is worth noting the subsequent impact of the Merrifield Solid Phase Method in facilitating the synthesis of agonists and antagonists of oxytocin [106]. The role of many oxytocin agonists and antagonists as invaluable pharmacology research tools in studies on the peripheral and central effects of oxytocin [50] should also be mentioned here.
Oxytocin has a narrow therapeutic window (i.e., the drug dosage which is effective is restricted) and is eliminated from the circulatory system within minutes [107]. Therefore, the most precise and reliable mode of delivering oxytocin is through infusing it directly into the blood. Administered orally, the nonapeptide can be destroyed by proteolytic enzymes in the gastrointestinal tract [107]. As discussed above, the most common therapeutic application is the stimulation of maternal labor, and we saw that the efficiency of the method is not accepted by everyone. Possible administration routes include intraveinous infusion or intranasal administration. However, there seem to be some contradictions in the statements that can be found in the scientific literature. For instance, oxytocin nasal sprays have been successfully employed in neurobehavioral research [35], but an attempt to stimulate breastfeeding by a nasal spray was demonstrated to be ineffective [108]. Furthermore, this approach lacked biochemical evidence indicating that oxytocin can enter the CNS in significant quantities through a nasal spray. According to earlier reports, oxytocin given intravenously does not enter the brain—it does not cross the blood–brain barrier in significant quantities (see e.g., Ref. 109). However, the evidence that oxytocin can cross the blood–brain barrier following nasal administration and/or intraveinous administration is quite compelling according to recent studies [42, 110, 111].
Towards an Oxytocin Receptor Therapy
For 40 years, there has been increasing interest concerning neurohypophysial peptide research, particularly concerning the design of more selective agonists and antagonists (peptide and nonpeptide) of oxytocin receptors. The following excellent reviews should be consulted for more complete descriptions, see Refs. [50, 104, 112].
Potent and selective peptide agonists were developed for the oxytocin uterine receptor in the rat: [Thr4]OT, [HO1][Thr4]OT, [Thr4, Gly7]OT, and [HO1][Thr4, Gly7]OT [50]. While [Thr4, Gly7]OT is not highly selective for the human oxytocin receptor, [HO1][Thr4]OT is, and might therefore be used to design therapeutic analogues of oxytocin.
Similarly, highly selective peptide antagonists were synthesized for the oxytocin receptor in the rat and in humans: desGly‐NH2,d(CH2)5[Tyr(Me)2,Thr4]OVT, desGly‐NH2,d(CH2)5[D‐Tyr2,Thr4]OVT, d(CH2)5[D‐Thi2,Thr4,Tyr‐NH2 9]OVT, and desGly‐NH2,d(CH2)5[D‐Trp2,Thr4,Dap5]OVT [50].
More specifically, selective human oxytocin receptor antagonists with high affinities were produced: desGly‐NH2,d(CH2)5[D‐2‐Nal2,Thr4]OVT, desGly‐NH2,d(CH2)5[2‐Nal2,Thr4]OVT, d(CH2)5[D‐2‐Nal2,Thr4,Tyr‐NH2 9]OVT, d(CH2)5[2‐Nal2,Thr4,Tyr‐NH2 9]OVT, and FE 200 440 (Barusiban) [50]. The rationale behind developing highly selective oxytocin receptor antagonists for humans is the necessity to find a compound with better and safer tocolytic properties than Atosiban for the prevention of premature labor. Atosiban is a peptidic antagonist, but as we shall discuss below, its effects are complex, not entirely specific to oxytocin receptors (i.e., affinity for the AVP receptor subtype V1a) and the peptide is quickly metabolized. Alternatively, nonpeptide antagonists have also been developed with the aim of creating new orally administered active substances for the treatment and prevention of premature labor.
At this stage of the discussion, it is important to distinguish between the central and peripheral actions of the various oxytocin agonists and antagonists.
Some promising compounds (oxytocin receptor agonists or antagonists) have peripheral therapeutic applications: L‐372,662 (antagonist [113]), GSK‐221,149 (antagonist [114]), SSR‐126,768 (antagonist [115]), and synthetic oxytocin (known as Pitocin or Syntocinon), which is used to induce labor and to help milk production [116].
Correspondingly, several nonpeptide oxytocin agonists or oxytocin antagonists have a central therapeutic relevance: antagonist L‐371,257 (CAS# 162042–44‐6) (Ki= 9.3 nM) [117, 118]—which does not cross the blood brain barrier when administered in the CNS [119], L‐368,899 (antagonist, CAS# 148927–60‐0, CNS effects after oral administration, see Refs. 120, 121), WAY‐162,720 ([119], which is a brain‐penetrant oxytocin receptor antagonist when administered peripherally), WAY‐267,464 (agonist, which has been successfully introduced as an anxiolytic in mice, see [122], US patent assigned to Wyeth Corp, [50, 123]) and Compound 27 (agonist, EC50= 33 nM, 25 times more selective over vasopressin receptors, [124]). Compound 27 has been classified in the list of drugs putatively having a central effect, but this should be taken carefully since its biological actions are still under investigation.
It is important to know that over 94 clinical trials on oxytocin‐related studies are currently listed by the ClinicalTrials.gov registry (National Institutes of Health, USA). Of note is that 12 of these relate to the nervous system effects of oxytocin.
If treatments based on the nonapeptide itself and its analogues (Demoxytocin and Carbetocin) do not seem to be ideal, targeting oxytocin receptors with more long‐acting, specific and selective nonpeptide ligands might constitute a more reliable and successful approach. Yet the major challenge in the near future will be to tackle the issue of the diversity of the oxytocin receptor signaling pathways, which involve coupling to not only Gq proteins, but also to Gs and Gi proteins. Furthermore, the activation of Gq proteins itself seems to trigger a multifaceted and complex response in which not only the PLC/InsP3/PKC pathway, but also the phosphatidylinositol‐3‐kinase/AKT/endothelial nitric oxide synthase pathway is stimulated, at least at the peripheral level [125]. Therefore, designing drugs targeting both the oxytocin receptor and selectively one specific pathway (by combining agents or addressing one particular cell type for instance) might become a major consideration in the development of the next oxytocin‐based therapies.
Peripheral Functions of the Hormone Oxytocin and its Receptors
Oxytocin research has been extensively pursued in endocrine models such as the myometrium and mammary glands [126]. Furthermore, an increasing amount of data provides evidence that oxytocin acts on a plethora of peripheral organs distinct from the usual endocrine systems, where the neuropeptide plays unexpected biological roles, for instance as a peripheral neuromodulator or growth factor. Therefore, the question emerges: what can we learn from the periphery in terms of oxytocin's actions that could be applicable to better understand the CNS?
Reproductive System
We shall start with one of the oldest applications of oxytocin as a proper drug, that is, oxytocin used as a therapeutic agent during deliveries. The nonapeptide (or more precisely the octapeptide amide as first described by Vincent du Vigneaud in 1953; [1]) is a stimulant widely employed to induce or augment maternal labor [105], especially after term, when adequate oxytocin receptors are present. Although it is prescribed routinely, emerging studies highlight the side effects caused by misuse of oxytocin treatment: patient hyperstimulation and an increased caesarean delivery rate. Particularly, there was a recent controversy about how clinicians should give oxytocin to patients in terms of dosage, timing and monitoring its effects [127]. The authors report that oxytocin is considered to be one of the high‐alert medications along with insulin, methotrexate and nitroprusside ([127]; see the corresponding reply in the same journal: [128]). Indeed, even though the relationship between oxytocin signaling and maternal labor seems to be well established and therapy with the synthetic hormone has been commonly used for many years, the range of potential risks linked to oxytocin infusion is still not completely known; this requires further clinical trials with a larger number of patients. However, as we discussed above, oxytocin is multifaceted and all its targets are difficult to control. One investigation has proposed that oxytocin administration during pregnancy might be responsible for the development of autism and other behavioral disorders, but this hypothesis still needs to be tested [129]. Noteworthy is that an antagonist of the oxytocin receptor (Atosiban) is given to delay premature birth [130, 131]. In fact, Atosiban treatment is now generally accepted as the preferred treatment of preterm labor throughout Europe. The use of Atosiban is still controversial, and some authors have reported the lack of positive effect [132] and have proposed the alternate use of nifedipine (a dihydropyridine calcium channel blocker), which was recently shown to be ineffective as well [133]. Similarly, another study has shown that neither Atosiban nor nifedipine combined with betamethasone administration have direct adverse effects on the fetus [134]. In fact, it was reported that Atosiban is a rather nonselective compound in the human myometrium, where it activates very efficiently the AVP V1a receptors [50].
One of the main and now well‐characterized peripheral oxytocin targets is the erectile tissues (corpus spongiosum and corpus cavernosum). Though it appears to be an indirect effect, oxytocin injected in the ventral tegmental area of rats induces penile erection, a phenomenon which is dependent on calcium influx, nitric oxide (NO) production and a cyclic guanosine monophosphate (GMP) increase in dopaminergic neurones that modulate oxytocinergic neurones projecting to the spinal cord [135]. Moreover, oxytocin is thought to be associated with ejaculation by increasing sperm number and contracting ejaculatory tissues (prostatic urethra, bladder neck, and ejaculatory duct; [136]). An interesting study determined that oxytocin‐stimulated ejaculation is specifically mediated by vasopressin V1a receptors, as shown by the application of the V1a antagonist, SR49059, on ejaculatory tissues [137]. The authors demonstrated V1a‐dependence in erectile tissues as well, and therefore proposed V1a antagonists as a putative therapy for premature ejaculation.
Oxytocin as a Trophic Hormone
Devost et al. recently demonstrated that oxytocin exerts a trophic effect on myometrial cells and that this effect is mediated by dephosphorylation and the activation of elongation factor eEF2 [138]. These authors furthermore established that this effect does not involve any of the pathways known to activate eEF2 but, instead, is mediated via the activation of PKC [139].
Another recent investigation revealed the involvement of the neuropeptide in the anabolism of bone mass. By the use of oxytocin and oxytocin receptors (oxytocin receptors being present on bone cells) in null male and female mice, the authors found that the hormone evokes the differentiation of osteoblasts, stimulates osteoclast formation, but inhibits the restorative activity of mature osteoclasts ex vivo[140]. These findings strongly support a previous study demonstrating oxytocin‐promoted osteogenesis in human mesenchymal stem cells and oxytocin‐induced reversion of bone loss in mice [141]. Thus, there might be some medical perspective for osteoporosis; in particular, oxytocin could be employed as an anabolic stimulus to restore the skeletal loss occurring during pregnancy and lactation or postmenopausal periods, which should now be explored in patients.
Cell Proliferation and Cancer
Oxytocin can serve as a factor in differentiation, and it is now recognized that the neurohormone can also act as a growth factor, regulating cell proliferation, with a prominent effect on cancer cells. Paola Cassoni's seminal work over the last 10 years sheds some light on the connection between tumors and the nonapeptide. The latter displays a dual effect: while oxytocin impairs the proliferation of neoplastic cells from the mammary and endometrial epithelium, nerves and bone, in vitro[142, 143, 144, 145] and in vivo[146], the hormone promotes growth in the trophoblast and endothelium [147, 148]. Noteworthily different signaling pathways are involved in these opposite actions: the cAMP‐protein kinase A (PKA) cascade (quite unusual for oxytocin signaling) seems to be responsible for the inhibitory effect [149] with a possible link to the vasopressin receptor type 2 [150], whereas the stimulatory effect would be sustained by a conventional increase in intracellular calcium concentration and tyrosine phosphorylation [147, 148]. A change in the membrane localization of oxytocin receptors from noncaveolar structures to lipid rafts can switch the effect from inhibition to proliferation in MDCK cells [151].
Oxytocin in the Heart
Recently, we reviewed the actions of oxytocin in the heart and reported that an oxytocin system is present in cardiac cells and that oxytocin signaling plays a role in cardiac differentiation via NO signaling, in atrial natriuretic peptide (ANP) release and in the expression of oestrogen receptors [19, 152]. In rats, endogenous oxytocin plays an important role in cardiovascular responses to stress [153]. The heart is also a suggested site of oxytocin production and action [152]. From the vascular point of view, postnatal treatment with oxytocin was able to decrease blood pressure in spontaneously hypertensive adult male rats [154]. Nonetheless, the neurohormone seems to be ineffective in female rats [155].
Role of Oxytocin in Pancreatic Cells
The investigation of Björkstrand et al. in 1996 suggests that oxytocin influences pancreatic hormone secretion by two different mechanisms: peripheral circulating oxytocin evokes a rise in glucagon and glucose levels, whereas central oxytocin (administered by intracerebroventricular injections) causes a rise in insulin levels probably due to the activation of vagal cholinergic neurones [156]. Quite interestingly, some authors observed a decrease in the gene expression of oxytocin and oxytocin receptors in the heart of young diabetic mice [157]. Hitherto, however, no attempts to develop an oxytocin‐based therapy against diabetes have been made.
Coupling of Oxytocin Receptors to Intracellular Cascades
As discussed above, the OTR that mediates the manifold actions of oxytocin is a member of the GPCR superfamily.
In contrast to arginine‐vasopressin, oxytocin was known to have only one type of receptor, which belongs to the rhodopsin‐type (class I) G protein (Gαq11)‐coupled receptor family and is coupled to PLC, which controls the generation of InsP3 and diacylglycerol (DAG), which, in turn, leads to the liberation of Ca2+ from intracellular stores and the activation of protein kinases type C (PKC), respectively. In fact, the OTRs couple to different G proteins (for review see Ref. 32). Indeed, the coupling of the oxytocin receptor to Gs and Gi proteins also takes place (Figure 2).
Figure 2.
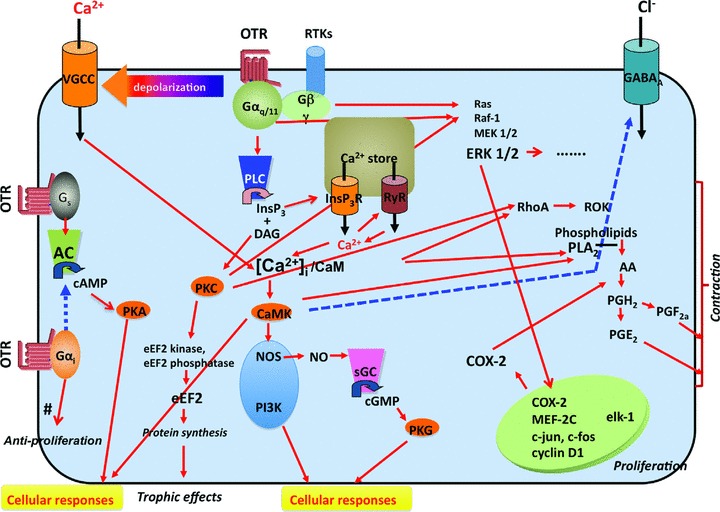
Schematic diagram of OTR‐linked signaling pathways. Oxytocin receptor (OTR) activation leads to three different GTP‐binding protein mechanisms. The major mechanism is mediated by the Gq/PLC/InsP3 pathway. When oxytocin binds to OTR, it activates Gαq/11 and then phospholipase C (PLC), which induces the cleavage of PIP2 to inositoltrisphosphate (InsP3) and diacylglycerol (DAG). InsP3 induces Ca2+ release from Ca2+ stores via InsP3R and, in some cells, causes Ca2+‐induced Ca2+ release (CICR) via the ryanodine receptor (RyR). The activation of Gq also causes membrane depolarization*, which, in turn, activates VGCCs and then facilitates Ca2+ entry through VGCCs. Thus, increased cytosolic Ca2+ ([Ca2+]i) stimulates CaMK after binding to the Ca2+ binding protein Calmodulin. The Ca2+/CaM complex then activates CaMK and causes various cellular responses, such as smooth muscle contractions, or induces the activation of several different types of enzymes, such as NOS or PI3K. DAG causes protein kinase C (PKC) activation and also various cellular responses. Additional pathways activated through the OTR include the MAP‐kinase (MAPK) and the Rho kinase pathways. The increased transcription of COX2 mediates the increased production and secretion of prostaglandins. The OTR‐mediated opening of Ca2+ channels is likely mediated through free Gβγ subunits. The OT receptor is known to be coupled with the other G proteins, Gs and Gi, both of which are linked with the AC pathway. The proliferative effects involve MAPK‐mediated activation of specific gene transcription. The trophic effects are mediated via a PKC‐mediated activation of eEF2. Activation of the Rho and MAP kinase pathways, the increase in intracellular Ca2+ and the increased prostaglandin secretion all contribute to the contractile effects. The antiproliferative effects observed in certain cells types appear to be mediated via αi G protein subunits. For further details, see the text and the references therein. The solid red lines and broken blue lines indicate activation and inhibition, respectively. Abbreviations: VGCC = Voltage‐gated Ca2+ channel; InsP3R = InsP3 receptor; RyR = Ryanodine receptor; PLC = Phospholipase C; DAG = Diacyl glycerol; Ca2+/CaM = Ca2+‐calmodulin complex; CaMK = Ca2+/Calmodulin‐dependent protein kinase; NOS = NO synthase; PLA2= Phospholipase A2; COX2 = Cyclooxygenase 2; AC = Adenylate cyclase; PI3K = Phosphoinositide 3‐kinase; ROK = Rho kinase. #The Gi mediated anti‐proliferative effect has been described as dependent on epidermal growth factor receptor (EGFR) transactivation and mitogen‐activated protein kinase (MAPK) activation via a PLC/PI3K/cellular sarcoma tyrosine kinase (c‐Src)‐dependent pathway that ultimately leads to a sustained activation of the cell cycle inhibitor [158].* The mechanisms of the oxytocin‐induced membrane depolarization have been explored in various types of neuronal cells, and they are classified as follows: 1. Suppression of voltage‐gated K+ currents 2. Activation of non‐selective cationic currents 3. Activation of sustained Na+‐dependent currents (could be the same as 2) Inhibition of GABAA receptors (this would depolarize if GABA acts as a tonic inhibitory modulator).
Moreover, several intracellular signaling pathways are activated via Gq. Beside the activation of PLC, intracellular Ca2+ is also increased by the opening of plasmalemmal Ca2+ channels. Oxytocin‐induced contractions are also mediated via the activation of the Rho kinase pathway. OTR activation leads to the stimulation of phospholipase A2 production and an increase in cyclooxygenase 2 levels, both resulting in increased prostaglandin production. The MAP‐kinase (MAPK) cascade is activated by different pathways, including trans‐activation of receptor tyrosine kinases and possibly different G protein‐linked pathways. The trophic effects of oxytocin have recently been shown to occur via a PKC‐mediated activation of eukaryotic elongation factor 2 [139]. The proliferative effects of oxytocin appear to be Gq‐linked and likely involve MAPK activation, leading to c‐fos and c‐jun induction. On the other hand, inhibition of cell growth has been reported to be Gi‐mediated [158]. As shown in Figure 2, the oxytocin‐mediated proliferative, trophic, contractile, and antiproliferative effects are supported by complex networks of signaling pathways. Not all of them are simultaneously active in every oxytocin responsive cell; the blend of oxytocin responses that occur in any given cell depends on the specific cell type as well as on the specific plasma membrane domains in which the receptor is located [158].
Such complex couplings of OTR to a diversity of intracellular cascades represent a double advantage with regards to physiological cellular reactions. First, it provides for a fine‐tuned control of all biochemical processes evoked by OTR stimulation. Indeed, the more steps there are in the signaling cascade, the more checkpoints there are to verify that each phase of the pathway has been correctly completed. Then, OTR activation leads to parallel signaling pathways that can converge on a common target. This redundancy is either important to amplify the initial signal or to supply an additional similar cascade in case the main cascade is impaired.
In terms of therapeutic consequences, the diversity of OTR signaling is extremely challenging. The coupling of OTR to different G proteins exhibiting opposite effects renders the definition of “agonist” and “antagonist” rather questionable. All OTR ligands have putatively the potential to stimulate dual signaling responses. Therefore, an “agonist” or “antagonist” can only be defined relative to the cellular context (e.g., cell type, stage of development, phosphorylation level, receptor subtype expression/trafficking level). The ability to design compounds that can discriminate between many diverse pathways and predominantly activate a specific intracellular reaction will be at the heart of future OTR‐based drug strategies.
Future Perspectives
Over the last years, ligand screening assays and studies of GPCRs have benefited from the development of fluorescent agonists and antagonists, particularly in the field of vasopressin and oxytocin signaling pathways. Fluorescent oxytocin agonists present quite high affinities and a strong selectivity for the human oxytocin receptor [159]. They appear to be useful tools to investigate receptor localization, desensitization and internalization of the oxytocin receptor ligand. Furthermore, they can be used to perform fluorescence recovery after photobleaching (FRAP) to track the diffusion of substances in tissues or cells. In addition, they are suitable for ligand–receptor interaction and structural organization studies, using fluorescence quenching, fluorescence polarization and fluorescence resonance energy transfer (FRET) [50]. As safe, very sensitive, homogenous and fast methods, they are considered as a strong basis for high‐throughput screening experiments [160].
Using bioluminescence resonance energy transfer (BRET) techniques, Zingg's group was able to demonstrate that the oxytocin receptor interacts with other GPCRs coexpressed in myometrial cells [161]. Heterodimerization with the β2 adrenergic receptor (β2AR) was also supported by BRET experiments. The authors also obtained evidence that this interaction has functional consequences: β2AR antagonists are able to allosterically modulate oxytocin receptor signaling and oxytocin receptor trafficking and, vice versa, an oxytocin antagonist can modulate β2AR signaling (personal communication).
Therefore, the development of drug design to target oxytocin signaling pathways may need to consider joint therapies with compounds being able to target several types of receptors at the same time or to consider substances involved in the oligomerization process of the oxytocin receptors.
Concluding Remarks
The story of oxytocin begins right before pregnancy, continues during birth and later, travels from the brain to the heart and throughout the entire body, triggering or modulating a full range of physiological functions and emotions: happiness, attraction, love, affection, and hatred after stress. These are all governed directly or indirectly, at least in part, by oxytocin. With this review, we aimed to highlight the newly discovered roles of oxytocin by covering both basic science and the therapeutic applications of new oxytocin analogues, and we attempt to summarize the recently discovered physiological effects of oxytocin. The nonapeptide appears to play a central role in social behavior, and emerging clinical trials seek to assess and define its therapeutic potential in the treatment of pathophysiological behaviors. Another promising therapeutic breakthrough in the next years could be the development of oxytocin‐based medications to treat altered nociception. Though there is some evidence for the involvement of oxytocin in pain, a certain amount of work still needs to be carried out, especially understanding the link between oxytocin and the DRGs. At the periphery, oxytocin also seems to be a key component in bone formation, glycaemia, male sexuality, cardiac differentiation, and nonregulated cellular proliferation. Therefore, there is a strong impetus to develop and establish new technological tools that will enable us to uncover oxytocin and its possibilities. We report here, for instance, major advances in the fields of transgenic animal engineering and the synthesis of fluorescent agonists and antagonists. Thus, we are now in a good position to more completely screen oxytocin function at the molecular, cellular and whole animal levels. Taken together, the insights gained from more than 100 years of research indicate that the success story of the hormone of “swift birth” will continue. The potential therapeutic uses for oxytocin and more long‐acting and specific analogues of oxytocin are huge. Chemical, physiopathological, psychological, philosophical and ethical studies will reinforce the development of new drugs involving the use of oxytocin, oxytocin agonists and antagonists for various human disorders such as autism, premature ejaculation, osteoporosis, diabetes and cancer.
Conflict of Interest
The authors state that they have no conflict of interest pertaining to this manuscript.
Acknowledgments
G. Dayanithi is supported by the “Centre National de la Recherche Scientifique”‐France, the Japanese Society for Promotion of Science Fellowship Program (#FY2008; S‐08216), and research facilities from the Institute of Experimental Medicine of the Academy of Sciences of the Czech Republic. This work was supported by the Grant Agency of the Czech Republic grants GACR 309/08/1381, GACR 305/08/1384, and GACR 309/09/1597; the Academy of Sciences of the Czech Republic grant AVOZ 50390512 and the Ministry of Education, Youth and Sports of the Czech Republic grants LC554 and 1M0538. We are very grateful to James Dutt, IEM ASCR, Prague, for critical reading and language editing of the manuscript.
Re‐use of this article is permitted in accordance with the Terms and Conditions set out at http://www3.interscience.wiley.com/authorresources/onlineopen.html
References
- 1. Du Vigneaud V. Trail of sulfur research: From insulin to oxytocin. Science 1956;123:967–974. [DOI] [PubMed] [Google Scholar]
- 2. Cazalis M, Dayanithi G, Nordmann J. The role of patterned burst and interburst interval on the excitation‐coupling mechanism in the isolated rat neural lobe. J Physiol 1985;369:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nordmann J, Dayanithi G. Release of neuropeptides does not only occur at nerve terminals. Biosci Rep 1988;8:471–483. [DOI] [PubMed] [Google Scholar]
- 4. Cazalis M, Dayanithi G, Nordmann JJ. Requirements for hormone release from permeabilized nerve endings isolated from the rat neurohypophysis. J Physiol 1987;390:71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cazalis M, Dayanithi G, Nordmann JJ. Hormone release from isolated nerve endings of the rat neurohypophysis. J Physiol 1987;390:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shibuya I, Dayanithi G, Ueta Y. Stimulus‐secretion‐coupling. Naibunpitsu tounyouka. Endocrinol Diabetol 2004;18:403–410. [Google Scholar]
- 7. Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol 1998;10:881–895. [DOI] [PubMed] [Google Scholar]
- 8. Neumann I, Russell J, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: A microdialysis study. Neuroscience 1993;53:65–75. [DOI] [PubMed] [Google Scholar]
- 9. Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol 1994;478(Pt 2):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dayanithi G, Widmer H, Richard P. Vasopressin‐induced intracellular Ca2+ increase in isolated rat supraoptic cells. J Physiol 1996;490(Pt 3):713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabatier N, Shibuya I, Dayanithi G. Intracellular calcium increase and somatodendritic vasopressin release by vasopressin receptor agonists in the rat supraoptic nucleus: Involvement of multiple intracellular transduction signals. J Neuroendocrinol 2004;16:221–236. [DOI] [PubMed] [Google Scholar]
- 12. Dayanithi G, Sabatier N, Widmer H. Intracellular calcium signaling in magnocellular neurones of the rat supraoptic nucleus: Understanding the autoregulatory mechanisms. Exp Physiol 2000;85:75S–84S. [DOI] [PubMed] [Google Scholar]
- 13. Ludwig M, Sabatier N, Bull P, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity‐dependent neuropeptide release from dendrites. Nature 2002;418:85–89. [DOI] [PubMed] [Google Scholar]
- 14. Sabatier N, Richard P, Dayanithi G. Activation of multiple intracellular transduction signals by vasopressin in vasopressin‐sensitive neurones of the rat supraoptic nucleus. J Physiol 1998;513(Pt 3):699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuriyama K, Nakashima T, Kawarabayashi T, Kiyohara T. Oxytocin inhibits nonphasically firing supraoptic and paraventricular neurons in the virgin female rat. Brain Res Bull 1993;31:681–687. [DOI] [PubMed] [Google Scholar]
- 16. Chini B, Manning M, Guillon G. Affinity and efficacy of selective agonists and antagonists for vasopressin and oxytocin receptors: An “easy guide” to receptor pharmacology. Prog Brain Res 2008;170:513–517. [DOI] [PubMed] [Google Scholar]
- 17. Widmer H, Ludwig M, Bancel F, Leng G, Dayanithi G. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. J Physiol 2003;548(Pt 1):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viero C, Dayanithi G. Neurosteroids are excitatory in supraoptic neurons but inhibitory in the peripheral nervous system: It is all about oxytocin and progesterone receptors. Prog Brain Res 2008;170:177–192. [DOI] [PubMed] [Google Scholar]
- 19. Dayanithi G, Viero C, Shibuya I. The role of calcium in the action and release of vasopressin and oxytocin from CNS neurones/terminals to the heart. J Physiol Pharmacol 2008;59(Suppl 8):7–26. [PubMed] [Google Scholar]
- 20. Theodosis DT, Poulain DA. Activity‐dependent neuronal‐glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience 1993;57:501–535. [DOI] [PubMed] [Google Scholar]
- 21. Hatton GI. Oxytocin and vasopressin neurones: Vive la difference. J Physiol 1997;500(Pt 2):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sykova E. Glial diffusion barriers during aging and pathological states. Prog Brain Res 2001;132:339–363. [DOI] [PubMed] [Google Scholar]
- 23. Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev 2008;88:1277–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A 2004;101:2151–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Theodosis D, Poulain D, Oliet S. Activity‐dependent structural and functional plasticity of astrocyte‐neuron interactions. Physiol Rev 2008;88:983–1008. [DOI] [PubMed] [Google Scholar]
- 26. Febo M, Shields J, Ferris C, King J. Oxytocin modulates unconditioned fear response in lactating dams: An fMRI study. Brain Res. 2009;1302:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Schwartz P, Rissman E. Distribution of estrogen receptor‐beta‐like immunoreactivity in rat forebrain. Neuroendocrinology 1997;66:63–67. [DOI] [PubMed] [Google Scholar]
- 28. Young, L , HH Z. Oxytocin In: Pfaff EaD AM, editor. Molecular mechanisms of hormones actions on behavior, San Diego , CA : Academic Press, 2009;783–802. [Google Scholar]
- 29. Ostrowski N. Oxytocin receptor mRNA expression in rat brain: Implications for behavioral integration and reproductive success. Psychoneuroendocrinology 1998;23:989–1004. [DOI] [PubMed] [Google Scholar]
- 30. Adan R, Van Leeuwen F, Sonnemans M, et al Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology 1995;136:4022–4028. [DOI] [PubMed] [Google Scholar]
- 31. Veinante P, Freund‐Mercier M. Distribution of oxytocin‐ and vasopressin‐binding sites in the rat extended amygdala: A histoautoradiographic study. J Comp Neurol 1997;383:305–325. [PubMed] [Google Scholar]
- 32. Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev 2001;81:629–683. [DOI] [PubMed] [Google Scholar]
- 33. Neumann ID. The advantage of social living: Brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front Neuroendocrinol 2009;30:483–496. [DOI] [PubMed] [Google Scholar]
- 34. Reidpath D, Chan K, Gifford S, Allotey P. ‘He hath the French pox’: Stigma, social value and social exclusion. Sociol Health Illn 2005;27:468–489. [DOI] [PubMed] [Google Scholar]
- 35. Kosfeld M, Heinrichs M, Zak P, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 2005;435:673–676. [DOI] [PubMed] [Google Scholar]
- 36. Kirsch P, Esslinger C, Chen Q, et al Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 2005;25:11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Domes G, Heinrichs M, Gläscher J, Büchel C, Braus D, Herpertz S. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 2007;62:1187–1190. [DOI] [PubMed] [Google Scholar]
- 38. DiCicco‐Bloom E, Lord C, Zwaigenbaum L, et al The developmental neurobiology of autism spectrum disorder. J Neurosci 2006;26:6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Israel S, Lerer E, Shalev I, et al The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One 2009;4:e5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lucht M, Barnow S, Sonnenfeld C, et al Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:860–866. [DOI] [PubMed] [Google Scholar]
- 41. Kavaliers M, Choleris E, Agmo A, et al Inadvertent social information and the avoidance of parasitized male mice: A role for oxytocin. Proc Natl Acad Sci U S A 2006;103:4293–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hollander E, Novotny S, Hanratty M, et al Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology 2003;28:193–198. [DOI] [PubMed] [Google Scholar]
- 43. Hollander E, Bartz J, Chaplin W, et al Oxytocin increases retention of social cognition in autism. Biol Psychiatry 2007;61:498–503. [DOI] [PubMed] [Google Scholar]
- 44. Yamasue H, Kuwabara H, Kawakubo Y, Kasai K. Oxytocin, sexually dimorphic features of the social brain, and autism. Psychiatry Clin Neurosci 2009;63:129–140. [DOI] [PubMed] [Google Scholar]
- 45. Leckman J, Goodman W, North W, et al The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology 1994;19:723–749. [DOI] [PubMed] [Google Scholar]
- 46. De Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol 1993;14:251–302. [DOI] [PubMed] [Google Scholar]
- 47. Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein R. Association between the oxytocin receptor (OXTR) gene and autism: Relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry 2008;13:980–988. [DOI] [PubMed] [Google Scholar]
- 48. Yoshida M, Takayanagi Y, Inoue K, et al Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci 2009;29:2259–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vitalo A, Fricchione J, Casali M, et al Nest making and oxytocin comparably promote wound healing in isolation reared rats. PLoS One 2009;4:e5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non‐peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: Research tools and potential therapeutic agents. Prog Brain Res 2008;170:473–512. [DOI] [PubMed] [Google Scholar]
- 51. Antoni F. Hypothalamic control of adrenocorticotropin secretion: Advances since the discovery of 41‐residue corticotropin‐releasing factor. Endocr Rev 1986;7:351–378. [DOI] [PubMed] [Google Scholar]
- 52. Rivier C, Plotsky P. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol 1986;48:475–494. [DOI] [PubMed] [Google Scholar]
- 53. Gibbs D. Stress‐specific modulation of ACTH secretion by oxytocin. Neuroendocrinology 1986;42:456–458. [DOI] [PubMed] [Google Scholar]
- 54. Link H, Dayanithi G, Föhr K, Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH‐releasing factor or arginine vasopressin‐induced ACTH secretion, respectively. Endocrinology 1992;130:2183–2191. [DOI] [PubMed] [Google Scholar]
- 55. Makara G, Sutton S, Otto S, Plotsky P. Marked changes of arginine vasopressin, oxytocin, and corticotropin‐releasing hormone in hypophysial portal plasma after pituitary stalk damage in the rat. Endocrinology 1995;136:1864–1868. [DOI] [PubMed] [Google Scholar]
- 56. Oki Y, Peatman T, Qu Z, Orth D. Effects of intracellular Ca2+ depletion and glucocorticoid on stimulated adrenocorticotropin release by rat anterior pituitary cells in a microperifusion system. Endocrinology 1991;128:1589–1596. [DOI] [PubMed] [Google Scholar]
- 57. Link H, Dayanithi G, Gratzl M. Glucocorticoids rapidly inhibit oxytocin‐stimulated adrenocorticotropin release from rat anterior pituitary cells, without modifying intracellular calcium transients. Endocrinology 1993;132:873–878. [DOI] [PubMed] [Google Scholar]
- 58. Nakashima T, Noguchi T, Furukawa T, et al Brain oxytocin augments stress‐induced long‐lasting plasma adrenocorticotropic hormone elevation in rats. Neurosci Lett 2002;321:161–164. [DOI] [PubMed] [Google Scholar]
- 59. Carter C. Developmental consequences of oxytocin. Physiol Behav 2003;79:383–397. [DOI] [PubMed] [Google Scholar]
- 60. Slattery D, Neumann I. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 2010;58:56–61. [DOI] [PubMed] [Google Scholar]
- 61. Engelmann M, Landgraf R, Wotjak C. The hypothalamic‐neurohypophysial system regulates the hypothalamic‐pituitary‐adrenal axis under stress: An old concept revisited. Front Neuroendocrinol 2004;25:132–149. [DOI] [PubMed] [Google Scholar]
- 62. Mantella R, Vollmer R, Rinaman L, Li X, Amico J. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am J Physiol Regul Integr Comp Physiol 2004;287:R1494–1504. [DOI] [PubMed] [Google Scholar]
- 63. Ochedalski T, Subburaju S, Wynn P, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic‐pituitary‐adrenal axis activity. J Neuroendocrinol 2007;19:189–197. [DOI] [PubMed] [Google Scholar]
- 64. Windle R, Kershaw Y, Shanks N, Wood S, Lightman S, Ingram C. Oxytocin attenuates stress‐induced c‐fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo‐pituitary‐adrenal activity. J Neurosci 2004;24:2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boutet C, Vercueil L, Schelstraete C, Buffin A, Legros J. Oxytocin and maternal stress during the post‐partum period. Ann Endocrinol (Paris) 2006;67:214–223. [DOI] [PubMed] [Google Scholar]
- 66. Kai‐Kai M, Anderton B, Keen P. A quantitative analysis of the interrelationships between subpopulations of rat sensory neurons containing arginine vasopressin or oxytocin and those containing substance P, fluoride‐resistant acid phosphatase or neurofilament protein. Neuroscience 1986;18:475–486. [DOI] [PubMed] [Google Scholar]
- 67. Horn A, Lightman S. Vasopressin‐induced turnover of phosphatidylinositol in the sensory nervous system of the rat. Exp Brain Res 1987;68:299–304. [DOI] [PubMed] [Google Scholar]
- 68. Yang Q, Wu Z, Li X, Li Z, Wei J, Hu Q. Modulation by oxytocin of ATP‐activated currents in rat dorsal root ganglion neurons. Neuropharmacology 2002;43:910–916. [DOI] [PubMed] [Google Scholar]
- 69. Zimmerman E, Nilaver G, Hou‐Yu A, Silverman A. Vasopressinergic and oxytocinergic pathways in the central nervous system. Fed Proc 1984;43:91–96. [PubMed] [Google Scholar]
- 70. Millan M, Millan M, Członkowski A, Herz A. Vasopressin and oxytocin in the rat spinal cord: Distribution and origins in comparison to [Met]enkephalin, dynorphin and related opioids and their irresponsiveness to stimuli modulating neurohypophyseal secretion. Neuroscience 1984;13:179–187. [DOI] [PubMed] [Google Scholar]
- 71. Lundeberg T, Meister B, Björkstrand E, Uvnäs‐Moberg K. Oxytocin modulates the effects of galanin in carrageenan‐induced hyperalgesia in rats. Brain Res 1993;608:181–185. [DOI] [PubMed] [Google Scholar]
- 72. Puder B, Papka R. Hypothalamic paraventricular axons projecting to the female rat lumbosacral spinal cord contain oxytocin immunoreactivity. J Neurosci Res 2001;64:53–60. [DOI] [PubMed] [Google Scholar]
- 73. Xu X, Wiesenfeld‐Hallin Z. Intrathecal oxytocin facilitates the spinal nociceptive flexor reflex in the rat. Neuroreport 1994;5:750–752. [DOI] [PubMed] [Google Scholar]
- 74. Yang J, Yang Y, Chen J, Liu W, Wang C, Lin B. Central oxytocin enhances antinociception in the rat. Peptides 2007;28:1113–1119. [DOI] [PubMed] [Google Scholar]
- 75. Yang J. Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine (Phila Pa 1976) 1994;19:867–871. [DOI] [PubMed] [Google Scholar]
- 76. Condés‐Lara M, Maie I, Dickenson A. Oxytocin actions on afferent evoked spinal cord neuronal activities in neuropathic but not in normal rats. Brain Res 2005;1045:124–133. [DOI] [PubMed] [Google Scholar]
- 77. Lundeberg T, Uvnäs‐Moberg K, Agren G, Bruzelius G. Anti‐nociceptive effects of oxytocin in rats and mice. Neurosci Lett 1994;170:153–157. [DOI] [PubMed] [Google Scholar]
- 78. Daddona M, Haldar J. The release of oxytocin from spinal cord synaptosomes by high KCl depolarizing stimulus: A calcium dependent process. Life Sci 1994;54:945‐949. [DOI] [PubMed] [Google Scholar]
- 79. Daddona M, Haldar J. Opioid modulation of oxytocin release from spinal cord synaptosomes. Neuroreport 1994;5:1833–1835. [DOI] [PubMed] [Google Scholar]
- 80. Condés‐Lara M, González N, Martínez‐Lorenzana G, Delgado O, Freund‐Mercier M. Actions of oxytocin and interactions with glutamate on spontaneous and evoked dorsal spinal cord neuronal activities. Brain Res 2003;976:75–81. [DOI] [PubMed] [Google Scholar]
- 81. Condés‐Lara M, Rojas‐Piloni G, Martínez‐Lorenzana G, Rodríguez‐Jiménez J, López Hidalgo M, Freund‐Mercier M. Paraventricular hypothalamic influences on spinal nociceptive processing. Brain Res 2006;1081:126–137. [DOI] [PubMed] [Google Scholar]
- 82. Condés‐Lara M, Rojas‐Piloni G, Martínez‐Lorenzana G, López‐Hidalgo M, Rodríguez‐Jiménez J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A‐delta and C fiber primary afferent excitation of spinal cord cells. Brain Res 2009;1247:38–49. [DOI] [PubMed] [Google Scholar]
- 83. Martínez‐Lorenzana G, Espinosa‐López L, Carranza M, et al PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain 2008;140:265–273. [DOI] [PubMed] [Google Scholar]
- 84. DeLaTorre S, Rojas‐Piloni G, Martínez‐Lorenzana G, Rodríguez‐Jiménez J, Villanueva L, Condés‐Lara M. Paraventricular oxytocinergic hypothalamic prevention or interruption of long‐term potentiation in dorsal horn nociceptive neurons: Electrophysiological and behavioral evidence. Pain 2009;144:320–328. [DOI] [PubMed] [Google Scholar]
- 85. Wilson L, Wayman C, Jackson V. Neuropeptide modulation of a lumbar spinal reflex: Potential implications for female sexual function. J Sex Med 2009;6:947–957. [DOI] [PubMed] [Google Scholar]
- 86. Breton J, Veinante P, Uhl‐Bronner S, et al Oxytocin‐induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in lamina I‐II which amplify GABAergic inhibition. Mol Pain 2008;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Murphy D, Wells S. In vivo gene transfer studies on the regulation and function of the vasopressin and oxytocin genes. J Neuroendocrinol 2003;15:109–125. [DOI] [PubMed] [Google Scholar]
- 88. Young Wr, Gainer H. Transgenesis and the study of expression, cellular targeting and function of oxytocin, vasopressin and their receptors. Neuroendocrinology 2003;78:185–203. [DOI] [PubMed] [Google Scholar]
- 89. Tsien R. The green fluorescent protein. Annu Rev Biochem 1998;67:509–544. [DOI] [PubMed] [Google Scholar]
- 90. Shimomura O, editor. Discovery of green fluorescent protein (GFP) Nobel Lecture. 2008. [DOI] [PubMed]
- 91. Young Wr, Iacangelo A, Luo X, King C, Duncan K, Ginns E. Transgenic expression of green fluorescent protein in mouse oxytocin neurones. J Neuroendocrinol 1999;11:935–939. [DOI] [PubMed] [Google Scholar]
- 92. Zhang B, Kusano K, Zerfas P, Iacangelo A, Young Wr, Gainer H. Targeting of green fluorescent protein to secretory granules in oxytocin magnocellular neurons and its secretion from neurohypophysial nerve terminals in transgenic mice. Endocrinology 2002;143:1036–1046. [DOI] [PubMed] [Google Scholar]
- 93. Ueta Y, Fujihara H, Dayanithi G, Kawata M, Murphy D. Specific expression of optically active reporter gene in arginine vasopressin‐secreting neurosecretory cells in the hypothalamic‐neurohypophyseal system. J Neuroendocrinol 2008;20:660–664. [DOI] [PubMed] [Google Scholar]
- 94. Ueta Y, Fujihara H, Serino R, et al Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology 2005;146:406–413. [DOI] [PubMed] [Google Scholar]
- 95. Fujio T, Fujihara H, Shibata M, et al Exaggerated response of arginine vasopressin‐enhanced green fluorescent protein fusion gene to salt loading without disturbance of body fluid homeostasis in rats. J Neuroendocrinol 2006;18:776–785. [DOI] [PubMed] [Google Scholar]
- 96. Suzuki H, Kawasaki M, Ohnishi H, et al Exaggerated response of a vasopressin‐enhanced green fluorescent protein transgene to nociceptive stimulation in the rat. J Neurosci 2009;29:13182–13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Katoh A, Fujihara H, Ohbuchi T, et al Specific expression of an oxytocin‐enhanced cyan fluorescent protein fusion transgene in the rat hypothalamus and posterior pituitary. J Endocrinol 2010;204:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fujihara H, Ueta Y, Suzuki H, et al Robust up‐regulation of nuclear red fluorescent‐tagged fos marks neuronal activation in green fluorescent vasopressin neurons after osmotic stimulation in a double‐transgenic rat. Endocrinology 2009;150:5633–5638. [DOI] [PubMed] [Google Scholar]
- 99. Oliet SH, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT. Neuron‐glia interactions in the rat supraoptic nucleus. Prog Brain Res 2008;170:109–117. [DOI] [PubMed] [Google Scholar]
- 100. Kovács G, Sarnyai Z, Szabó G. Oxytocin and addiction: A review. Psychoneuroendocrinology 1998;23:945–962. [DOI] [PubMed] [Google Scholar]
- 101. Zak P, Stanton A, Ahmadi S. Oxytocin increases generosity in humans. PLoS One 2007;2:e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Opar A. Search for potential autism treatments turns to ‘trust hormone’. Nat Med 2008;14:353. [DOI] [PubMed] [Google Scholar]
- 103. Young L. Being human: Love: Neuroscience reveals all. Nature 2009;457:148. [DOI] [PubMed] [Google Scholar]
- 104. Lee H, Macbeth A, Pagani J, Young Wr. Oxytocin: The great facilitator of life. Prog Neurobiol 2009;88:127–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Den Hertog C, De Groot A, Van Dongen P. History and use of oxytocics. Eur J Obstet Gynecol Reprod Biol 2001;94:8–12. [DOI] [PubMed] [Google Scholar]
- 106. Manning M. Impact of the Merrifield solid phase method on the design and synthesis of selective agonists and antagonists of oxytocin and vasopressin: A historical perspective. Biopolymers 2008;90:203–212. [DOI] [PubMed] [Google Scholar]
- 107. Rowland M, Tozer TN. Why clinical pharmacokinetics? In: Wilkins LW, editor. Clinical pharmacokinetics: Concepts and applications. Philadelphia , PA : Lippincott Williams and Wilkins; 1995;1–10. [Google Scholar]
- 108. Fewtrell M, Loh K, Blake A, Ridout D, Hawdon J. Randomised, double blind trial of oxytocin nasal spray in mothers expressing breast milk for preterm infants. Arch Dis Child Fetal Neonatal Ed 2006;91:F169–F174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Leng G. Oxytocin In: Fink G, editor. Encyclopedia of stress. San Diego , CA : Academic Press, 2000;109–114. [Google Scholar]
- 110. Shamay‐Tsoory S, Fischer M, Dvash J, Harari H, Perach‐Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biol Psychiatry 2009;66:864–870. [DOI] [PubMed] [Google Scholar]
- 111. Guastella A, Howard A, Dadds M, Mitchell P, Carson D. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 2009;34:917–923. [DOI] [PubMed] [Google Scholar]
- 112. Gimpl G. Oxytocin receptor ligands: A survey of the patent literature. Exp Opin Therap Patents 2008;18:1239–1251. [Google Scholar]
- 113. Bell I, Erb J, Freidinger R, et al Development of orally active oxytocin antagonists: Studies on 1‐(1‐[4‐[1‐(2‐methyl‐1‐oxidopyridin‐3‐ylmethyl)piperidin‐4‐yloxy]‐2‐ methoxybenzoyl]piperidin‐4‐yl)‐1,4‐dihydrobenz[d][1,3]oxazin‐2‐one (L‐372,662) and related pyridines. J Med Chem 1998;41:2146–2163. [DOI] [PubMed] [Google Scholar]
- 114. McCafferty G, Pullen M, Wu C, et al Use of a novel and highly selective oxytocin receptor antagonist to characterize uterine contractions in the rat. Am J Physiol Regul Integr Comp Physiol 2007;293:R299–R305. [DOI] [PubMed] [Google Scholar]
- 115. Serradeil‐Le Gal C, Valette G, Foulon L, et al SSR126768A (4‐chloro‐3‐[(3R)‐(+)‐5‐chloro‐1‐(2,4‐dimethoxybenzyl)‐3‐methyl‐2‐oxo‐2,3‐dihydro‐1H‐indol‐3‐yl]‐N‐ethyl‐N‐(3‐pyridylmethyl)‐benzamide, hydrochloride): A new selective and orally active oxytocin receptor antagonist for the prevention of preterm labor. J Pharmacol Exp Ther 2004;309:414–424. [DOI] [PubMed] [Google Scholar]
- 116. Hayes E, Weinstein L. Improving patient safety and uniformity of care by a standardized regimen for the use of oxytocin. Am J Obstet Gynecol. 2008;198:622.e1–7. [DOI] [PubMed] [Google Scholar]
- 117. Williams P, Clineschmidt B, Erb J, et al 1‐(1‐[4‐[(N‐acetyl‐4‐piperidinyl)oxy]‐2‐methoxybenzoyl]piperidin‐4‐ yl)‐4H‐3,1‐benzoxazin‐2(1H)‐one (L‐371,257): A new, orally bioavailable, non‐peptide oxytocin antagonist. J Med Chem 1995;38:4634–4636. [DOI] [PubMed] [Google Scholar]
- 118. Wyatt P, Allen M, Chilcott J, et al Identification of potent and selective oxytocin antagonists. Part 1: Indole and benzofuran derivatives. Bioorg Med Chem Lett 2002;12:1399–1404. [DOI] [PubMed] [Google Scholar]
- 119. Ring R, Malberg J, Potestio L, et al Anxiolytic‐like activity of oxytocin in male mice: Behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. [DOI] [PubMed] [Google Scholar]
- 120. Boccia M, Goursaud A, Bachevalier J, Anderson K, Pedersen C. Peripherally administered non‐peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non‐human primates. Horm Behav 2007;52:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Williams P, Anderson P, Ball R, et al 1‐((7,7‐Dimethyl‐2(S)‐(2(S)‐amino‐4‐(methylsulfonyl)butyramido)bicyclo [2.2.1]‐heptan‐1(S)‐yl)methyl)sulfonyl)‐4‐(2‐methylphenyl)piperaz ine (L‐368,899): An orally bioavailable, non‐peptide oxytocin antagonist with potential utility for managing preterm labor. J Med Chem 1994;37:565–571. [DOI] [PubMed] [Google Scholar]
- 122. Rahman Z, Resnick, L , Rosenzweig‐Lipson SJ, Ring RH, inventors; Wyeth Corp., assignee . Methods of treatment using oxytocin receptor agonists. USA 2007; 2007‐05‐24. [Google Scholar]
- 123. Ring R, Schechter L, Leonard S, et al Receptor and behavioral pharmacology of WAY‐267464, a non‐peptide oxytocin receptor agonist. Neuropharmacology 2010;58:69–77. [DOI] [PubMed] [Google Scholar]
- 124. Pitt G, Batt A, Haigh R, et al Non‐peptide oxytocin agonists. Bioorg Med Chem Lett 2004;14:4585–4589. [DOI] [PubMed] [Google Scholar]
- 125. Cattaneo M, Lucci G, Vicentini L. Oxytocin stimulates in vitro angiogenesis via a Pyk‐2/Src‐dependent mechanism. Exp Cell Res 2009;315:3210–3219. [DOI] [PubMed] [Google Scholar]
- 126. Soloff M, Beauregard G, Potier M. Determination of the functional size of oxytocin receptors in plasma membranes from mammary gland and uterine myometrium of the rat by radiation inactivation. Endocrinology 1988;122:1769–1772. [DOI] [PubMed] [Google Scholar]
- 127. Clark S, Simpson K, Knox G, Garite T. Oxytocin: New perspectives on an old drug. Am J Obstet Gynecol 2009;200:35.e1–6. [DOI] [PubMed] [Google Scholar]
- 128. Voutsos L. Oxytocin: Less opinion, more studies. Am J Obstet Gynecol 2009;201:e9. [DOI] [PubMed] [Google Scholar]
- 129. Wahl R. Could oxytocin administration during labor contribute to autism and related behavioral disorders?—A look at the literature. Med Hypotheses 2004;63:456–460. [DOI] [PubMed] [Google Scholar]
- 130. Akerlund M, Carlsson A, Melin P, Trojnar J. The effect on the human uterus of two newly developed competitive inhibitors of oxytocin and vasopressin. Acta Obstet Gynecol Scand 1985;64:499–504. [DOI] [PubMed] [Google Scholar]
- 131. Akerlund M. Targeting the oxytocin receptor to relax the myometrium. Expert Opin Ther Targets 2006;10:423–427. [DOI] [PubMed] [Google Scholar]
- 132. Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev 2005;3(CD004452):1–52. [DOI] [PubMed] [Google Scholar]
- 133. Lyell D, Pullen K, Mannan J, et al Maintenance nifedipine tocolysis compared with placebo: A randomized controlled trial. Obstet Gynecol 2008;112:1221–1226. [DOI] [PubMed] [Google Scholar]
- 134. De Heus R, Mulder E, Derks J, Visser G. The effects of the tocolytics atosiban and nifedipine on fetal movements, heart rate and blood flow. J Matern Fetal Neonatal Med 2009;22:485–490. [DOI] [PubMed] [Google Scholar]
- 135. Succu S, Sanna F, Cocco C, et al Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: Role of nitric oxide and cyclic GMP. Eur J Neurosci 2008;28:813–821. [DOI] [PubMed] [Google Scholar]
- 136. Thackare H, Nicholson H, Whittington K. Oxytocin—its role in male reproduction and new potential therapeutic uses. Hum Reprod Update 12:437–448. [DOI] [PubMed] [Google Scholar]
- 137. Gupta J, Russell R, Wayman C, Hurley D, Jackson V. Oxytocin‐induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br J Pharmacol 2008;155:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Devost D, Girotti M, Carrier M, Russo C, Zingg H. Oxytocin induces dephosphorylation of eukaryotic elongation factor 2 in human myometrial cells. Endocrinology 2005;146:2265–2270. [DOI] [PubMed] [Google Scholar]
- 139. Devost D, Carrier M, Zingg H. Oxytocin‐induced activation of eukaryotic elongation factor 2 in myometrial cells is mediated by protein kinase C. Endocrinology 2008;149:131–138. [DOI] [PubMed] [Google Scholar]
- 140. Tamma R, Colaianni G, Zhu L, et al Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci U S A 2009;106:7149–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Elabd C, Basillais A, Beaupied H, et al Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008;26:2399–2407. [DOI] [PubMed] [Google Scholar]
- 142. Cassoni P, Sapino A, Negro F, Bussolati G. Oxytocin inhibits proliferation of human breast cancer cell lines. Virchows Arch 1994;425:467–472. [DOI] [PubMed] [Google Scholar]
- 143. Cassoni P, Sapino A, Stella A, Fortunati N, Bussolati G. Presence and significance of oxytocin receptors in human neuroblastomas and glial tumors. Int J Cancer 1998;77:695–700. [DOI] [PubMed] [Google Scholar]
- 144. Cassoni P, Fulcheri E, Carcangiu M, Stella A, Deaglio S, Bussolati G. Oxytocin receptors in human adenocarcinomas of the endometrium: Presence and biological significance. J Pathol 2000;190:470–477. [DOI] [PubMed] [Google Scholar]
- 145. Novak J, Judkins M, Chernin M, et al A plasmin‐derived hexapeptide from the carboxyl end of osteocalcin counteracts oxytocin‐mediated growth inhibition [corrected] of osteosarcoma cells. Cancer Res 2000;60:3470–3476. [PubMed] [Google Scholar]
- 146. Cassoni P, Sapino A, Papotti M, Bussolati G. Oxytocin and oxytocin‐analogue F314 inhibit cell proliferation and tumor growth of rat and mouse mammary carcinomas. Int J Cancer 1996;66:817–820. [DOI] [PubMed] [Google Scholar]
- 147. Cassoni P, Sapino A, Munaron L, et al Activation of functional oxytocin receptors stimulates cell proliferation in human trophoblast and choriocarcinoma cell lines. Endocrinology 2001;142:1130–1136. [DOI] [PubMed] [Google Scholar]
- 148. Cassoni P, Sapino A, Deaglio S, et al Oxytocin is a growth factor for Kaposi's sarcoma cells: Evidence of endocrine‐immunological cross‐talk. Cancer Res 2002;62:2406–2413. [PubMed] [Google Scholar]
- 149. Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA‐MB231 human breast‐cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Cancer 1997;72:340–344. [DOI] [PubMed] [Google Scholar]
- 150. Fay M, Du J, Longo K, North W. Oxytocin does not induce a rise in intracellular free calcium in human breast cancer cells. Res Commun Mol Pathol Pharmacol 1999;103:115–128. [PubMed] [Google Scholar]
- 151. Guzzi F, Zanchetta D, Cassoni P, et al Localization of the human oxytocin receptor in caveolin‐1 enriched domains turns the receptor‐mediated inhibition of cell growth into a proliferative response. Oncogene 2002;21:1658–1667. [DOI] [PubMed] [Google Scholar]
- 152. Jankowski M, Hajjar F, Kawas S, et al Rat heart: A site of oxytocin production and action. Proc Natl Acad Sci U S A 1998;95:14558–14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wsol A, Cudnoch‐Jedrzejewska A, Szczepanska‐Sadowska E, Kowalewski S, Puchalska L. Oxytocin in the cardiovascular responses to stress. J Physiol Pharmacol 2008;59(Suppl 8):123–127. [PubMed] [Google Scholar]
- 154. Petersson M, Uvnäs‐Moberg K. Postnatal oxytocin treatment of spontaneously hypertensive male rats decreases blood pressure and body weight in adulthood. Neurosci Lett 2008;440:166–169. [DOI] [PubMed] [Google Scholar]
- 155. Petersson M, Lundeberg T, Uvnäs‐Moberg K. Oxytocin decreases blood pressure in male but not in female spontaneously hypertensive rats. J Auton Nerv Syst 1997;66:15–18. [DOI] [PubMed] [Google Scholar]
- 156. Björkstrand E, Eriksson M, Uvnäs‐Moberg K. Evidence of a peripheral and a central effect of oxytocin on pancreatic hormone release in rats. Neuroendocrinology 1996;63:377–383. [DOI] [PubMed] [Google Scholar]
- 157. Gutkowska J, Broderick T, Bogdan D, Wang D, Lavoie J, Jankowski M. Downregulation of oxytocin and natriuretic peptides in diabetes: Possible implications in cardiomyopathy. J Physiol 2009;587(Pt 19):4725–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rimoldi V, Reversi A, Taverna E, et al Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin‐1 enriched domains. Oncogene 2003;22:6054–6060. [DOI] [PubMed] [Google Scholar]
- 159. Terrillon S, Cheng L, Stoev S, et al Synthesis and characterization of fluorescent antagonists and agonists for human oxytocin and vasopressin V(1)(a) receptors. J Med Chem 2002;45:2579–2588. [DOI] [PubMed] [Google Scholar]
- 160. Albizu L, Teppaz G, Seyer R, et al Toward efficient drug screening by homogeneous assays based on the development of new fluorescent vasopressin and oxytocin receptor ligands. J Med Chem 2007;50:4976–4985. [DOI] [PubMed] [Google Scholar]
- 161. Devost D, Zingg H. Homo‐ and hetero‐dimeric complex formations of the human oxytocin receptor. J Neuroendocrinol 2004;16:372–377. [DOI] [PubMed] [Google Scholar]


