Abstract
Objective
HIV+ elite controllers are a unique group of rare individuals who maintain undetectable viral loads in the absence of antiretroviral therapy. We studied immune responses in these subjects to inform vaccine development, with the goal of identifying the immune correlates of protection from HIV.
Methods
We compared markers of cellular activation, HIV-specific immune responses, and regulatory T (Treg) cell frequencies in 4 groups of subjects: HIV-negative healthy controls, elite controllers (HIV RNA level <75 copies/ml), individuals on highly active antiretroviral therapy (HAART), and subjects with HIV RNA level >10,000 copies/ml (non-controllers).
Results
Elite controllers possessed significantly lower levels of activated HIV-specific CD8+ T cells and of recently divided HIV-specific CD4+ T cells than non-controllers, while these differences were not seen in the respective CMV-specific T cell populations. Elite controllers also mounted a stronger and broader cytokine and chemokine response following HIV-specific stimulation than individuals on HAART and non-controllers. Finally, we found that HAART suppressed subjects had elevated Treg cell frequencies, while elite controllers and non-controllers maintained normal percentages of Treg cells.
Conclusion
Elite controllers maintain high levels of HIV-specific immune responses with low levels of HIV-specific T cell activation, and do not have elevated Treg cell levels. Based on these data an ideal HIV vaccine would induce strong HIV-specific immune responses while minimizing HIV-specific T cell activation.
Keywords: Cellular immunity, activation, regulatory T cells, pathogenesis, HIV
Introduction
HIV infection is characterized by chronic immune activation, uncontrolled viral replication and a loss of CD4+ T cells, resulting in progression to AIDS. A population of rare individuals (approximately 1% of the HIV infected population) has been shown to maintain clinically undetectable plasma viral loads (<75 copies/ml) in the absence of therapy (“elite controllers”) [1, 2]. Elite controllers have decreased T cell activation [3, 4] and stronger HIV-specific CD4+ T cell IFNγ and IL-2 responses targeted towards the Gag protein than individuals who do not control HIV replication [5].
It is well documented that there is an increase in the level of CD4+ and CD8+ T cells expressing “activation” markers such as CD38, HLA-DR and/or Ki67 expression in HIV infection [3, 6–9]. For reasons that have not yet been fully defined, a higher frequency of these cells is associated with more rapid disease progression, independent of viral load and CD4+ T cell counts [3, 10–12]. Recent work by our group found that despite having undetectable viral loads, elite controllers had higher levels of immune activation than HIV seronegatives and HAART suppressed individuals [2, 3]. The mechanism accounting for this higher than expected level of immune activation is not known, but may be due to higher levels of HIV production, higher levels of microbial translocation, and/or lower levels of immunoregulatory cells.
Since immune activation appears to play an adverse role in HIV infection, cells with the ability to decrease inflammation may be beneficial. Regulatory T (Treg) cells are a unique population of CD4+ T cells that have the ability to suppress the activation and proliferation of T cells [13–16]. This population of CD4+ T cells has traditionally been identified by the expression of CD25 and the transcription factor FoxP3, and more recently in combination with other markers including CD127 and CD152 [13, 17–23]. Studies of the role of Treg cells in HIV infection show conflicting results. Some studies have shown an increase in the number of Treg cells in HIV infection [24–26], while others have shown a decrease [27–32]. Higher levels of Treg function could prevent some of the harmful effects of immune activation on disease progression, while lower levels could allow a strong and durable antigen-specific T cell response.
Finally, cytokine and chemokine profiles may differ between elite controllers and non-controllers. However, there is considerable variability in the levels of cytokines produced between infected individuals [33–38]. Studies of the earliest phase of HIV infection in plasma donors acquiring HIV revealed a striking pro-inflammatory cytokine cascade beginning within days of the first appearance of viremia [39, 40]. While there have been numerous studies of cytokine profiles in HIV infection, broad analysis of the cytokine responses in elite controllers has not been reported.
Despite strong and growing interest in the elite controllers as a model for understanding the optimal HIV-specific host response, no study to our knowledge has attempted to fully characterize the nature of the T cell response in these individuals. In our current study we assessed the activation and proliferation status of antigen-specific CD4+ and CD8+ T cells, the cytokine and chemokine response to HIV and CMV stimulation, and the number of Treg cells in four groups of subjects. Our sample cohort included HIV seronegative individuals and three classes of HIV infected individuals: elite controllers, HAART suppressed individuals and non-controllers. One sample from each individual was assessed for all parameters at the same time point, with the goal of identifying immunological factors that drive the maintenance of robust T cell responses and control of viral replication in elite controllers in the absence of therapy.
Methods
Study subjects
Blood samples were obtained from HIV infected individuals enrolled in the University of California, San Francisco (UCSF) SCOPE cohort and from 20 healthy HIV seronegative individuals. All subjects underwent informed consent under protocols approved by the UCSF Committee on Human Research. HIV infected subjects were divided into three groups: (1) Elite controllers: at least two viral loads <75 copies/ml spanning ≥6 months (median of 10 undetectable viral loads/subject). A viral load >1000 copies was recorded in 3 subjects over 4.5 years prior to study sample acquisition. Three controllers had ART exposure 7, 10, and 14 years prior to study. (2) HAART suppressed: antiretroviral treated with at least two viral loads <75 copies/ml spanning ≥12 months. (3) Non-controllers: untreated with an index viral load >10,000 copies/ml (Table 1). Since advanced immunodeficiency can cause T cell dysfunction, we excluded from our analysis any subject with a CD4+ T cell count <300 cells/µl.
Table 1.
Subject Characteristics.
| Age a (years) |
Female No. (%) |
CD4 count a (cells/µl) |
Viral load a (copies/ml) |
|
|---|---|---|---|---|
|
HIV seronegative n = 20 |
43 (31–64) | 8 (40) | - | - |
|
Elite controller n = 18 |
48 (34–64) | 7 (39) | 764 (423–1546) | <75 |
|
HAART suppressed n = 20 |
53 (35–80) | 2 (10) | 725 (401–1276) | <75 |
|
Non-controller n = 20 |
44 (25–44) | 1 (5) | 588 (308–1108) | 23,210 (10,430–130,775) |
Median and (range) displayed
Stimulation of PBMC
Cryopreserved PBMC were rapidly thawed into RPMI supplemented with 10% heat inactivated human AB serum (Sigma-Aldrich), 10 mM Hepes and 50 IU/ml penicillin/streptomycin (UCSF Cell Culture Facility). One million cells/well were stimulated in 96 well “U” bottom plates (Falcon, BD Labware) for one hour at 37°C, 5% CO2, with either 5µg/ml of an HIV-1 p55 or CMV pp65 peptide pool (123 and 138 peptides, respectively, 15 amino acids long with 11 amino acid overlap, NIH AIDS Research & Reference Reagent Program), 200ng/ml of staphylococcal enterotoxin B (SEB; Sigma-Aldrich) as a positive control, or were left unstimulated as a negative control. Brefeldin A (Sigma-Aldrich) and GolgiStop (BD Biosciences) were added together at final concentrations of 10µg/ml and 8µM, respectively, prior to overnight incubation at 37°C, 5% CO2. Duplicate cultures without inhibitors were set up when cell numbers allowed for analysis of cytokines/chemokines. Supernatants were harvested between 18–24 hours and frozen for batch analysis.
Flow cytometric analysis
Unless otherwise noted, all reagents were obtained from BD Pharmingen or BD Biosciences. Activation was assessed with the following panel: CD3-Pacific Blue, CD4-Alexa-fluor 700, CD8-APC-H7, CD45RA-PE-Cy5.5 (Caltag Laboratories), CD27-APC, HLA-DR-PE-Cy5, CD38-PE, IFNγ-PE-Cy7, Ki67-FITC and aqua amine-reactive dye (Invitrogen-Molecular Probes). The Treg panel consisted of: CD3-Pacific Blue, CD4-Alexa-fluor 700, CD25-PE-Cy7, CD127-PE, CD152-APC, FoxP3-Alexa-fluor 488 and aqua amine-reactive dye. Gating on CD4+CD25+CD127−CD152+ T cells has been shown to define Treg cells [18, 19, 23, 41]. Our results were consistent with these prior studies showing that this population was also FoxP3+ (data not shown). Activation panel intracellular staining was accomplished using Cytofix and Cytoperm reagents (BD Biosciences) and Treg intracellular staining was performed using a Foxp3 staining buffer set (eBioscience) according to the respective manufacturer’s instructions. Compensation controls included CompBeads or cells stained with equivalent quantities of test antibody. Fluorescence minus one (FMO) and isotype stained controls were used to set gating. Gates for IFNγ were set using unstimulated negative control samples.
The Treg panel was acquired on an LSRII flow cytometer and the activation panel on a FACS Aria. Instrument set-up was standardized to reduce batch-to-batch variation. Pre-optimized target channel voltages were set using mid-range FL1 rainbow fluorescent particles (BD Biosciences). Single stained compensation tubes were checked to ensure each stain was the brightest in its own channel. A median of 150,000 viable CD3+ events was collected for each panel. Data was analyzed using FlowJo 8.7.3 software (TreeStar).
Multiplex cytokine and chemokine analysis
Supernatants were assayed using the high-sensitivity LincoPlex kit (Millipore) for IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 p70, IL-13, IFNγ, granulocyte-macrophage colony stimulating factor (GM-CSF) and tumor necrosis factor-α (TNFα), and the standard-sensitivity Milliplex Map kit (Millipore) for IFNα2, IL-15, IL-17, inducible protein -10 (IP-10), monocyte chemotactic protein-1 (MCP-1), MCP-3, macrophage-derived chemokine (MDC), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, soluble CD40 ligand (sCD40L) and TNFβ following the manufacturer’s protocols. Samples were acquired on a Labscan 100 analyzer (Luminex) using Bio-Plex manager 4.1 software (Bio-Rad).
Statistical analysis
Non-parametric tests were used because the data were not normally distributed. The Kruskal-Wallis test followed by Dunn’s test for multiple comparisons was used to assess differences in the distribution of values for HIV seronegative individuals and infected groups. Spearman’s rank test was used to determine the correlation between Treg number and age using Prism 5 software (Graphpad). To examine the association between HAART therapy and the frequency of Treg cells, multivariate linear regression models were generated that included therapy (HAART therapy vs. untreated), age, and CD4+ T cell count as independent variables using Stata 10.1 SE software (StataCorp LP). Heat maps of the cytokine data were generated using Aabel 3 software (Gigawiz). Results were considered statistically significant if p<0.05.
Results
Cohort characteristics
Subjects from four groups were studied, HIV negatives, HIV controllers, HAART suppressed and HIV non-controllers, including 18 HIV controllers and 20 of each of the other groups. Most subjects were men, and the median age for the respective groups was 43, 48, 53 and 44 years (Table 1). The median CD4+ T cell counts for the controllers, HAART-suppressed and non-controllers were 764, 725 and 588 cells/µl (CD4+ T cell counts were not available for the HIV seronegatives).
Elite controllers have the lowest levels of HIV-specific T cell activation
For unclear reasons, at least a subset of elite controllers is able to maintain vigorous HIV-specific T cell responses for extended periods, with some patients now known to be able to maintain control for up to 30 years. It is known that elite controllers have high frequencies of HIV-specific CD4+ and CD8+ T cells and intermediate levels of general T cell activation when compared to HIV uninfected or HAART suppressed subjects (low activation) and non-controllers (high activation) [3, 5], and we confirmed those findings in the current study (data not shown). However, we hypothesized that HIV-specific cells might be relatively less activated in elite controllers compared to non-controller subjects. We defined activated HIV-specific cells as those co-expressing CD38 and HLA-DR (gated on IFNγ+ cells following HIV p55 stimulation, Fig. 1a). We found significantly lower levels of activated CD8+ but not CD4+ HIV-specific T cells among elite controllers and HAART suppressed individuals compared to non-controllers (Fig. 1b,c). Elite controllers also showed the lowest levels of recently divided HIV-specific CD4+ T cells (expressing Ki67), and their numbers were significantly lower than non-controllers and HAART suppressed subjects (Fig. 1d). There was no significant difference in the turnover of HIV-specific CD8+ T cells among the HIV+ groups (Fig. 1e).
Figure 1. Elite controllers have lower levels of activated and recently divided HIV-specific T cells than non-controllers.
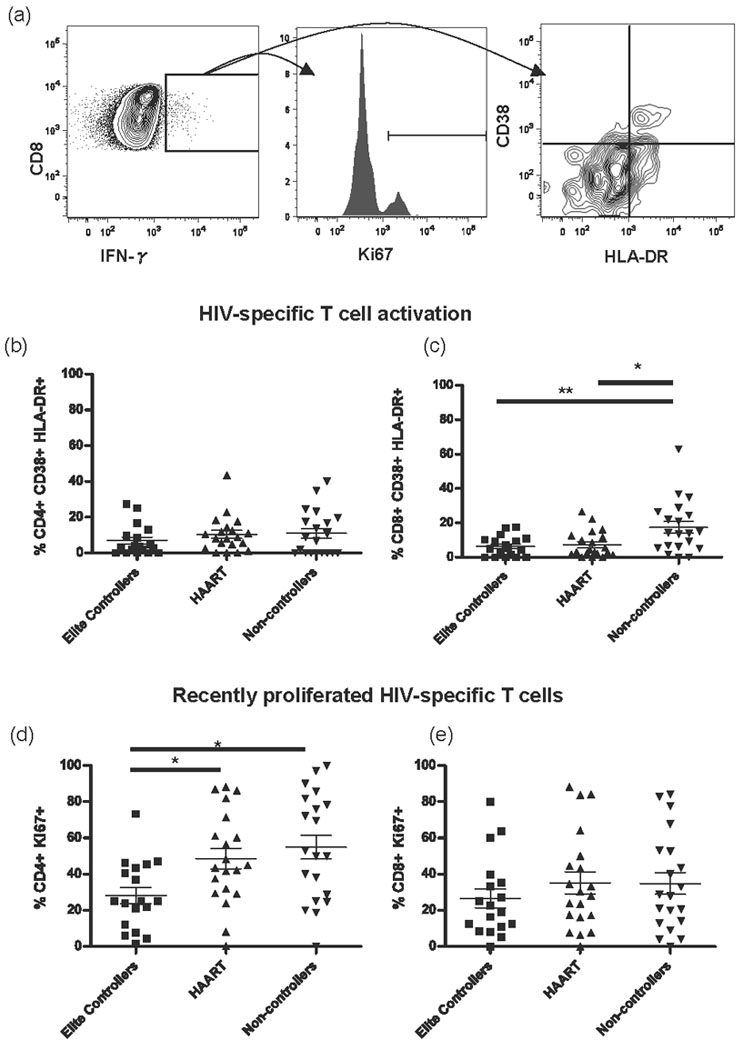
(a) Representative flow cytometry plots of HIV-specific CD8+ T cells illustrate the gating strategy, showing HIV-specific cells (left panel), recently divided cells in a smoothed histogram of Ki67 expression (middle panel), and activation status by CD38 and HLA-DR co-expression (right panel). (b) The percentage of activated CD4+ IFNγ+ T cells and (c) CD8+ IFNγ+ T cells co-expressing CD38 and HLA-DR following HIV p55 stimulation. (d) The percentage of recently divided CD4+ IFNγ+ Ki67+ T cells and (e) CD8+ IFNγ+ Ki67+ T cells following p55 stimulation. * p<0.05, ** p<0.01.
The lower levels of HIV-specific CD8+ T cell activation in elite controllers could have been due to decreased antigen exposure in subjects with lower viral load, as recently suggested [42–44]. To test this we measured activation of CMV-specific T cells and found no significant differences compared to non-controllers (Fig. 2a,b). These results imply that the increased activation seen in HIV-specific CD8+ T cells from non-controllers is due to greater antigenic stimulation compared to elite controllers. It has recently been suggested that CD4+ T cell proliferation (measured by BrdU staining) is determined by both CD4+ T cell depletion and HIV viral burden [43]. One would expect that response to CD4+ T cell depletion would occur equally in HIV-and CMV-specific CD4+ T cells, while HIV viral burden would induce more proliferation in HIV-specific CD4+ T cells in an antigen-specific fashion or equally affect HIV- and CMV-specific T cells through direct mechanisms such as infection of activated cells or induction of bystander apoptosis. Consistent with viral burden rather than CD4+ T cell depletion driving proliferation, frequencies of recently divided CMV-specific T cells were lower compared to HIV-specific T cells among non-controllers (p<0.001 and p<0.05 for CD4+ and CD8+ T cells, respectively). Additionally, the CMV-specific T cells showed no increase in recently divided cells in non-controllers compared to elite controllers (Fig. 2c,d), illustrating no global increase in proliferation of antigen-specific cells in non-controllers compared to elite controllers.
Figure 2. Elite controllers do not have lower levels of activated and recently divided CMV-specific T cells than non-controllers.
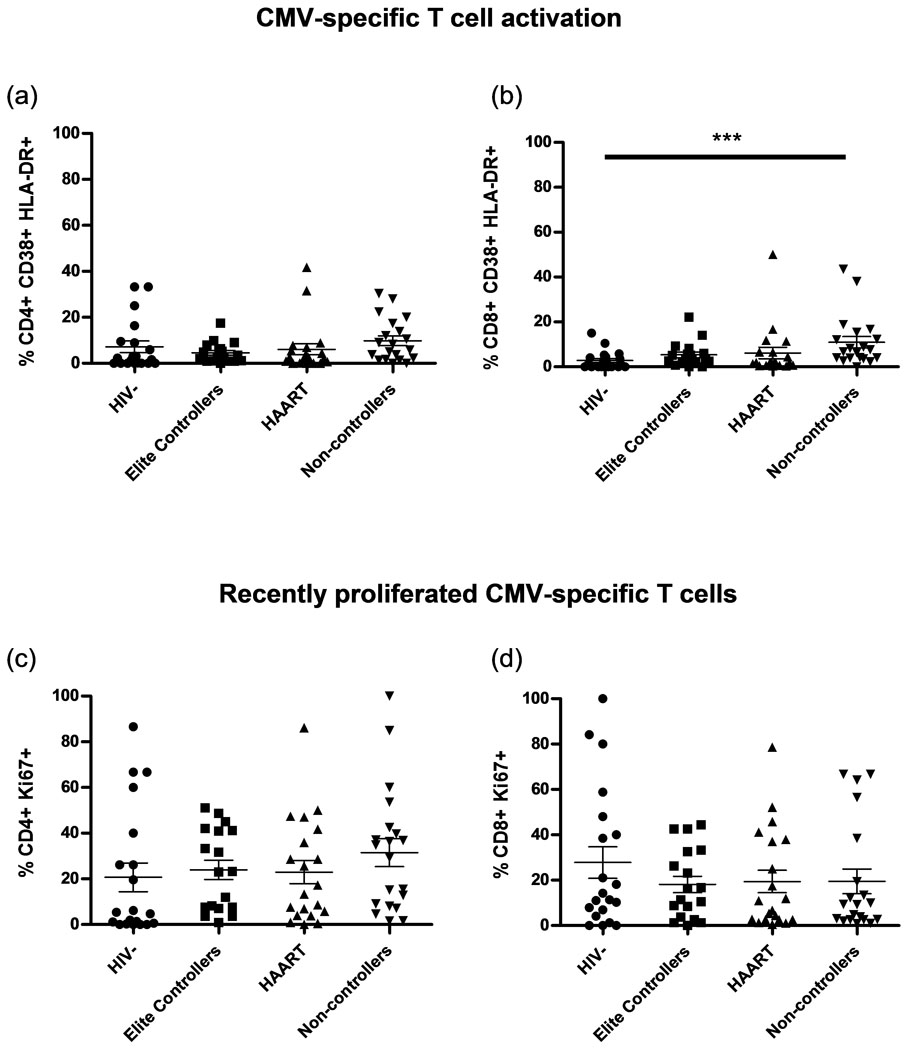
(a) The percentage of activated CD4+ IFNγ+ T cells and (b) CD8+ IFNγ+ T cells co-expressing CD38 and HLA-DR following pp65 stimulation. (c) The percentage of recently divided CD4+ IFNγ+ Ki67+ T cells and (d) CD8+ IFNγ+ Ki67+ T cells following pp65 stimulation. *** p<0.001.
Elite controllers mount broad and vigorous HIV-specific cytokine responses
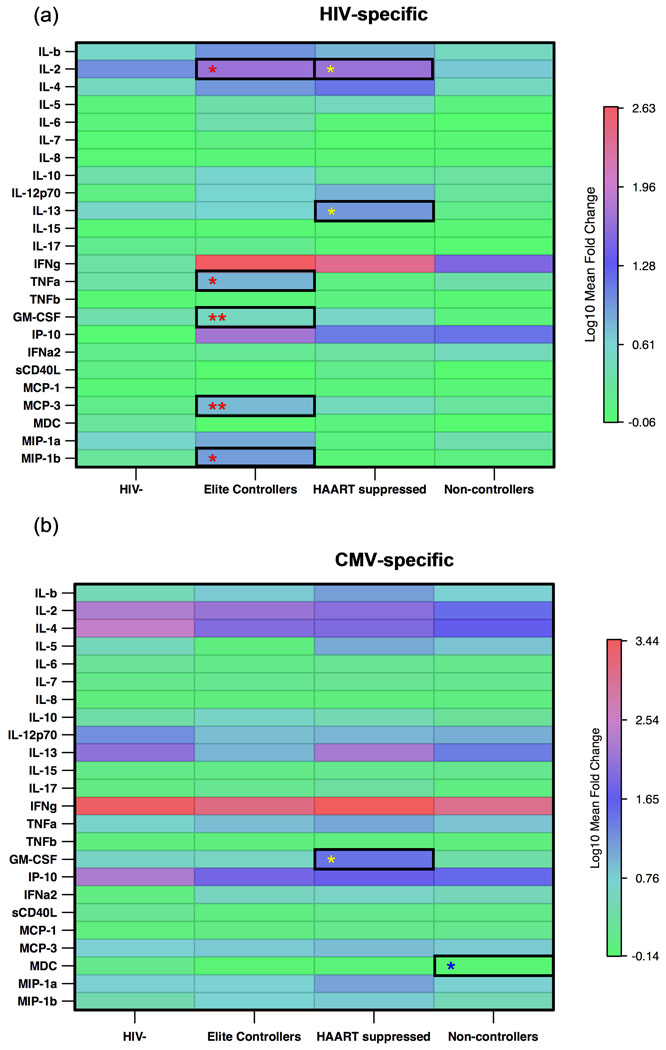
Elite control of HIV infection has been shown to correlate with the presences of cells able to secrete IFNγ, IL-2, TNFα, and MIP-1β. We aimed to extend these observations by focusing on other factors that might be preferentially expressed in T cells from elite controllers. Stimulation with p55 induced pro-inflammatory cytokines and chemokines, including IL-2, IFNγ, TNFα, IP-10, GM-CSF, MCP-3 and MIP-1β. PBMC from elite controllers produced significantly more IFNγ, GM-CSF, IP-10, MCP-3, TNFα and IL-2 than the HIV seronegative individuals after p55 stimulation (Fig. 3a). In contrast, the HAART suppressed group only produced significantly more IFNγ and IP-10 upon p55 stimulation compared to the HIV seronegative individuals. The HAART suppressed group also produced more IL-2 and IL-13 than the non-controllers (Fig. 3a). The non-controllers produced the lowest levels of HIV-induced cytokines, only secreting IFNγ and IP-10 (Fig. 3a). The non-controllers had the lowest mean-fold increase in IFNγ compared to elite controllers and HAART suppressed individuals (mean-fold change of 31.69 vs. 424.23 for elite controllers and 246.50 for HAART suppressed). The non-controllers also secreted less TNFα, GM-CSF and MCP-3 than the elite controllers (Fig. 3a). Collectively, these data demonstrate the elite controllers can produce a wide breadth of cytokines and chemokines after HIV stimulation, including antiviral factors such as MIP-1β.
Figure 3. Elite controllers mount a broader and stronger cytokine and chemokine response following HIV-specific stimulation than non-controllers.
Heat map showing the log10 mean fold-change in cytokines and chemokines following (a) HIV p55 stimulation and (b) CMV pp65 stimulation. Progressive increases in log10 mean fold-change are represented by blue to red colors. Significant p values are indicated by asterisks (* p< 0.05, ** p< 0.001), with a red * representing significant differences between the elite controllers and the non-controllers, a yellow * representing significant differences between the HAART suppressed group and the non-controllers, a blue * representing significant differences between non-controllers and HIV uninfected subjects, and a black * representing significant differences between elite controllers and the HAART suppressed group.
Stimulation of PBMC with the CMV pp65 peptide pool induced cytokines and chemokines to the same degree in all groups, with the exception of non-controllers who produced significantly less MDC than HIV seronegative individuals and less GM-CSF than HAART suppressed individuals (Fig. 3b). Therefore the alterations in the cytokine and chemokine environment were HIV-specific and not a result of general immune activation resulting from a chronic viral infection.
Elite controllers have no elevation in the number of Treg cells
The decreased levels of activation and proliferation seen in HIV-specific T cells from elite controllers may have been due to higher magnitude Treg cell levels in the elite controllers. We defined Treg cells as CD4+CD25+CD127−CD152+ (Fig. 4a). The frequency and absolute number of these cells in the elite controllers was comparable to that in HIV seronegatives and non-controllers, while the HAART suppressed patients showed an elevated percentage of Treg cells compared to HIV seronegatives and elevated absolute Treg cell numbers compared to non-controllers (Fig. 4b,c). We found no significant correlation between subject age and absolute Treg cell count among HIV+ samples (Fig. 4d). However, when all samples (both HIV+ and HIV−) were analyzed, a positive correlation between the frequency of Treg cells and age was found (data not shown, p<0.05). The HAART suppressed group, which had the highest frequency and absolute number of Treg cells, also had the oldest median age of 53 years (range 35 to 80 years), significantly older than the median age of the HIV seronegative individuals and the HIV+ non-controllers (Table 1, p<0.05 for both comparisons). Multivariable linear regression analysis showed that after adjusting for age and CD4 count, HAART was significantly associated with a 0.60% increase in the frequency of Treg cells on average (p=0.03). Age (p=0.63) and CD4 count (p=0.60) were not significantly associated with an increase in the frequency of Treg cells.
Figure 4. Elite controllers maintain normal numbers of Treg cells.
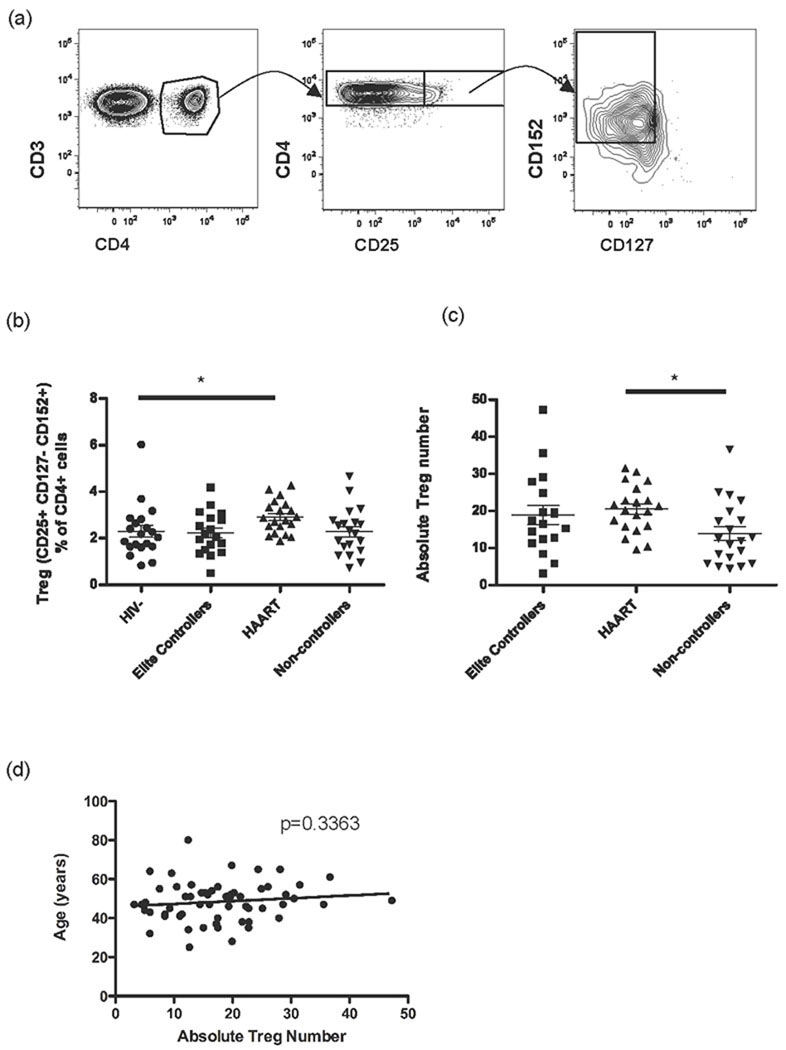
(a) A representative flow cytometry plot of Treg cells is shown to illustrate the gating strategy showing live PBMCs (left panel) gated on CD3+ CD4+ cells (middle panel) and finally on CD25+ cells (right panel). (b) The frequency of Treg cells (CD25+ CD127− CD152+) as a percentage of viable CD3+ CD4+ cells. (c) The absolute number of Treg cells in HIV infected individuals. (d) Correlation between the absolute number of Treg cells and age for all HIV infected individuals. * p<0.05.
Discussion
We found that elite controllers maintained lower levels of activated HIV-specific CD8+ and recently divided HIV-specific CD4+ T cells compared to non-controllers, and this was not explained by increased proportions of Treg cells in elite controllers. Decreased antigen-specific T cell activation was limited to the HIV-specific subsets of cells and was not found in the respective CMV-specific populations. Additionally, the elite controllers possessed the strongest and broadest HIV-specific immune responses, with seven cytokines and chemokines induced by HIV stimulation (IL-2, IFNγ, TNFα, GM-CSF, IP-10, MCP-3 and MIP-1β). In summary, elite controllers maintained the strongest HIV-specific immune responses, with markers of inflammation on HIV-specific cells significantly lower than those in subjects unable to control viral replication.
Elite controllers had the ability to mount a strong and broad antiviral cytokine response when stimulated with a p55 peptide pool, producing IL-2, IFNγ, TNFα, IP-10, GM-CSF, MIP-1β and MCP-3. Whilst the non-controllers also induced some of the same cytokines as elite controllers (IL-2, IFNγ, TNFα, GM-CSF, IP-10 and MCP-3), they produced significantly less of each than elite controllers. The lower quantities of antiviral cytokines may reflect weak antiviral T cell responses and be associated with exhaustion of adaptive immune responses. Antiretroviral therapy was clearly associated with a reduced ability to secrete a broad cytokine and chemokine response, with these individuals only secreting significant amounts of IL-2, IFNγ and IP-10. Interestingly, HAART suppression was associated with the production of IL-13, a cytokine involved in B cell growth and differentiation that can inhibit macrophage inflammatory cytokine production. The reduction of inflammatory cytokines could also be responsible for the reduction in T cell activation and proliferation seen during HAART treatment. Our multiplex cytokine testing data support previous reports of viral control being associated with polyfunctional T cell responses [42, 45, 46], and the cytokines identified above further clarify which specific responses are associated with control of viral replication during chronic HIV infection.
The increase in the number of Treg cells following HAART that we observed is consistent with some previous studies. Weiss et al. found an expanded number of Treg cells in HIV+ individuals receiving HAART, with a Treg cell phenotype similar to that of normal donors and cancer patients [26]. Lim et al. also observed an increase in the number of Treg cells identified by an increase in FoxP3 mRNA expression in individuals who suppressed viremia with HAART [47]. Kolte et al. found that both absolute Treg cell numbers and the percentage of Treg cells were increased after one and five years of receiving HAART and were associated with an increase in the thymic output of naïve Treg cells [48]. Two other studies showed no effect of HAART on Treg cell numbers despite suppression of viral replication and immunological recovery [49, 50]. The precise mechanism of Treg cell expansion during HAART remains unknown and requires further investigation. An increase in the peripheral Treg cell pool by proliferation, increased survival of Treg cells or an increase in the thymic generation of Treg cells all could be responsible [41, 51–53]. As we saw no correlation between the number of Treg cells and HIV-specific or CMV-specific T cell responses (data not shown), it would appear that Treg cells do not strongly interfere with HIV-specific immune responses, raising the possibility of inducing these cells to ameliorate the effects of immune activation in the setting of high viral loads during chronic HIV infection.
Whilst our data mostly agree with those of Chase et al. [54], we did see a difference in which HIV infected group had the highest number of Treg cells. Elite controllers in the Chase et al. study had the highest number of Treg cells, whereas we saw the highest number of Treg cells in our HAART suppressed group. One possible explanation for this is confounding by age, since older individuals have higher Treg cell numbers [55–57]. In both our study and the Chase study the groups with the highest number of Treg cells were also the oldest. In the Chase study elite controllers were the oldest (median age = 54 years), while their HAART suppressed group was the youngest (median age = 46 years). In contrast, our HAART suppressed group was the oldest (median age = 53 years) and the elite controllers were younger (median age = 48 years). Multivariate analysis of our data, which controlled for confounding by age, showed that the increase in Treg cells was due to the therapy and not age. Whether this would be the case in the Chase et al. study was not addressed [54].
In conclusion, lower levels of HIV-specific T cell activation and in vivo proliferation combined with stronger, broader HIV-specific cytokine responses likely play a role in the control of HIV infection by elite controllers. However, elite controllers do not completely clear the virus [2] and may eventually lose their elite status and progress towards the development of AIDS [1, 3]. A therapeutic vaccine or immune modulation that could reduce immune activation, potentially by the induction of Treg cells, and generate a more appropriate balance of immune responses (such as those seen in elite controllers) may allow non-controllers to decrease HIV replication and delay the progression to AIDS.
Acknowledgements
This work was supported in part by grants from the NIAID Center for HIV/AIDS Vaccine Immunology (CHAVI) AI-067854, the UCSF/Gladstone Center for AIDS Research (P30 AI27763, NIAID (AI069994, AI44595), the UCSF Clinical and Translational Science Institute (UL1 RR024131-01) and American Foundation for AIDS Research (106710-40-RGRL). The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NAID, NIH: HIV-1 consensus B Gag peptides (#8117), HCMV pp65 peptide pool (#11549). The UCSF AIDS Specimen Bank provided cryopreserved PBMC from the SCOPE cohort. We thank P.W. Hunt and J.M. McCune for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
R.E.O. planned the studies, performed experiments, analyzed data, and wrote the manuscript. J.W.H. and D.F.H. performed experiments, M.C.L. planned the studies, H.H.B. performed statistical analyses, J.N.M. provided clinical samples, M.R.K. maintained the database, S.G.D. planned the studies and provided clinical samples, and P.J.N. planned the studies and wrote the manuscript.
References
- 1.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Hatano H, Delwart EL, Norris PJ, Lee TH, Dunn-Williams J, Hunt PW, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R, et al. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol. 2005;79:14169–14178. doi: 10.1128/JVI.79.22.14169-14178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida M, Cordero M, Almeida J, Orfao A. Relationship between CD38 expression on peripheral blood T-cells and monocytes, and response to antiretroviral therapy: a one-year longitudinal study of a cohort of chronically infected ART-naive HIV-1+ patients. Cytometry B Clin Cytom. 2007;72:22–33. doi: 10.1002/cyto.b.20144. [DOI] [PubMed] [Google Scholar]
- 7.Benito JM, Lopez M, Lozano S, Ballesteros C, Martinez P, Gonzalez-Lahoz J, Soriano V. Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4+ T cells under successful highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;38:373–381. doi: 10.1097/01.qai.0000153105.42455.c2. [DOI] [PubMed] [Google Scholar]
- 8.Kestens L, Vanham G, Vereecken C, Vandenbruaene M, Vercauteren G, Colebunders RL, Gigase PL. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–441. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Eggena MP, Barugahare B, Okello M, Mutyala S, Jones N, Ma Y, et al. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006;120:163–170. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 12.Kolber MA. CD38+CD8+ T-cells negatively correlate with CD4 central memory cells in virally suppressed HIV-1-infected individuals. Aids. 2008;22:1937–1941. doi: 10.1097/QAD.0b013e32830f97e2. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 14.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 16.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 18.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. FOXP3 expressing CD127lo CD4+ T cells inversely correlate with CD38+ CD8+ T cell activation levels in primary HIV-1 infection. J Leukoc Biol. 2008;83:254–262. doi: 10.1189/jlb.0507281. [DOI] [PubMed] [Google Scholar]
- 21.O'Garra A, Vieira P. Twenty-first century Foxp3. Nat Immunol. 2003;4:304–306. doi: 10.1038/ni0403-304. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 23.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, et al. HIV-1- driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenorio AR, Martinson J, Pollard D, Baum L, Landay A. The relationship of T-regulatory cell subsets to disease stage, immune activation, and pathogenspecific immunity in HIV infection. J Acquir Immune Defic Syndr. 2008;48:577–580. doi: 10.1097/QAI.0b013e31817bbea5. [DOI] [PubMed] [Google Scholar]
- 26.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 27.Baker CA, Clark R, Ventura F, Jones NG, Guzman D, Bangsberg DR, Cao H. Peripheral CD4 loss of regulatory T cells is associated with persistent viraemia in chronic HIV infection. Clin Exp Immunol. 2007;147:533–539. doi: 10.1111/j.1365-2249.2006.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chase AJ, Sedaghat AR, German JR, Gama L, Zink MC, Clements JE, Siliciano RF. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol. 2007;81:12748–12757. doi: 10.1128/JVI.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Q, Zhang L, Wang R, Jeffrey J, Washburn ML, Brouwer D, et al. FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2−/−gammaC−/− mice in vivo. Blood. 2008;112:2858–2868. doi: 10.1182/blood-2008-03-145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao Y, Fu J, Xing S, Fu B, Zhang Z, Shi M, et al. The decrease of regulatory T cells correlates with excessive activation and apoptosis of CD8+ T cells in HIV-1-infected typical progressors, but not in long-term non-progressors. Immunology. 2009;128:e366–e375. doi: 10.1111/j.1365-2567.2008.02978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozos A, Garrido M, Carreras J, Plana M, Diaz A, Alos L, et al. Redistribution of FOXP3-positive regulatory T cells from lymphoid tissues to peripheral blood in HIV-infected patients. J Acquir Immune Defic Syndr. 2007;46:529–537. [PubMed] [Google Scholar]
- 33.Barcellini W, Rizzardi GP, Borghi MO, Fain C, Lazzarin A, Meroni PL. TH1 and TH2 cytokine production by peripheral blood mononuclear cells from HIV-infected patients. Aids. 1994;8:757–762. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Fan J, Bass HZ, Fahey JL. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993;151:5031–5040. [PubMed] [Google Scholar]
- 35.Graziosi C, Pantaleo G, Gantt KR, Fortin JP, Demarest JF, Cohen OJ, et al. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 36.Klein SA, Dobmeyer JM, Dobmeyer TS, Pape M, Ottmann OG, Helm EB, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. Aids. 1997;11:1111–1118. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Miedema F, Petit AJ, Terpstra FG, Schattenkerk JK, de Wolf F, Al BJ, et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vyakarnam A, Matear P, Meager A, Kelly G, Stanley B, Weller I, Beverley P. Altered production of tumour necrosis factors alpha and beta and interferon gamma by HIV-infected individuals. Clin Exp Immunol. 1991;84:109–115. doi: 10.1111/j.1365-2249.1991.tb08132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris PJ, Pappalardo BL, Custer B, Spotts G, Hecht FM, Busch MP. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV Type 1 infection. AIDS Res Hum Retroviruses. 2006;22:757–762. doi: 10.1089/aid.2006.22.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim A, Tan D, Price P, Kamarulzaman A, Tan HY, James I, French MA. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. Aids. 2007;21:1525–1534. doi: 10.1097/QAD.0b013e32825eab8b. [DOI] [PubMed] [Google Scholar]
- 48.Kolte L, Gaardbo JC, Skogstrand K, Ryder LP, Ersboll AK, Nielsen SD. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naive Tregs. Clin Exp Immunol. 2009;155:44–52. doi: 10.1111/j.1365-2249.2008.03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaardbo JC, Nielsen SD, Vedel SJ, Ersboll AK, Harritshoj L, Ryder LP, et al. Regulatory T cells in human immunodeficiency virus-infected patients are elevated and independent of immunological and virological status, as well as initiation of highly active anti-retroviral therapy. Clin Exp Immunol. 2008;154:80–86. doi: 10.1111/j.1365-2249.2008.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim AY, Price P, Beilharz MW, French MA. Cell surface markers of regulatory T cells are not associated with increased forkhead box p3 expression in blood CD4+ T cells from HIV-infected patients responding to antiretroviral therapy. Immunol Cell Biol. 2006;84:530–536. doi: 10.1111/j.1440-1711.2006.01467.x. [DOI] [PubMed] [Google Scholar]
- 51.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–237. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- 52.Antons AK, Wang R, Oswald-Richter K, Tseng M, Arendt CW, Kalams SA, Unutmaz D. Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J Immunol. 2008;180:764–773. doi: 10.4049/jimmunol.180.2.764. [DOI] [PubMed] [Google Scholar]
- 53.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol. 2008;82:8307–8315. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kozlowska E, Biernacka M, Ciechomska M, Drela N. Age-related changes in the occurrence and characteristics of thymic CD4(+) CD25(+) T cells in mice. Immunology. 2007;122:445–453. doi: 10.1111/j.1365-2567.2007.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, et al. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol. 2007;188:117–127. doi: 10.1016/j.jneuroim.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Tenorio AR, Spritzler J, Martinson J, Gichinga CN, Pollard RB, Lederman MM, et al. The effect of aging on T-regulatory cell frequency in HIV infection. Clin Immunol. 2009;130:298–303. doi: 10.1016/j.clim.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]