Abstract
The accumulation of di-carbonyl compounds, methylglyoxal (MG) and glyoxal (G) has been observed in diabetic conditions. They are formed from non-oxidative mechanisms in anaerobic glycolysis and lipid peroxidation and act as advanced glycation endproduct (AGE) precursors. The objective of this study was to monitor and characterize the AGE formation of human immunoglobulin G (hIgG) by MG and G, utilizing UV-Fluorescence spectroscopy, circular dichroism (CD) and MALDI-Mass Spectrometry. Human IgG was incubated over time with MG and G at different concentrations. Formation of AGE was monitored by UV and fluorescence spectroscopy. The effect of AGE formation on secondary structure of hIgG has been studied by CD. Comparison of AGE profile for MG and G was performed by MALDI-Mass Spectrometry. Both MG and G formed AGE with MG being almost twice as reactive as G. The combination of these techniques is a convenient method for evaluating and characterizing the AGE proteins.
Keywords: Advanced glycation endproducts, Immunoglobulin G, methylglyoxal, glyoxal, circular dichroism, MALDI-MS
Introduction
A major complication of diabetics is their increased risk to infections [1–2]. In the human body, B-cells are responsible for protecting against foreign bodies or infections. The function of the B-cells is mediated by immunoglobulins, proteins that have an antibody function, of which Immunoglobulin G (IgG) is the most abundant and widely distributed. Immunoglobulins act as recognizing blocks or structures that identify a foreign body or infection and initiate an immune response [3].Any alteration to its structure can hinder the function of immunoglobins and affect its contribution in the humoral immunity. Non-enzymatic glycation of biomolecules is a major issue that leads to several complications in the diabetics. Glycation affects protein structure and function and thus makes it important for characterization [4]. Extensive reports have appeared on characterizing, quantifying and for monitoring the structural changes of glycated proteins [5–7].
Immunoglobulins are rich in lysine residues, making them a potential target for glycation. Glycation of immunoglobulins occur under physiological conditions and are increased in diabetes [8–12]. Several studies on the effects of glycation by glucose or fructose on immunoglobulin function have reported that the binding ability of glycated antibodies to their respective antigens has been impaired, thereby compromising the immune response [13–16].
Di-carbonyl compounds, glyoxal (G) and methyl glyoxal (MG) are highly reactive metabolites that can act as precursors of advanced glycation endproducts (AGE) [17–19]. Studies from our laboratory have demonstrated that glyoxal and methyl glyoxal can be potent glycators of DNA nucleosides and nucleotides [20–21]. Also there is an overproduction of MG in diabetic conditions due to excess glucose metabolism [19, 22, and 28]. It has been reported that MG disrupts immune responses by damaging different components of the immune system [22].
The aim of this study was two fold. First, was to demonstrate the in vitro AGE formation of human Immunoglobulin G (hIgG) by methylglyoxal and glyoxal and secondly, to characterize the AGE adducts utilizing UV and Fluorescence spectroscopy, circular dichroism (CD) and MALDI-Mass Spectrometry.
In this report, we describe the effect of varying glucose-derived carbonyl intermediates, glyoxal and methylglyoxal, concentrations and incubation time on the in vitro AGE formation of human IgG and compare their reactivity using CD and MALDI-Mass profiles.
Materials and methods
Chemicals and supplies
Analytical grade glyoxal (G), methylglyoxal (MG), human immunoglobulin G (hIgG), sodium phosphate monobasic, sodium phosphate dibasic were purchased from Sigma Chemical Company (St. Louis, MO, USA). All buffers were prepared with Milli-Q purified distilled water (Millipore, Bedford, MA, USA).Disposable UV-transparent cuvettes (12.5 mm × 12.5 mm × 36 mm) were obtained from Fisher Scientific (New Lawn, NJ, USA). Unless otherwise indicated, all other reagents and solvents were of analytical grade and were purchased from Sigma-Aldrich chemical (St. Louis, MO, USA).
Preparation of buffers and reaction mixtures
Unless otherwise indicated, 0.2 M phosphate buffer (pH 7.4) containing 0.02% sodium azide was used in all reactions. Reaction mixtures included varying amounts of glyoxal and methylglyoxal (i.e., 5, 20 or 40 mM) in the presence of constant amount of immunoglobulin G (5 mg/ml). Controls included methylglyoxal or glyoxal at 5, 20 and 40 mM concentration or hIgG (5 mg/ml) in the absence of sugar. All reaction mixtures and controls were incubated in the dark at 37 °C for 30 days and then frozen until analysis.
UV and fluorescence spectroscopy
The acquisition of UV spectra was performed at a wavelength of 280 nm with an Ultrospec 2100 pro UV/Visible spectrophotometer (GE) and fluorescence spectra at an excitation of 340 nm and an emission wavelength of 420 nm using a LS 55 luminescence spectrometer (Perkin-Elmer) equipped with a thermal cell to maintain the temperature of all samples at 25±1 °C. Unless otherwise indicated all UV/fluorescence readings were performed in triplicate.
Matrix-Assisted laser desorption/ionization time-of-flight mass spectrometry
MALDI measurements were performed on a Protein Chip System (Ciphergen Biosystems, Copenhagen, Denmark) operating in the positive linear mode. A nitrogen laser illuminates the sample and initiates the process of ionization and desorption inside the protein chip reader. Sinapinic acid, dissolved in acetonitrile/water (50:50 v:v) at a concentration of 50 mM was used as the matrix. Samples for measurement were dissolved in 0.1% formic acid at a concentration of about 0.1 mg/ml. Equal amounts of sample and matrix solutions were added and about 1–2 μl of the mixture was deposited on the gold plate and allowed to dry before introduction into the mass spectrometer. Analysis was done using the protein chip software.
Circular Dichroism
Circular dichroism (CD) studies were carried out on a Jasco J-810 Spectropolarimeter equipped with a Peltier thermoelectric type temperature control system and flow through HPLC cell. The instrument was controlled by Jasco’s Spectra Manager™ software. The concentration of hIgG was adjusted to 200 μg/ml with 0.2 M phosphate buffer, pH 7.4. The measurement range was 190–260 nm and temperature was kept constant at 25 °C. Cells having path lengths of 0.1 cm were used and analysis was done at a scanning speed of 50 nm/min. A total of ten spectra were obtained and averaged.
Results
UV and fluorescence spectroscopy
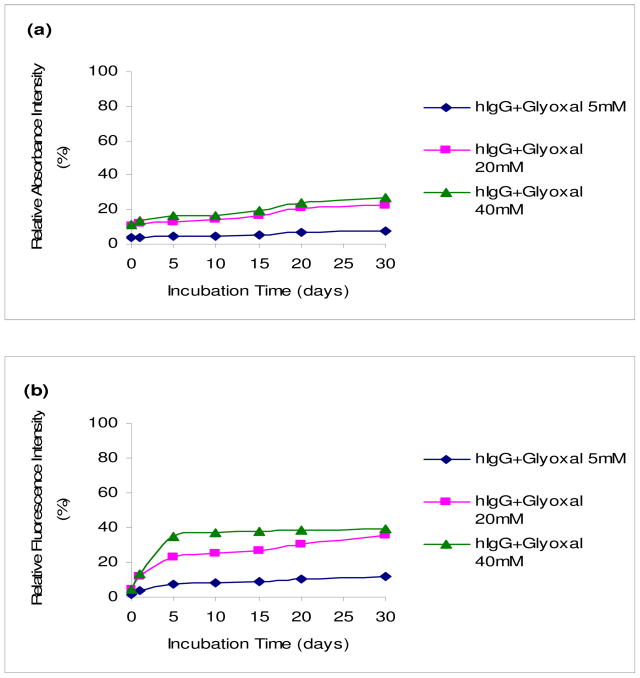
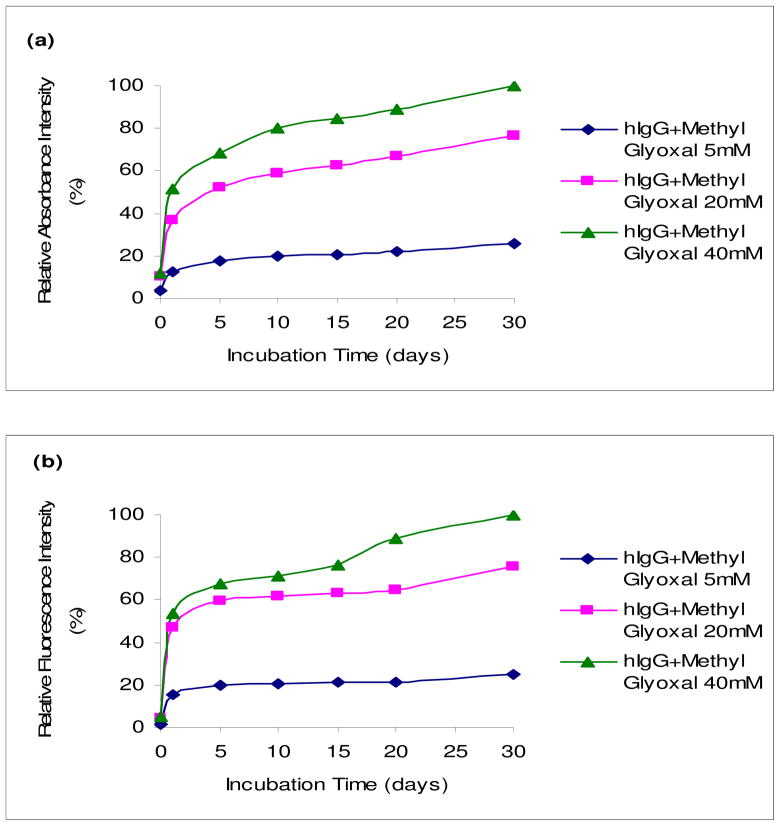
The in vitro nonenzymatic glycation of hIgG was monitored over time with UV and fluorescence spectroscopy. In a series of experiments, emphasis was placed on incubating a constant concentration (5 mg/ml) of hIgG with increasing concentrations of glyoxal or methylglyoxal (5, 20 and 40 mM). Figures 1 and 2 show the relative UV and fluorescence spectral profiles of hIgG with glyoxal and methylglyoxal at 37°C for 30 days. All readings in each figure were compared with the highest reading acquired with methylgyoxal, which was set at 100%.
Fig. 1.
UV and fluorescence spectral profiles of hIgG (5mg/ml) with glyoxal (5, 20 and 40 mM) at 37°C for 30 days. UV spectral profiles (a) measured at 280 nm. Fluorescence spectral profiles (b) measured at an excitation wavelength of 340 nm and an emission wavelength of 420 nm. All points in graphs (a) and (b) represent the average of duplicate measurements, and every reading was found to be within 5% of its counterpart duplicate. All readings were compared to the highest reading acquired with methylglyoxal set at 100%.
Fig. 2.
UV and fluorescence spectral profiles of hIgG (5mg/ml) with methylglyoxal (5, 20 and 40 mM) at 37°C for 30 days. UV spectral profiles (a) measured at 280 nm. Fluorescence spectral profiles (b) measured at an excitation wavelength of 340 nm and an emission wavelength of 420 nm. All points in graphs (a) and (b) represent the average of duplicate measurements, and every reading was found to be within 5% of its counterpart duplicate. All readings were compared to the highest reading that was set at 100%.
The UV spectral profiles substantiated with the fluorescence spectral profiles and demonstrate that the formation of AGEs increased with time and concentrations of glyoxal and methylglyoxal. It was also found that methylglyoxal was more reactive than glyoxal by using UV and fluorescence readings as a measure of AGEs. The control sugar solutions of glyoxal and methylglyoxal did not show any increase in UV or fluorescence readings and similarly, as with the control hIgG. This indicates that the changes seen with hIgG containing either glyoxal or methylglyoxal (Figures 1 and 2) were due to the formation of AGEs (data not shown).
From the UV and fluorescence data, it appears that the AGE formation increases with the increase in concentration and incubation time confirming that the formation of glycated products was a concentration-dependent and time-dependent process. Methylglyoxal was found to be more reactive than glyoxal. Another observation was that UV readings were less pronounced compared to their fluorescence counterparts.
Matrix-Assisted laser desorption/ionization time-of-flight mass spectrometry
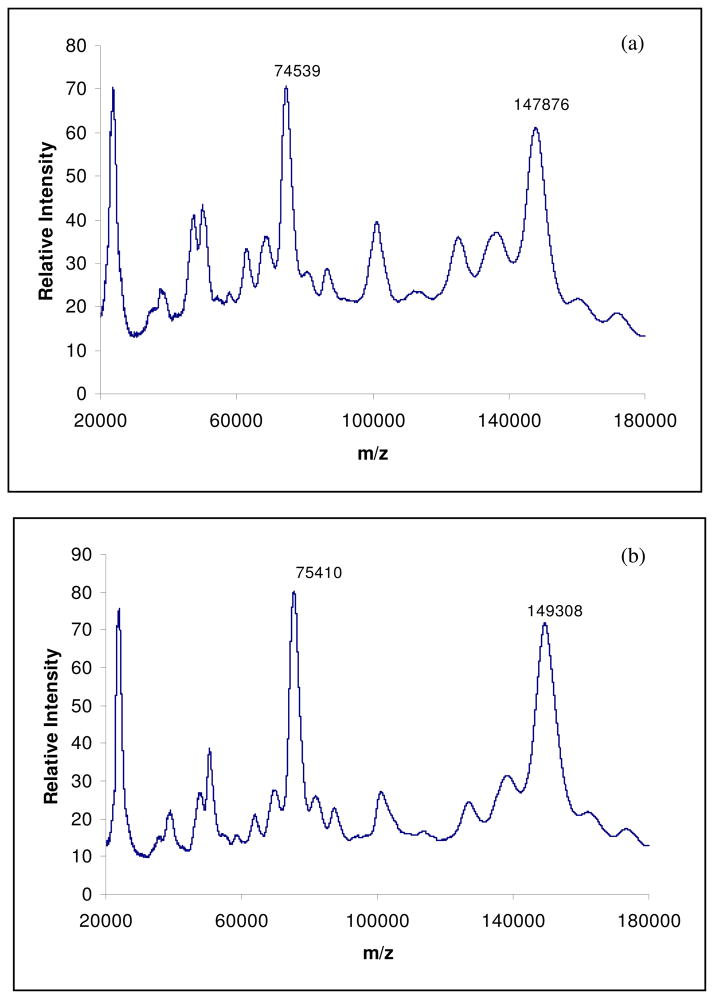
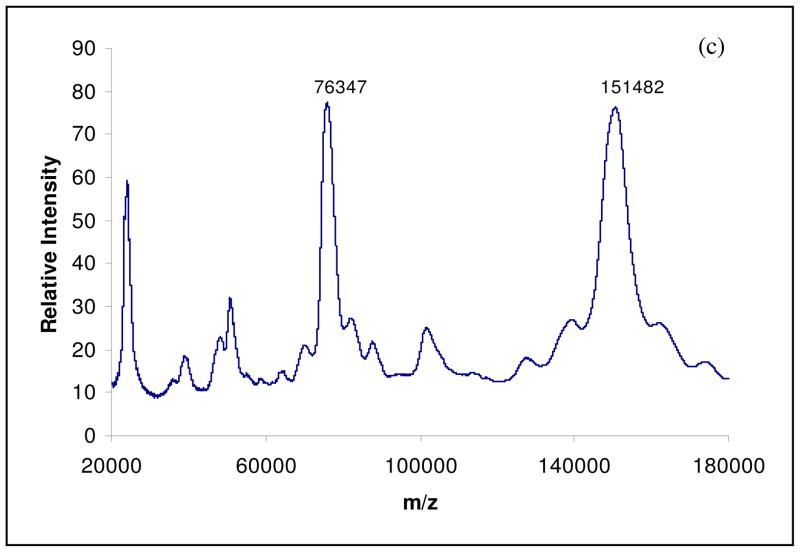
MALDI/MS (Matrix-Assisted Laser Desorption/ionization and Electrospray Ionization) technique has been used to evaluate both in vitro and in vivo AGE formation in different proteins [23 – 26]. The MALDI spectrum of control hIgG is shown in figure 3a. It has two main peaks at m/z 147,876 and 74,539, corresponding to singly and doubly charged hIgG molecules. The spectrum of hIgG incubated with glyoxal (40 mM) for 30 days at 37°C is shown in figure 3b. It consists of two prominent peaks at m/z 149,308 and 75,410. The mass difference (ΔM) between the control hIgG and the hIgG incubated with glyoxal is found to be 1432 Da and this difference corresponds to approximately 35 glyoxal molecules condensed on the active sites of the hIgG molecule. In figure 3c, spectrum of hIgG incubated with Methylglyoxal (40 mM) for 30 days at 37°C, a mass increase of nearly 4 kDa is observed, corresponding to the addition of approximately 73 methylglyoxal molecules on the hIgG molecule. Also, comparing the figures 3b and 3c, it can be assumed that methylglyoxal is twice as reactive as glyoxal. Thus, the development of MALDI-TOF/MS technique is an easy and specific diagnostic tool for the analysis of glycated proteins and peptides.
Fig. 3.
MALDI mass spectra of control hIgG (a), hIgG incubated with Glyoxal (40 mM) for 30 days at 37°C (b) and hIgG incubated with Methylglyoxal (40 mM) for 30 days at 37°C (c).
Circular Dichroism
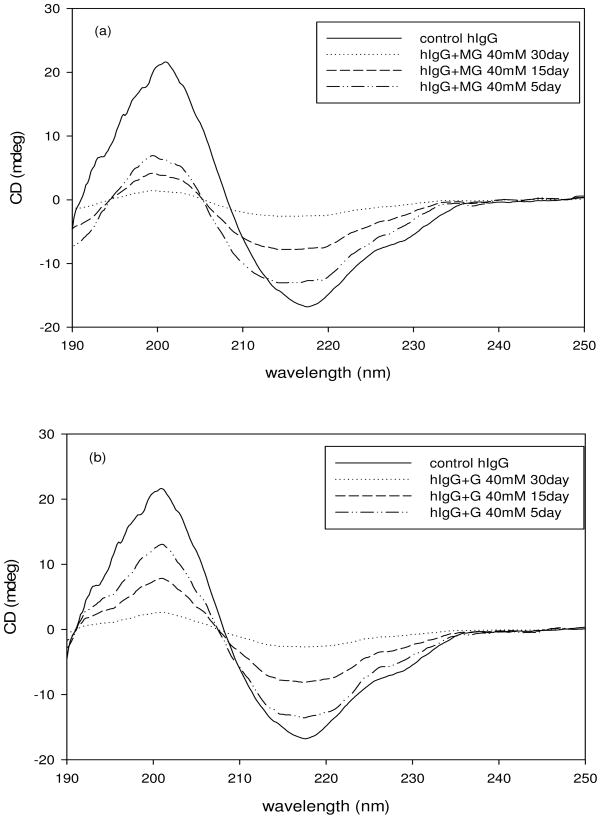
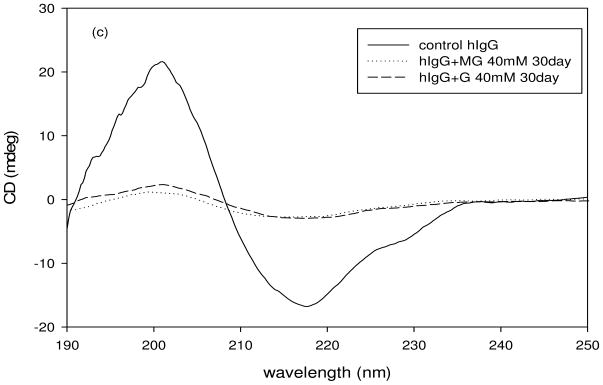
Circular dichroism spectra of the control hIgG and AGE modified hIgG indicates changes in the secondary structure of the glycated hIgG. The spectra were taken over a range of 190–260 nm wavelength at a constant temperature (25 °C). The final spectra were an average of 10 scans taken at a speed of 50 nm/min. The CD spectra of control hIgG and of hIgG in the presence of 40 mM MG and G are shown in figure 4. The spectrum of control hIgG demonstrated that the protein was primarily β-sheeted with minima at 217 nm and zero intensity at 208 nm. From figures 4a and 4b we can see that upon incubation with MG and G respectively, the secondary structure of hIgG is changing from a well defined β-sheet to a random conformation with increase in the duration of incubation time. This was revealed by the upward shift in the spectra from 210 nm to 220 nm of the glycated protein relative to the native protein.
Fig. 4.
Circular Dichroism spectra of hIgG (5mg/ml) that was incubated with methylglyoxal (40 mM) at 37°C for 30 days (a and c) and with Glyoxal (40 mM) at 37°C for 30 days (b and c).
The CD shape constant (S, a ratio of ellipticity at 200 nm against 217 nm) was calculated to evaluate the effect of glycation on the secondary structure of hIgG incubated with MG and G (Table 1). The value of S gives information about the nature of the β-sheets in the secondary structure [29]. An S value of 1 indicates weakly packed β-sheets, greater than 1 indicates strongly packed β-sheets and lesser than 1 indicates loosely packed β-sheets [29]. From table 1 it can be seen that glycation changed the secondary structure from a tightly packed β-sheet to a loosely packed one. From table 1 and figure 4c it can be said that MG had a more destabilizing effect on hIgG compared to G.
Table 1.
S values of control hIgG and hIgG incubated with G and MG (40 mM) for 30 days at 37 °C.
| Sample | S |
|---|---|
| Control hIgG | 1.26 |
| hIgG + G 40 mM | 0.906 |
| hIgG + MG 40 mM | 0.501 |
Conclusions
Glycation of immunoglobulins is of great importance as it is directly involved in suppressing the immune response in the body. The Fc and Fab fragments of the immunoglobulin contain a common domain called the immunoglobulin fold, which is composed of beta sheets and a disulfide linkage. This beta sheet secondary structure is important for immunoglobulin function and any changes to this structure result in loss of antibody activity [27]. The glycation of hIgG could affect its secondary structure and thereby influence its immunological response. Many in vitro and in vivo studies have been done using either glucose or fructose [6, 10–12]. Recent reports have demonstrated that glucose derived carbonyl compounds (such as glyoxal and methylglyoxal) also involve AGE formation in proteins and nucleosides. MG has been reported to be the root cause of immune suppression by glycating the cellular components in diabetes [22].
This report demonstrates that both methylglyoxal and glyoxal glycate human IgG in vitro under physiological conditions to form AGE products. Methylglyoxal was found to be more reactive than glyoxal and that AGE formation was affected by an increase in both the concentration and incubation time. UV and fluorescence readings of mixtures containing hIgG with methylglyoxal and glyoxal showed a linear increase with concentration and incubation time. This increase in the rate of AGE formation is a characteristic feature of glycated molecules formation.
The MALDI-TOF mass spectroscopic data proved that there is an increase in the mass of protein after glycation with respect to incubation time and concentration (data not shown). The mass difference between the glycated and control hIgG can be attributed to the number of molecules attached to the protein and showed that the methylglyoxal and glyoxal were potent glycators of hIgG. Methylglyoxal was twice as reactive as glyoxal, a finding substantiated by comparing the CD profiles and MALDI-TOF/MS data of mixtures containing hIgG with methylglyoxal and glyoxal.
CD studies provided an effective method in studying the effect of glycation on the secondary structure and stability of proteins. CD findings revealed that the glycation of hIgG with methylglyoxal and glyoxal has a destabilizing effect on its secondary structure. The shape constant provides a further insight into the changes of the packing of β-sheets in the secondary structure of hIgG upon glycation. From the CD spectra and the shape constant values it can be said that glycation caused a disruption in the secondary structure of hIgG and prominently methylglyoxal had more destabilizing effect, confirming it to be more reactive than glyoxal. Thus glycation of human immunoglobulin G leads to structural modification of the molecule as evidenced from the CD spectra and shape constant values. This could result in diminished immunoactivity and render the host prone to infection or sepsis. Thus, glycation of hIgG may influence occurrence of infections or sepsis in diabetes.
We have demonstrated that by a combination of spectroscopic techniques, it is easier to evaluate the glycation process between different sugars and proteins. The nonenzymatic reactions of different biomolecules with glucose derived carbonyl compounds such as methylglyoxal and glyoxal warrant further investigation, as these compounds also can act as potent glycators and also play an important role in promoting the changes in their structure and/or function and contribute to the pathology in diabetes.
Acknowledgments
This research was made possible by the use of Research and Bioinformatics Core Facilities supported jointly by NCRR/NIH Grant # P20 RR016457 and the Network institutions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13:R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moustschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infection. Diabetes Metab. 1992;18:187–201. [PubMed] [Google Scholar]
- 3.Yong PF, Chee R, Grimbacher B. Hypogammaglobulinaemia. Immunol Allergy Clin North Am. 2008;28:691–713. doi: 10.1016/j.iac.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Nakajou K, Wantanabe H, Kragh-Hansen U, Maruyama T, Otagiri M. The effect of glycation on the structure, function and biological fate of human serum albumin as revealed by recombinant mutants. Biochem Biophys Acta. 2003;1623:88–97. doi: 10.1016/j.bbagen.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.De Sa PFG, Treubig JM, Brown PR, Dain JA. The use of capillary electrophoresis to monitor Maillard Reaction Products (MRP) by glyceraldehyde and the epsilon amino group of lysine. Food Chem. 2001;72:379–384. [Google Scholar]
- 6.Dutta U, Cohenford MA, Dain JA. Monitoring the effect of glyceraldehyde glycation on the secondary structure human serum albumin and immunoglobulin G: An analysis based on circular dichromism, thermal melting profiles and UV-fluorescence spectroscopy. Anal Chim Acta. 2006;558:187–194. [Google Scholar]
- 7.Schmitt A, Schmitt G, Munch J. Gasic-Milencovic, Characterization of advanced glycation end products for biochemical studies: side chain modifications and fluorescence characteristics. Anal Biochem. 2005;338:201–215. doi: 10.1016/j.ab.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Danze PM, Tarojorman A, Rousseaux J, Fossati P, Daulrevaux M. Evidence for an increased glyctaion of IgG in diabetic patients. Clin Chim Acta. 1987;166:143–153. doi: 10.1016/0009-8981(87)90416-5. [DOI] [PubMed] [Google Scholar]
- 9.Lapolla A, Fedele D, Traldi P. Diabetes and Mass spectrometry. Diabetes Metab Res Rev. 2001;17:99–112. doi: 10.1002/dmrr.189. [DOI] [PubMed] [Google Scholar]
- 10.Cohenford MA, Urbanowski JC, Shepard DC, Dain JA. Nonenzymatic glycosylation of human serum IgG. Immunol Comm. 1983;12:189–200. doi: 10.3109/08820138309066868. [DOI] [PubMed] [Google Scholar]
- 11.Vrodoljak A, Trescec A, Benko B, Hecimovic D, Simic M. In vitro glycation of human immunoglobulin G. Clin Chim Acta. 2004;345:105–111. doi: 10.1016/j.cccn.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Jairajpuri DS, Fatima S, Saleemuddin M. Immunoglobulin glycation with fructose: A comparative stduy. Clin Chim Acta. 2007;378:86–92. doi: 10.1016/j.cca.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Kaneshige H. Non enzymatic glycosylation of serum IgG and its effect on antibody activity in patients with diabetes mellitus. Diabetes. 1987;36:822–828. doi: 10.2337/diab.36.7.822. [DOI] [PubMed] [Google Scholar]
- 14.Dolhofer-Bliesener R, Gerbitz KD. Impairment by glycation of immunoglobulin G Fc fragment function. Scad J Clin Lab Invest. 1990;50:739–746. doi: 10.3109/00365519009091067. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy DM, Skillen AW, Self CH. Glycation of monoclonal antibodies impairs their ability to bind antigen. Clin Exp Immunol. 1994;98:245–251. doi: 10.1111/j.1365-2249.1994.tb06133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodarzi MT, Ghahramany S, Mirmomeni MH. In vitro Glycation of human IgG and its effect on interaction with anti-IgG. Iran J Allergy Asthma Immunol. 2004;3:181–187. [PubMed] [Google Scholar]
- 17.Fukunaga M, Miyata S, Higo S, Hamada Y, Ueyama S, Kasuga M. Methylglyoxal induces apoptosis through oxidative stress mediated activation of p38 mitogen-activated proetin kinase in rat Schwann cells. Ann NY Acad Sci. 2005;1043:151–157. doi: 10.1196/annals.1333.019. [DOI] [PubMed] [Google Scholar]
- 18.Karachalias M, Babaei-Jadidi R, Ahmed N, Thornalley PJ. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem Soc Trans. 2003;31:1423–1425. doi: 10.1042/bst0311423. [DOI] [PubMed] [Google Scholar]
- 19.Thornalley PJ. Pharmacology of methylglyoxalase: formation, modification of proteins and nucleic acids, and enzymatic detoxification-a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 20.Li YY, Dutta U, Cohenford MA, Dain JA. Nonenzymatic glycation of guanosine 5′-triphosphate by glyceraldehyde: an in vitro study of AGE formation. Bioorg Chem. 2007;35:417–429. doi: 10.1016/j.bioorg.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Li YY, Dutta U, Cohenford MA, Dain JA. The structural modification of DNA nucleosides by nonenzymatic glycation: an in vitro study based on the reactions of glyoxal and methylglyoxal with 2′-deoxyguanosine. Anal Bioanal Chem. 2008;390:679–688. doi: 10.1007/s00216-007-1682-4. [DOI] [PubMed] [Google Scholar]
- 22.Price CL, Knight SC. Methylglyoxal: possible link between hyperglycaemia and immune suppression? Trends in Endocrinology and Metabolism. 2009;20:312–317. doi: 10.1016/j.tem.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Lapolla A, Fedele D, Aronica R, Garbeglio M, D’Alpaos M, Plebani M, Seraglia R, Traldi P. A highly specific method for the characterization of glycation and glyco-oxidation products of globin. Rapid Commun Mass Spectrom. 1997;11:613–617. doi: 10.1002/(SICI)1097-0231(199704)11:6<613::AID-RCM907>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Lapolla A, Fedele D, Aronica R, Garbeglio M, D’Alpaos M, Seraglia R, Traldi P. Evaluation of IgG glycation levels by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 1997;11:1342–1346. doi: 10.1002/(SICI)1097-0231(199708)11:12<1342::AID-RCM972>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 25.Lapolla A, Fedele D, Plebani M, Aronica R, Garbeglio M, D’Alpaos M, Seraglia R, Traldi P. Evaluation of glycated globins by matrix-assisted laser desorption/ionization mass spectrometry. Clin Chem. 1999;45:288–290. [PubMed] [Google Scholar]
- 26.Lapolla A, Fedele D, Garbeglio M, Martano L, Tonani R, Seraglia R, Favretto D, Fedrigo MA, Traldi P. Matrix-assisted laser desorption/ionization mass spectrometry, enzymatic digestion, and molecular modeling in the study of nonenzymatic glycation of IgG. J Am Soc Mass Spectrom. 2000;11:153–159. doi: 10.1016/S1044-0305(99)00134-8. [DOI] [PubMed] [Google Scholar]
- 27.Bork P, Holm L, Sander C. The immunoglobulin fold. Structural classification, sequence patterns and common core. J Mol Biol. 1994;242:309–20. doi: 10.1006/jmbi.1994.1582. [DOI] [PubMed] [Google Scholar]
- 28.Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems--role in ageing and disease. Drug Metabol Drug Interact. 2008;23:125–50. doi: 10.1515/dmdi.2008.23.1-2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li SQ, Bomser JA, Zhang QH. Effects of pulsed electric fields and heat treatment on stability and secondary structure of bovine immunoglobulin G. J Agric Food Chem. 2005;53:663–670. doi: 10.1021/jf048923r. [DOI] [PubMed] [Google Scholar]