Abstract
Objective
Mesenchymal stem cells (MSCs) possess potent immuno-modulatory activity but whether they evade immune surveillance in an allogeneic transplant setting remains controversial. Herein we evaluated whether administration of major histocompatibility (MHC) class I mismatched MSCs induce an immune response in rhesus macaques.
Methods
MSCs from a male donor were injected intra-cranially at two different doses into eight immuno-competent female infant rhesus macaques. Blood cell counts and circulating levels of lymphocyte subpopulations were quantified prior to surgery and at 10, 30, and 90–180 days post-surgery by flow cytometry. Immuno-reactivity of recipient PBMNCs to donor MSCs was evaluated in vitro and allo-antibody production in vivo was determined by ELISA and flow cytometry.
Results
MSC transplantation induced transient but significant increases in circulating white blood cells, lymphocytes, and neutrophils in most transplant recipients but not sham-operated control animals. Flow cytometric analysis revealed a strong correlation between expansion of CD8+ve, CD16+ve, and CD8+ve/CD16+ve lymphocyte subpopulations in peripheral blood, the dose of administered MSCs, and degree of antigenic mismatch between donor and recipient. MSC-specific allo-antibodies were also detected in several transplant recipients. However, PBMNCs harvested from transplant recipients post-surgery exhibited no lytic activity against donor MSCs in vitro upon re-challenge.
Conclusions
MSCs induced an allo-graft response in rhesus macaques that involved principally CD8+ve, CD16+ve, and CD8+ve/CD16+ve lymphocyte subpopulations and was cell dose and haplotype dependent. This study demonstrates that MSCs are weakly immunogenic in vivo when transplanted across MHC class I barriers.
Keywords: Stem cell transplantation, mesenchymal stem cells, mesenchymal stromal cells, immunogenicity, non-human primates, intra-cranial
Introduction
Mesenchymal stem cells (MSCs) derived from adult bone marrow have recently been shown to possess potent immuno-modulatory activities both in vitro and in vivo. For example, the cells can inhibit proliferation of T cells stimulated in vitro, modulate their function by inducing anergy or a regulatory phenotype, and also regulate B cell function and dendritic cell maturation (1). Additionally, the cells have been reported to prolong the survival of skin allo-grafts in baboons (2), prevent rejection of allogeneic tumor cells in immuno-competent mice (3), ameliorate experimental autoimmune encephalomyelitis (4), and control acute, steroid-resistant graft versus host disease (GvHD) in human bone marrow transplant patients (5). These effects have been attributed, in part, to their ability to secrete a broad array of paracrine acting factors that affect immune cell function (6–10).
However, data demonstrating if MSCs are transplantable across major histocompatibility (MHC) class I barriers remains ambiguous. For example, Eliopoulos et al. (11) reported that erythropoietin-expressing MSCs transplanted to allogeneic mice failed to produce a significant increase in hematocrit due to their rejection by a host cellular immune response involving CD8+ve T cells and natural killer (NK) cells. Other groups have reported that allogeneic MSCs injected into naïve mice (12, 13) and swine (14) induced an immune response despite exhibiting immuno-suppressive effects in vitro. MSCs also failed to protect mice against GvHD (15). In contrast, allogeneic MSCs administered to human patients appear to be well tolerated (16). Therefore, it is difficult to determine whether disparities between human and animal studies reflect species specific differences in innate and adaptive immunity (17) or other confounding variables related to patient pre-conditioning.
MSCs have also gained traction as cellular vector to treat neurologic disorders (18) but their immunological properties following direct intra-cranial transplantation are largely unexplored. The latter is significant due to the unique anatomical features of the CNS, which has no direct lymphatic drainage and is protected by a blood-brain barrier that becomes progressively less permeable from fetal development through adulthood (19–21). Consequently, the proportion of T lymphoblasts that gain entry to the CNS is far lower than seen in other organs (22, 23). However, microglia and per-vascular macrophages resident in the brain also function in antigen presentation, inflammatory processes, and removal of apoptotic cells and cellular debris (24, 25). Therefore, it is unclear how infusion of allogeneic MSCs into the CNS would affect the host immune response.
In the present study we transplanted MHC class I-positive MSCs derived from a single male rhesus macaque into the CNS of eight unrelated healthy immuno-competent female infants and monitored their immune status over a period of up to six months post-transplant. Our results indicate that MSC administration induced a host immune response characterized by a transient increase in circulating levels of CD8+ve, CD16+ve, and CD8+ve/CD16+ve lymphocyte subpopulations, which are known to function in acute allo-graft rejection. Moreover, the magnitude of the immune response was directly related to the dose of injected MSCs and to a lesser extent on the degree of mismatch between the MSC donor and recipient. Since graft rejection may profoundly affect the therapeutic efficacy of transplanted MSCs, these results have important implications in the design of cell-based therapies for neurologic disorders in human patients.
Materials and methods
Animal subjects
Female rhesus macaques (Macaca mulatta) were housed in standard infant cages individually and allowed social contact with each other on a regular basis. All aspects of animal care were approved by the Institutional Animal Care and Use Committee of Tulane University. Serological testing revealed that the animals were negative for simian immunodeficiency virus, hepatitis B virus, and simian T cell leukemia virus.
Cell isolation and culture
MSCs were elaborated from the bone marrow of a male rhesus macaque raised in the virus-free colony at the New England National Primate Research Center as previously described (26, 27). MSCs used for injections were collected at second passage and suspended in PBS at 12.5 × 103 cells/μl (low dose) or 22.5 × 103 cells/μl (high dose) prior to transplantation. Peripheral blood was collected from all transplant recipients and shams 1 week prior to surgery and at 10–14, 30, and 90–180 days post-surgery. PBMNCs isolated from blood were depleted of red blood cells using the ACK lysis buffer (Invitrogen, CA USA) and purified by density gradient centrifugation. Major blood cell subpopulations were quantified in each sample using a Hematology Analyzer Advia 120 (Bayer, PA, USA). PBMNCs collected from the MSC donor and each transplant recipient/sham was used to isolate genomic DNA, which was analyzed by PCR using primers specific to the Rhesus macaque Mamu alleles A1, A2, A8, A11, B1, B3, B4, B17, and DRBw201 as described previously (28).
Surgical procedures
Surgery was performed when infants were 7.6 ± 0.8 weeks of age. Animals were immobilized with ketamine (10 mg/kg), administered buprenorphine (0.01 mg/kg), acepromazine (0.02 mg/kg) and glycopyrolate (15g/kg), and maintained on isoflurane/O2 during the surgery. The anesthetized animals were placed in a stereo-tactic frame (KOPF, CA, USA) and administered eight injections (25μl each) of MSCs or PBS at a rate of 1.2μl/min. Injections were targeted to the caudate nucleus using stereo-tactic coordinates determined from MRI scans performed one to two weeks prior to surgery. In the latter case, animals were sedated with telazol and then a total of sixty coronal (1 mm) and 15 sagittal images (3 mm) were obtained using a GE Signa 1.5 Tesla machine (GE Medical Systems, WI, USA). A unique aspect of the animal’s dentition was identified and recorded with respect to anterior-posterior (AP), dorso-ventral (DV), and lateral (Lat) coordinates. Post-operatively animals were administered analgesics for 5 days and cephalexin for 2 weeks.
Animal health surveillance
All transplant recipients were subjected to routine physical examinations on a regular basis pre- and post-surgery. Animal body weight was measured weekly during the first 2 months of age, then monthly until sacrifice. Animal body temperature was measured on the day of surgery, each day thereafter up to 7 days post-surgery, and then weekly for up to 1 month post-surgery.
Flow cytometry
MSCs (2.5 × 105) were suspended in 100 μl of wash buffer (0.1% sodium azide, 1.0% BSA in PBS) containing 5 μg of the appropriate primary antibody specific for HLA class I or HLA-DR (Invitrogen) and incubated for 40 min on ice. Cells were washed twice with 200 μl of wash buffer and incubated for 20 min in wash buffer (100 μl) containing 1 μg of a fluorochrome-conjugated secondary antibody (BD Biosciences, CA, USA). The extent of cell labeling was evaluated using a Beckman Coulter Model Epics XL flow-cytometer (Beckman Coulter, CA, USA). Isotype controls were run in parallel using the same concentration of each antibody tested. PBMNCs were washed in PBS and stained with human antibodies that cross-react with the following rhesus antigens; CD3, CD20, CD25, CD28, and Foxp3 (BD Biosciences) and CD4, CD8, and CD16 (Miltenyi Biotec Inc., CA, USA). Stained cells were analyzed using a FACSCalibur Flow Cytometer (BD Biosciences).
In vitro cyto-toxicity assay
PBMNCs harvested from the indicated transplant recipients were co-cultured with donor or third party rhesus MSCs or human MSCs and the extent of cell lysis of the target cells quantified using the Cyto-Tox 96® non-radioactive cyto-toxicity assay kit (Promega, WI, USA). Three different quantities of PBMNCs (1 × 105, 5 × 104 or 2.5 × 104 cells) were co-cultured in multi-well plates (0.32 cm2) with 1 × 104 MSCs/well for 4 hours at 37°C and then levels of cytosolic lactate dehydrogenase released into the media was quantified using a colorimetric assay. Some samples were incubated overnight to increase the sensitivity of the assay. The percentage of cell-mediated toxicity was then calculated by comparing effector/target cells reactions to control samples (media alone, PBMNCs alone, MSCs alone) using the formula provided by the manufacturer. All samples were run in triplicate.
Detection of allo-antibodies
Donor MSCs were incubated for 1 hour in the presence of 1:2 diluted sera isolated from each transplant recipient or sham. Cells were washed and stained with the FITC-conjugated antibody specific to the λ-chain of rhesus immuno-globulins (Miltenyi Biotec Inc.) and the extent of cell staining analyzed by FACS. Additionally, serum and/or plasma collected from four low dose and two high dose recipients at 90–180 days post-transplant was incubated for 30 min in an ELISA assay designed to detect IgG antibodies to Human Leukocyte Antigens (HLA) Class I (QuikScreen ELISA, GTi Diagnostics, WI, USA). After washing, plates were incubated for 30 m with an IgG-specific, alkaline phosphatase-conjugated secondary antibody followed by addition of para-nitrophenylphosphate. Color generation was quantified at 410 nm using a spectrophotometer and values were compared to that derived from an HLA Class I negative control sera. All assays were run in duplicate.
Results
Intracranial administration of MHC class I mismatched male MSCs into female infant rhesus macaques
Previously we reported that MSCs injected intra-cranially into rhesus macaques durably engrafted and localized to specific anatomical brain regions but that cell dose and the age of the transplant recipient significantly impacted overall engraftment levels (27). Additionally, a cursory examination of circulating lymphocyte subpopulations failed to reveal evidence of a host immune response to the allogeneic MSCs. In the present study eight female infant macaques were administered either 2.5×106 (low dose) or 5×106 (high dose) male MSCs, which represented a 5 and 10-fold higher cell dose, respectively, than compared to that administered in our previous study (27). In addition, two sham-operated females received injections of an equivalent volume of PBS. MSCs derived from rhesus macaques were shown previously to be similar to human cells with respect to their surface phenotype and differentiation potential (26). Flow cytometric analysis confirmed that the transplanted donor MSCs used in this study were MHC class I positive but lacked detectable expression of MHC class II receptors (Suppl. Fig. 1). Haplotype analysis also revealed that the male MSC donor expressed the Mamu A8 allele as did one of the transplant recipients (HP32) (Table 1). Alternatively, five of the remaining transplant recipients expressed the A1, A2, or B1 allele, two expressed A8 together with one additional A or B allele, and one recipient (HM72) lacked expression of all class I alleles surveyed.
Table 1.
Experimental parameters and post-surgical clinical assessments.
| Animal # | MSC Dose | Donor→Recipient MHC class I alleles | Clinical Symptoms | ||
|---|---|---|---|---|---|
| Pre-Surgery | Post-Surgery | ||||
| 2 weeks | 4 weeks | ||||
| HM56 | 2.5×106 | A8→A2 | None | Hematoma on skull | Papule crusting rash on chest & inguinal area |
| HM66 | 2.5×106 | A8→A2/A8 | None | Umbilical hernia | None |
| HM72 | 2.5×106 | A8→DBRw201 | None | Inflammation at injection site | None |
| HM67 | 2.5×106 | A8→B1 | None | None | None |
| HP06 | 5×106 | A8→A2 | None | Mild macular rash on chest | Papule rash on abdomen & neck |
| HP07 | 5×106 | A8→A1 | None | Mild macular rash on chest | Papule rash on abdomen & neck |
| HN91 | 5×106 | A8→A8,B1,DBRw201 | None | None | None |
| HP32 | 5×106 | A8→A8 | None | None | None |
| HR86 | -------- | NA→A2,DBRw210 | None | None | None |
| HV88 | -------- | NA→A2,A8,B1 | Dermatitis | None | None |
The MHC class I haplotype of the MSC donor and each transplant recipient and sham were determined based on the identification of the 9 Mamu-alleles A1, A2, A8, A11, B1, B3, B4,B17, and DRBw201. Pre-surgical clinical assessments were made at the day of surgery. All clinical assessments were performed by the staff veterinarian assigned to the project. NA, not applicable.
The day after surgery all animals returned to their normal activities. Moreover, all animals exhibited steady weight gain throughout the study period and all body weight measurements fell within the normal range for age-matched controls (Suppl. Fig. 2A) (29). The average body temperature of each transplant recipient, which was monitored up to 1 month post-transplant, also fell within the normal range (99 to 1030F) for rhesus macaques (Suppl. Fig. 2B). However, two low dose (HM56, HM72) and three high dose (HN91, HP06, and HP07) transplant recipients exhibited inflammation and/or swelling around the injection site or the appearance of a skin rash by 2 to 4 weeks post-surgery (Table 1). Similar symptoms were not evident in the sham-operated controls. One animal (HM66) also developed a small umbilical hernia at two weeks post-surgery that was unrelated to the transplantation procedure and one sham-operated control (HV88) had dermatitis prior to the surgery which recurred at 3 months post-surgery.
Transient increases in circulating leukocyte populations following intra-cranial MSC administration
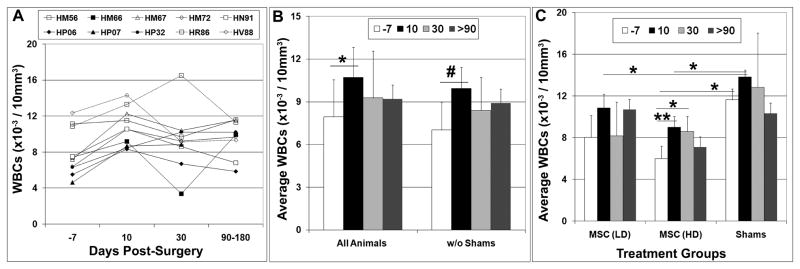
Analysis of peripheral blood collected at 10, 30, and 90–180 days post-surgery revealed increased numbers of circulating white blood cells (WBCs) in most transplant recipients as compared to values measured prior to surgery, although the variation between animals was large (Fig. 1A). Averaging these data for all animals revealed that WBC counts increased significantly (p<0.05) by 10 days post-surgery as compared to pre-surgical levels and this difference became more highly significant if the shams were excluded from the analysis (Fig. 1B). However, WBC counts diminished by 30 days post-transplant to levels that were not significantly different from pre-surgical values and remained as such when reanalyzed at 90–180 days post-surgery. Analyzing WBC counts as a function of treatment group further revealed a significant (p<0.05) increase at 10 and 30 days post-surgery as compared to pre-surgical levels only in animals that received a high dose of MSCs (Fig 1C). However, this difference was no longer significant when levels were re-evaluated at 90–180 days post-transplant. WBC counts were also significantly higher (p<0.05) in shams as compared to the high dose treatment group at 7 days prior to surgery and significantly higher (p<0.05) than both treatment groups at 10 days post-surgery (Fig. 1C). As noted above one sham (HV88) had scaly dermatitis around the mouth and fingers prior to surgery but its baseline WBC count was only slightly higher than the other sham (HR86) (12.38 vs. 10.89 × 10−3 cells/10mm3). Moreover, transplant recipient HM56 also had a baseline WBC count of 11.2 × 10−3 cells/10mm3, which was in the range of the two shams. Therefore, because animals were grouped randomly prior to establishing the baseline data we believe the high values observed in the sham-operated control group was coincidental and due principally to natural variation seen between animals. Despite the higher baseline levels, no significant difference in WBC counts pre- and post-surgery was evident in the sham-operated controls at all time points examined (Fig. 1C).
Figure 1. WBC counts quantified prior to and following intra-cranial MSC administration.
A–C) The total number of WBCs in the peripheral blood of infant macaques was determined by counting one week prior to intra-cranial MSC administration (−7) and at 10, 30, and 90–180 days post-transplantation. Statistical comparisons in (C) were based on treatment groups. Plotted values represent the mean ± SD. Students T test, *,p<0.05; **,p<0.01; #, p<0.005. LD, low dose; HD, high dose.
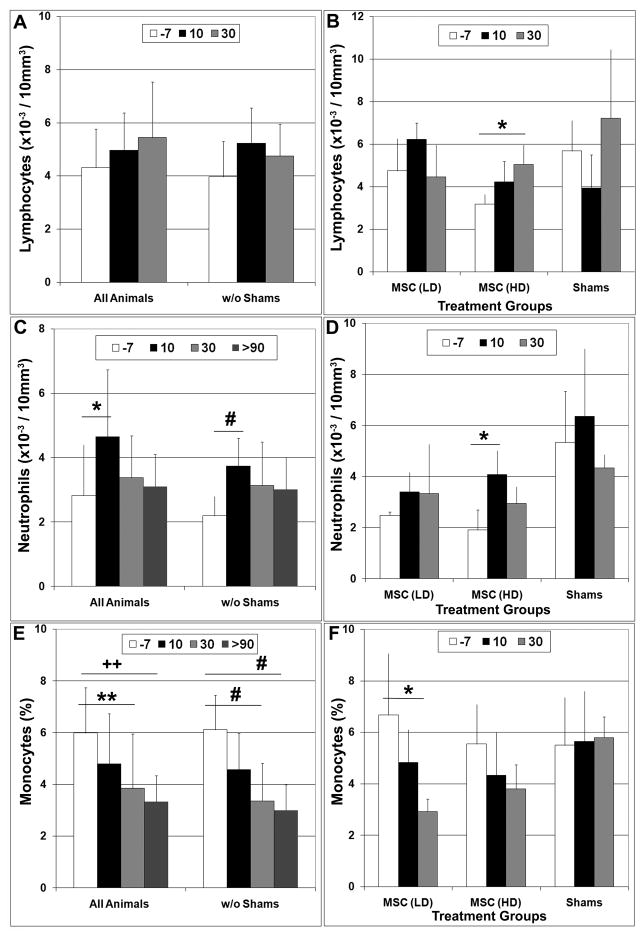
Flow cytometric analysis of peripheral blood revealed a trend toward increased numbers of circulating lymphocytes in all transplant recipients at 10 days post-surgery but this difference failed to reach statistical significance (Fig 2A). In contrast, the average number of circulating neutrophils was significantly (p<0.05) higher at 10 days post-surgery vs. pre-surgical levels in all animals and the difference became more significant (p<0.005) when the shams were excluded from the analysis (Fig 2C). Analyzing the data based on treatment group revealed that lymphocyte and neutrophil counts were significantly (p<0.05) elevated at 30 and 10 days post-transplant, respectively, as compared to pre-surgical levels in animals administered a high dose of MSCs (Fig 2B, D). Baseline neutrophil counts were found to be ~2-fold higher in the sham-operated controls as compared to the low and high dose treatment groups but this difference was not statistically significant (p>0.2). In contrast, the percentage of circulating monocytes showed a precipitous decline in all transplant recipients such that by 30 days post-surgery values were significantly (p<0.01) lower than compared to pre-surgical levels and remained so at 90–180 days post-transplant (Fig 2E). This trend was evident in both treatment groups but reached statistical significance (p<0.05) only in animals administered a low dose of MSCs (Fig 2F). At present it is unclear why monocyte levels were diminished following MSC administration but it may reflect some unique aspect of their immuno-suppressive activity. Correlating MSC engraftment levels with changes in the host immune response may provide insight into this phenomenon. No significant difference in lymphocyte, neutrophil, or monocyte counts was observed in sham-operated controls at all time points examined (Fig 2B, D, F).
Figure 2. Intra-cranial MSC administration altered levels of circulating lymphocyte subpopulations in a dose dependent manner.
The total number of circulating lymphocytes (A, B), neutrophils (C, D), and the percentage of monocytes (E, F) in peripheral blood of infant macaques was quantified by flow cytometry one week prior to MSC transplantation (−7) and at 10 and 30 days post-surgery. A, C, E) Data analysis included all animals or only animals injected with MSCs (w/o shams). B, D, F) Data analysis was based on treatment group. Plotted values represent the mean ± SD. Students T test, *,p<0.05; **,p<0.01; #, p<0.005; ++,p<0.001. LD, low dose; HD, high dose.
Intra-cranial MSC administration altered levels of CD8, CD16, and CD28 sub populations in peripheral blood
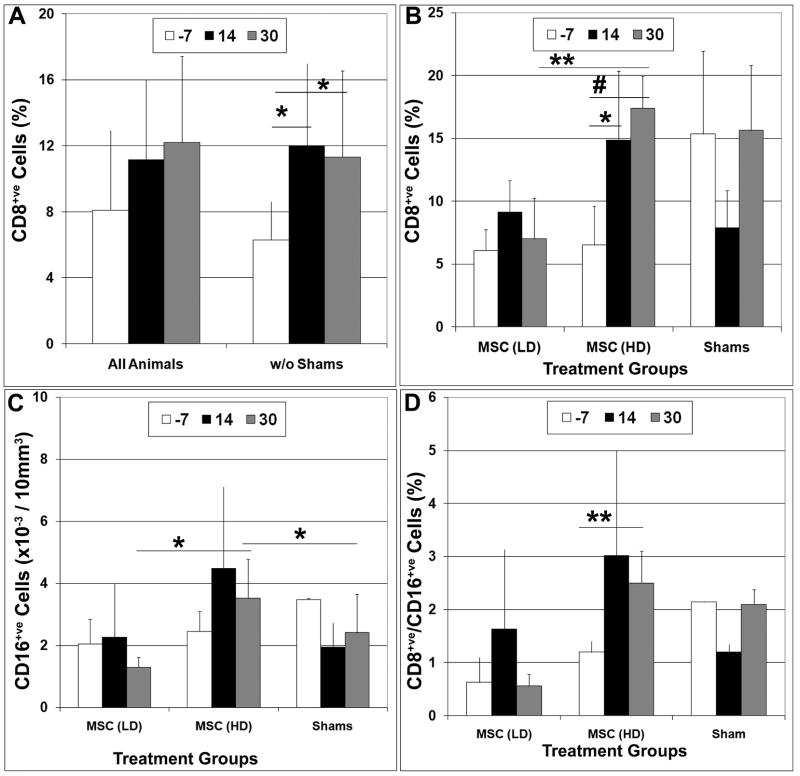
Flow cytometric analysis further revealed that the percentage of CD8+ve cells in peripheral blood was significantly higher (p<0.05) at 10 and 30 days post-transplant as compared to pre-surgical levels in all transplant recipients (Fig. 3 and 4A). Analyzing these data based on treatment group further revealed that these differences were only significant in animals administered a high dose of MSCs (Fig. 4B). Although baseline levels of CD8+ve cells were higher in shams vs. both the low and high dose treatment groups this difference was not statistically significant (p>0.28). Circulating levels of CD16+ve cells were also elevated at 10 and 30 days post-transplant in the high dose treatment group but these changes did not reach statistical significance when compared to pre-surgical values. However, levels observed at 30 days post-transplant were significantly greater (p<0.05) than that observed at the same time point in the other treatment groups (Fig. 4C). Circulating levels of CD8+ve/CD16+ve cells were also highly elevated at 10 and 30 days post-transplant in the high dose treatment group, and values measured at 30 days post-transplant were significantly greater as compared to pre-surgical levels (Fig. 4D). Similarly, the percentage of CD28+ve cells was also significantly (p<0.05) elevated at 10 days post-surgery vs. pre-surgery in the high dose treatment group but levels of CD25 and Fox3P showed no significant change either as a function of time post-transplantation or treatment group (data not shown). No significant changes in any of the aforementioned subpopulations were noted in the low dose or sham-operated groups at all time points examined.
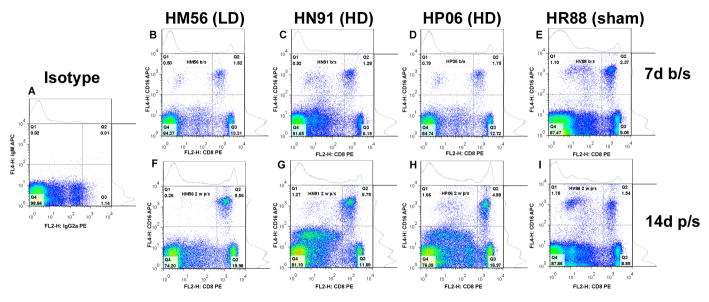
Figure 3. Flow cytometric analysis of CD8 and CD16 subpopulations in peripheral blood.
PBMNCs were collected from all animals enrolled in the study one week prior to MSC transplantation (7 d b/s) (B–E) and at 14 days post-transplantation (14 d p/s) (F–I). Cells were stained with antibodies against CD8 and CD16 and then analyzed by flow cytometry. Representative dot plots are illustrated for one low dose MSC recipient (HM56; B, F), two high dose MSC recipients (HN91; C,G and HP06; D,H) and one sham operated control (HR88; E,I). PBMNCs stained with the appropriate isotype controls are also illustrated (A).
Figure 4. Intracranial MSC administration resulted in transient increases in circulating levels of CD8+ve, CD16+ve, and CD8+ve/CD16+ve subpopulations in peripheral blood.
The total number of CD8+ve (A, B), CD16+ve (C), and CD8+ve/CD16+ve (D) subpopulations were determined by flow cytometric analysis of PBMNCs harvested from infant macaques one week prior to intracranial MSC administration (−7) and at 14 and 30 days post-transplantation. A) Data were analyzed by comparing all study subjects or only those animals injected with MSCs (w/o shams). B-D) Data were analyzed based on treatment group. Plotted values represent the mean ± SD for each subpopulation. Students T test, *,p<0.05; **,p<0.01; #, p<0.005. LD, low dose; HD, high dose.
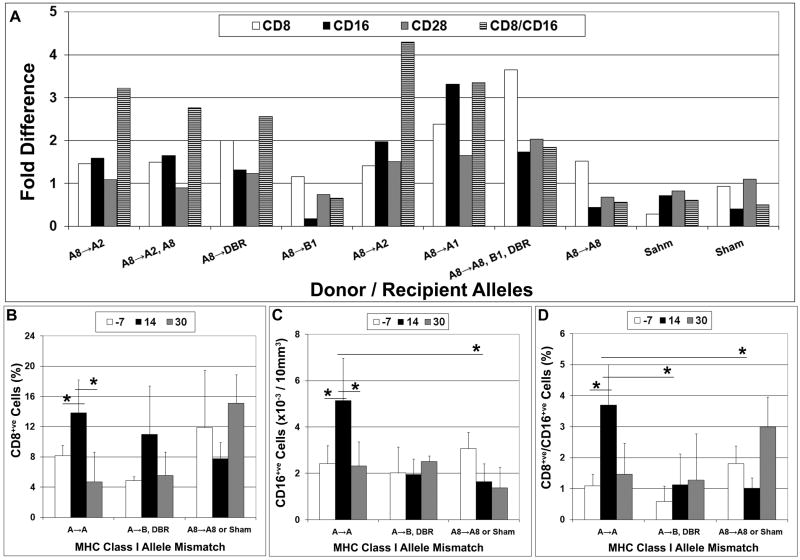
The degree of antigenic mismatch influences the cellular allo-response
Consistent with data showing the importance of HLA matching in transplantation biology (30, 31) our data indicate that circulating levels of CD8+ve, CD16+ve, and CD8+ve/CD16+ve populations also varied significantly as a function of time post surgery based on the degree of antigenic mismatch between the donor and host, even though our haplotype analysis included only a subset of MHC class I alleles (Fig. 5A). For example, circulating levels of all three subpopulations were significantly (p<0.05) elevated at 10 days post-transplant as compared to pre-surgical levels in recipients mismatched at the Mamu A allele but not the B or DBR alleles or in the sham-operated controls (Fig 5B-D). In this analysis both cohorts of mismatched (A and DBR) transplant recipients evaluated included at least one high and low dose transplant recipient (see Table 1). Moreover, one low dose (HM67) recipient mismatched at B1 and one high dose (HP32) that expressed A8 exhibited only a minor elevation in CD8 levels and no change in the levels of CD16+ve, CD28+ve, or CD8+ve/CD16+ve lymphocyte subpopulations post-transplantation (Fig. 5A). Therefore, the degree of mismatch between donor and recipient also appeared to significantly impact the magnitude of the host immune response.
Figure 5. The allo-graft response induced by MSC administration was dependent upon the degree of antigenic mismatch between donor and host.
Flow cytometric analysis was used to quantify levels of circulating CD8+ve, CD16+ve, and CD8+ve/CD16+ve cells one week prior to intracranial MSC administration (−7) and at 14 and 30 days post-transplantation. A) Fold change in circulating levels of lymphocyte subpopulations at 14 days post-transplant vs. pre-surgical levels. B-D) Changes in CD8+ve (B), CD16+ve (C), and CD8+ve/CD16+ve (D) levels in peripheral blood as a function of time post MSC administration. Statistical analyses were performed on test subjects grouped according to their degree of antigenic mismatch to the donor MSCs (A→A: HM56, HM66, HP06, HP07; A→B,DBR: HM67, HM72, HN91; A8→A8, HP32; A8→NA, HR86, HV88). *,p<0.05.
MSC transplantation elicited production of allo-antibodies in some transplant recipients
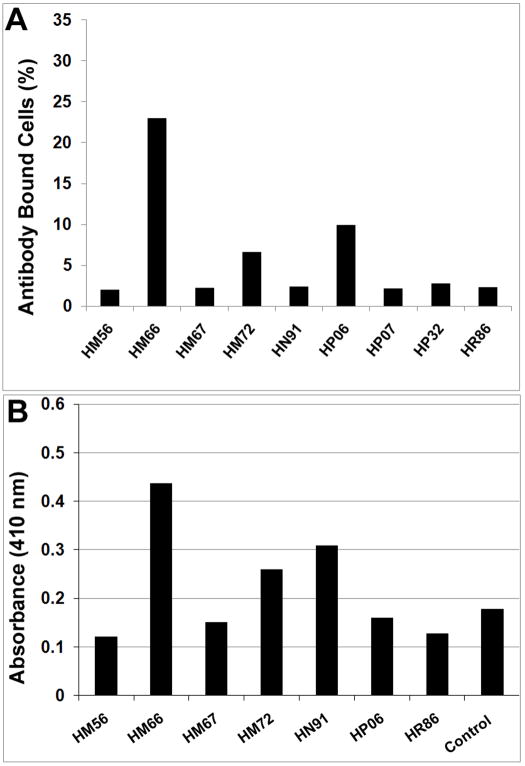
When donor MSCs were incubated with serum collected from the indicated transplant recipients and stained with a FITC-conjugated antibody specific to the λ-chain of rhesus immuno-globulins, serum derived from animal HM66, which received a low dose of MSCs, produced the highest degree of immuno-reactivity (Fig. 6A). However, serum from recipients HM72 and HP06 also produced a positive response as compared to the sham-operated control (HR86). Co-culture of serum and/or plasma from a subset of transplant recipients with a panel of HLA Class I glycoproteins also detected cross reactive allo-antibodies in two recipients, HM66 and HN91 (Fig. 6B). Therefore, at least one transplant recipient generated allo-antibodies against the donor MSCs as indicated by two independent assays.
Figure 6. Detection of allo-antibodies in the serum of MSC transplant recipients.
A) Donor MSCs were incubated with serum collected from the indicated animals at approximately 6 months post-surgery and then stained with a FITC-conjugated antibody specific to the λ-chain of rhesus immuno-globulins. The extent of cell labeling was then quantified by flow cytometry. B). Serum and/or plasma derived from the indicated animals was incubated in an ELISA plate coated with 40 different highly purified HLA Class I glycoproteins. After washing, plates were incubated with an IgG-specific, alkaline phosphatase-conjugated secondary antibody. After addition of the appropriate substrate, color development was quantified using a spectrophotometer and all values were compared to that from an HLA Class I negative control sera. All assays were run in duplicate. *,p<0.05; #, p<0.005.
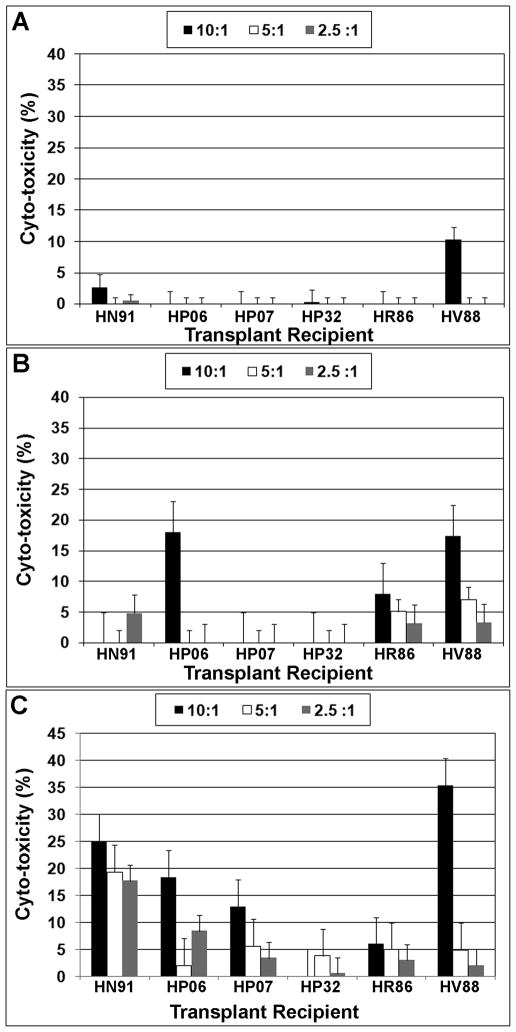
When PBMNCs obtained from each high dose transplant recipient were co-cultured with donor MSCs essentially no lytic activity was detected (Fig. 7A). This result indicates that the donor MSCs failed to induce memory T lymphocyte responses in vivo. Similarly, third party rhesus MSCs also failed to stimulate a strong lytic response even at high stimulator to responder cell ratios (Fig. 7B), consistent with previous studies demonstrating that the cells are resistant to lysis by alloreactive cytotoxic T-lymphocytes (32). In contrast, PBMNCs derived from all animals exhibited a measurable degree of lytic activity against human MSCs and this response was dose dependent (Fig. 7C).
Figure 7. Lack of in vivo sensitization of recipient PBMNCs to donor MSCs upon rechallenge in vitro.
PBMNCs isolated from peripheral blood of the indicated transplant recipients or shams at 6 months post-transplant were incubated at different ratios with donor MSCs (A), MSCs derived from an unrelated rhesus macaque (B), or human MSCs (C) and the amount of cytosolic lactate dehydrogenase (LDH) released into the media was quantified by ELISA. Cyto-toxicity was determined as the percentage increase over controls, which consisted of target and effector cell populations incubated in media alone without stimulation.
Discussion
This study demonstrates that allogeneic MSCs injected intra-cranially into immuno-competent rhesus macaques resulted in transient increases in circulating levels of neutrophils and CD8+ve, CD16+ve, and CD8+ve/CD16+ve lymphocyte subpopulations in peripheral blood. Despite the small sample size of the study, changes in the abundance of these cell populations pre- and post-surgery were found to be significant in animals administered a high dose of MSCs and/or mismatched at the major Mamu A allele. Moreover, although baseline levels of these cells were often higher in the sham-operated controls as compared to the other treatment groups, they failed to show significant changes in abundance after administration of PBS at all time points examined. Therefore, we interpret these data to indicate that transient changes in levels of circulating immune cells reflected a specific host response to the administered MSCs. Moreover, the data indicate that the magnitude of the immune response was dependent upon the dose of administered cells and/or the degree of mismatch between the recipient and donor. These results are consistent with our previous study wherein infusion of 5 to 10-fold lower doses of allogeneic MSCs into macaques failed to alter neutrophil and lymphocyte levels in peripheral blood (27). They are also in good agreement with studies conducted in baboons showing that allogeneic MSCs exhibit a shorter half-life in vivo compared to autologous cells (33) and stimulate production of allo-antibodies following repeated high dose administration (34).
It is estimated that up to 10% of all CD8+ve T cells can be stimulated by direct recognition of allo-antigens, thereby accounting for more than 90% of the total allo-response in acute graft rejection (35–38). Co-stimulation of accessory cell surface receptors on T cells also plays an important role in optimizing the cellular response to antigen stimulation. However, in the absence of co-stimulation T cell responses may still occur in the presence of high antigen concentration but are quantitatively weaker, more transient, and not characterized by elevated levels of pro-inflammatory cytokines or robust lymphocyte proliferation (39), consistent with outcomes reported in this study. Specifically, high dose administration of MSCs altered levels of CD8, CD16, and CD28 expressing cell populations but not CD25 (IL-2 receptor) and allo-specific antibodies were detected only in a subset of transplant recipients. Moreover, donor MSCs failed to promote a strong lytic response from recipient PBMNCs upon re-challenge in vitro. This finding is similar to studies by Sundin et al. (40) demonstrating that lymphocytes harvested from humans infused with allogeneic MSCs failed to exhibit evidence of in vivo sensitization upon re-challenge in vitro. Nevertheless, several studies have shown that MSCs activate expression in T cells of molecules associated with immune regulation, such as INF-γ and IL-2 but block their proliferation in vitro due to active suppressive mechanisms (40, 41). Based on our findings we propose that these suppressive mechanisms may not function efficiently in vivo and as such MSCs induce a weak allo-response in vivo that is cell dose dependent. The latter may explain why MSC engraftment levels are consistently low following administration of large numbers of cells in vivo (12–14, 27, 42). These data also suggest that mixed lymphocyte reactions conducted in vitro may not be predictive of the weak allograft response in vivo.
NK cells also play a role in allo-graft rejection. This activity is regulated by various inhibitory and activating receptors expressed on the surface of cells and dictated by the degree of MHC incompatibility between the donor and host and/or by the concentration of the allo-antigen, e.g. cell dose (43, 44). Both factors may contribute to the host immune response to allogeneic MSCs. For example, our analysis revealed an increased fraction of CD16+ve cells within the lymphocyte gate, indicative of antibody-mediated activation of cyto-toxic NK cells, which correlated with the presence of MSC donor-specific allo-antibodies in several MSC recipients. Moreover, recent studies have shown that IL-2-activated NK cells efficiently lyse autologous and allogeneic MSCs due to their expression of ligands (ULBPs, PVR, and Nectin-2) that activate the NK receptors NKp30, NKG2D, and DNAM-1 (45). Our analysis also detected elevated levels of CD8+ve/CD16+ve cells following MSC administration. Although CD16 is commonly expressed on monocytes, neutrophils, and NK cells it is also present on CD3+ve T cells that function as effectors of antibody-dependent cellular cytotoxicity (46) and rare subsets of TCRαβ CD8+ve T cells (47). Both populations exhibit functional properties of NK cells and respond to CD16 receptor triggering independent of their TCR profile. Recent studies have shown that chimpanzees posses large numbers of circulating CD8+ve NK cells that express high levels of killing enhancing receptors on their surface (48). Therefore, based on these findings, we conclude that allogeneic MSCs induce an allo-graft response in vivo that is cell dose dependent, affected by the degree of antigenic mismatch between donor and host, and mediated by CD16+ve and CD8+ve/CD16+ve lymphocyte subpopulations that play a well-described role in allo-graft rejection. Finally, our data demonstrates that the CNS affords no specific protection for allogeneic MSCs from the host immune system.
Supplementary Material
An aliquot of donor MSCs was collected prior to intra-cranial injection, stained with antibodies specific for HLA class I (gray) or HLA-DR (black) and the extent of cell labeling quantified by flow cytometry. The fraction of cells that were immuno-reactive to the HLA class I antibody is represented by the gray shaded region. The gray tracing represents staining with an isotype control. Regions denoted by the black lines represent cells stained with and HLA-Dr antibody and the appropriate Isotype control.
A) The body weight of each transplant recipient and sham was continuously monitored from birth up to approximately 3 months of age. B) The body temperature of each infant was recorded on the day of surgery, each day thereafter for 7 days, and then weekly for up to 3 weeks post-surgery.
Acknowledgments
The study was supported by the following grant: NIH 1R01NS052301-01A2 and funding from the Louisiana Gene Therapy Research Consortium. The authors would like to thank Dr. Marcelo Kuroda for insightful discussions of our work and Dr. Marion Ratterree for coordinating the animal breeding.
Footnotes
Conflict of Interest Disclosure
No financial interest/relationships with financial interests relating to the topic of this article have been declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 3.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 4.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 6.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 7.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 9.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone-marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase- mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 10.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immuno-suppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I-and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 12.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EGA, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a non-myeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badillo AT, Beggs KJ, Javazon EH, Tebbets JC, Flake AW. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral immune response. Biol Blood Marrow Transplant. 2007;13:412–422. doi: 10.1016/j.bbmt.2006.12.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783–790. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 15.Sudres M, Norol F, Trenado A, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 16.Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. doi: 10.1016/j.molmed.2010.02.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mestas J, Hughes CC. Of mice and men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 18.Isakova IA, Phinney DG. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 19.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2:269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 20.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 21.Stonestreet BS, Patlak CS, Pettigrew KD, Reilly CB, Cserr HF. Ontogeny of blood- brain barrier function in ovine fetuses, lambs, and adults. Am J Physiol. 1996;271:1594–1601. doi: 10.1152/ajpregu.1996.271.6.R1594. [DOI] [PubMed] [Google Scholar]
- 22.Engelhardt B. The blood-central nervous system barriers actively control immune cell entry into the central nervous system. Curr Pharm Des. 2008;14:1555–1565. doi: 10.2174/138161208784705432. [DOI] [PubMed] [Google Scholar]
- 23.Phillips LM, Lampson LA. Site-specific control of T cell traffic in the brain: T cell entry to brainstem vs. hippocampus after local injection of IFN-gamma. J Neuroimmunol. 1999;96:218–227. doi: 10.1016/s0165-5728(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 24.Becher B, Prat A, Antel JP. Brain-Immune Connection: immuno-regulatory properties of CNS-resident cells. Glia. 2000;29:293–304. [PubMed] [Google Scholar]
- 25.Rock RB, Gekker G, Hu S, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isakova IA, Baker K, Dufour J, Gaupp D, Phinney DG. Preclinical evaluation of adult stem cell engraftment and toxicity in the CNS of rhesus macaques. Mol Ther. 2006;13:1173–1184. doi: 10.1016/j.ymthe.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Isakova IA, Baker K, Dutreil M, Dufour J, Gaupp D, Phinney DG. Age- and dose- related effects on MSC engraftment levels and anatomical distribution in the central nervous systems of nonhuman primates: identification of novel MSC subpopulations that respond to guidance cues in brain. Stem Cells. 2007;25:3261–3270. doi: 10.1634/stemcells.2007-0543. [DOI] [PubMed] [Google Scholar]
- 28.Kaizu M, Borchardt GJ, Glidden CE, et al. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics. 2007;59:693–703. doi: 10.1007/s00251-007-0233-7. [DOI] [PubMed] [Google Scholar]
- 29.Fortman JD, Hewett TA, Bennett BT. The laboratory nonhuman primate. Boca Raton, FL: CRC Press; 2002. [Google Scholar]
- 30.Doxiadis II, Smits JM, Schreuder GM, et al. Association between specific HLA combinations and probability of kidney allograft loss: the taboo concept. Lancet. 1996;348:850–853. doi: 10.1016/s0140-6736(96)02296-9. [DOI] [PubMed] [Google Scholar]
- 31.Claas FH, Dankers MK, Oudshoorn M, et al. Differential immunogenicity of HLA mismatches in clinical transplantation. Transplant Immunol. 2005;14:187–191. doi: 10.1016/j.trim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 33.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 34.Beggs KJ, Lyubimov A, Borneman JN, et al. Immunological consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 35.Cabellero A, Fernandez N, Lavado R, Bravo MJ, Miranda JM, Alonso A. Tolerogenic response: Allorecognition pathways. Transplant Immunology. 2006;17:3–6. doi: 10.1016/j.trim.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Whitelegg A, Barber LD. The structural basis of T-cell allorecognition. Tissue Antigens. 2004;63:101–108. doi: 10.1111/j.1399-0039.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 37.Sherman LA, Chattopadhyay S. The molecular basis of allorecognition. Annu Rev Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 38.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 39.Gudmundsdottir H, Turka LA. T-cell costimulatory blockade: new therapies for transplant rejection. J Am Soc Nephrol. 1999;10:1356–1365. doi: 10.1681/ASN.V1061356. [DOI] [PubMed] [Google Scholar]
- 40.Sundin M, Barrett JA, Ringden O, et al. HSCT recipients have specific tolerance to MSC but not the MSC donor. J Immunother. 2009;32:755–764. doi: 10.1097/CJI.0b013e3181ab1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 42.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 43.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;230:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 44.Bix M, Liao N-S, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC- deficient hematopoietic cells by irradiated MHC-mismatched mice. Nature. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 45.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta I. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2 induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 46.Lanier LL, Kipps TJ, Phillips JH. Functional properties of a unique subset of cytotoxic CD3+ T lymphocytes that express Fc receptors for IgG (CD16/Leu-11 antigen) J Exp Med. 1985;162:2089–2106. doi: 10.1084/jem.162.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjorkstrom N, Gonzalez V, Malmberg K-J, et al. Elevated numbers of FcγRIIIA+ (CD16+) effector CD8 T cells with NK cell-like function in chronic hepatitis C virus infection. J Immunol. 2008;181:4219–4228. doi: 10.4049/jimmunol.181.6.4219. [DOI] [PubMed] [Google Scholar]
- 48.Rutjens E, Mazza S, Biassoni R, et al. CD8(+) NK cells are predominant in chimpanzees and characterized by high NCR expression and cytokine production and preserved in chronic HIV-1 infection. Eur J Immunol. doi: 10.1002/eji.200940062. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An aliquot of donor MSCs was collected prior to intra-cranial injection, stained with antibodies specific for HLA class I (gray) or HLA-DR (black) and the extent of cell labeling quantified by flow cytometry. The fraction of cells that were immuno-reactive to the HLA class I antibody is represented by the gray shaded region. The gray tracing represents staining with an isotype control. Regions denoted by the black lines represent cells stained with and HLA-Dr antibody and the appropriate Isotype control.
A) The body weight of each transplant recipient and sham was continuously monitored from birth up to approximately 3 months of age. B) The body temperature of each infant was recorded on the day of surgery, each day thereafter for 7 days, and then weekly for up to 3 weeks post-surgery.