Abstract
Viral respiratory tract infections are common and usually selflimited illnesses. For patients at risk of asthma, or with existing asthma, viral respiratory tract infections can have a profound effect on the expression of disease or loss of control. New evidence has shown that wheezing episodes early in life due to human rhinoviruses are a major risk factor for the later diagnosis of asthma at age 6 years. For those with existing asthma, exacerbations are a major cause of morbidity, can need acute care, and can, albeit rarely, result in death. Viral respiratory tract infections, predominantly those caused by human rhinoviruses, are associated with asthma exacerbations. There is also evidence that deficiencies in antiviral activity and the integrity of the airway epithelial barrier could make individuals with asthma more likely to have severe viral respiratory infections of the lower airway, and thus increase the risk of exacerbation. In view of the effect of respiratory viruses on many aspects of asthma, efforts to understand the mechanisms and risk factors by which these airway infections cause changes in airway pathophysiology are a first step towards improved treatment.
Introduction
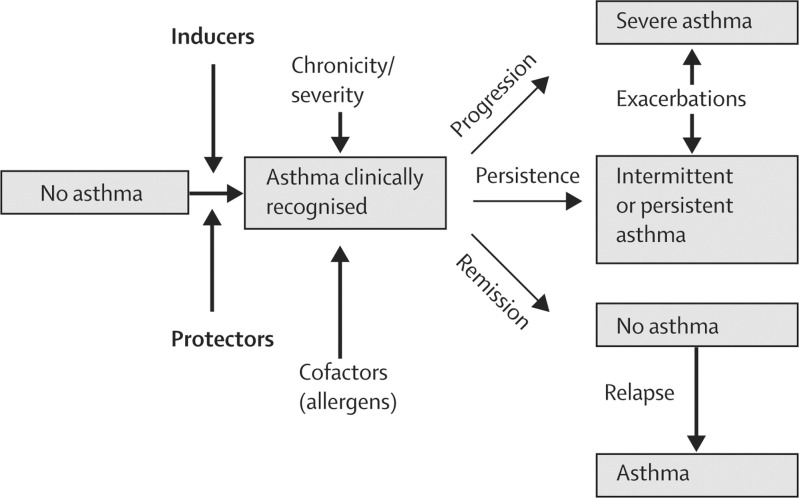
Viral respiratory tract infections can have profound effects on important aspects of asthma (figure 1 ).1 In early life, many children have wheezing episodes associated with respiratory infections. There are many pathogens associated with such wheezing events, including respiratory syncytial virus (RSV), human rhinovirus (HRV), metapneumovirus, parainfluenza, and coronavirus. For most infants, wheezing episodes with respiratory infections diminish with age, but for some individuals wheezing episodes in early life can mark the beginning of asthma.
Figure 1.
Role of infections in asthma
Infections in early life can be inducers of wheezing or protectors against the development of allergic disease.
Contrary to studies that link infections in early life with subsequent respiratory morbidity,2 the hygiene hypothesis3, 4, 5 proposed that infections, including respiratory infections in early life, were protective towards the eventual development of allergic diseases and possibly asthma; a proposal lent support by findings that show a low prevalence of allergies and asthma in children in day care or those with older siblings.3, 4 According to the hypothesis, frequent challenge with infections leads to stimulation of an individual's protective immunity (ie, Th1 immunity). More recent studies5 suggest that exposure to nonpathogenic microbes could be more important than exposure to pathogenic microbes in directing healthy immune development and reducing the risk for allergic diseases.
Respiratory viral infections can have severe adverse outcomes in patients with established asthma; viral respiratory infections are associated with nearly 80% of asthma exacerbation episodes.6, 7, 8, 9, 10 The association between viral respiratory infections and asthma exacerbations is seen in both children and adults. Whether respiratory infections determine disease progression or the eventual severity of disease is less clear.11 In this Review we discuss the role of viral respiratory infections in the expression of asthma in early life and how they contribute to asthma exacerbations.
Role of viral infections in onset of asthma
Wheezing illnesses are associated with viral respiratory infections in patients of all ages.6, 7, 8, 12, 13 By studying the association between wheezing episodes and respiratory infections in early life, we can attempt to determine the potential relation between these events and the eventual development of asthma. This relation has been best studied with respiratory infections due to RSV or HRV.
In infants, RSV bronchiolitis has many similarities to acute asthma: wheezing, rapid breathing, small airway inflammation, and, in some children, respiratory compromise.12 About a third of children with an initial wheezing episode due to bronchiolitis will have recurrent episodes of wheezing.13 This association has prompted speculation that severe episodes of RSV bronchiolitis in infancy initiate the development of asthma.
Sigurs and colleagues14 explored the association between an RSV infection sufficient to cause admission to hospital and the eventual development of asthma. Beginning in 1989, they identified 52 infants who were receiving treatment in hospital for RSV bronchiolitis and assessed their disease progression until they were 13 years old. The patients were matched with 93 healthy individuals of similar age and sex. When the children were 7·5 years old, those with a family history of both asthma and bronchiolitis had high rates of asthma (38%; 8 of 21 patients) compared with those with a family history of asthma but not bronchiolitis (none of 27 patients). Data from this study suggest that severe RSV infections together with a family history of asthma increase the likelihood of an individual developing asthma.
These findings accord with the widespread idea that both genetic (a family history of asthma) and environmental (RSV infection) factors combine to determine expression of disease (asthma). This gene–environment idea is lent support by a large epidemiological study15 done in Tennessee, USA, in which investigators noted that infants who were born roughly 120 days before the time of the year at which RSV prevalence was at its peak had the highest rate of admissions to hospital for wheezing illnesses. Follow-up revealed that the timing of birth with respect to the peak of the winter bronchiolitis season was also associated with a high risk for developing asthma. These findings suggest that infants who are at the highest risk for severe viral bronchiolitis are also most likely to develop asthma. Moreover, they suggest that several viral wheezing illnesses during infancy contribute to the development of asthma. At least two prospective birth cohort studies16, 17 have also documented an association between wheezing illnesses due to RSV infection in early life and the subsequent expression of persistent wheezing and asthma when a child begins school.
An association between RSV infection and asthma has not, however, been seen in all investigations. For example, longitudinal data from Tucson Children's Respiratory Study18 indicate that RSV infections of the lower respiratory tract before a child is 3 years of age are associated with subsequent wheezing and asthma in early childhood, but not beyond age 11 years. Furthermore, Thomsen and co-workers19 assessed the association between admission to hospital due to RSV infection and development of asthma based on a twin registry in Denmark. They concluded that severe RSV infections do not cause asthma but, instead, that a genetic predisposition to both RSV bronchiolitis and asthma exists.
HRVs are the cause of the common cold and are the viral pathogens that most frequently cause respiratory tract infections, but they are not limited to the upper airway tract. Studies in several countries have implicated HRV infections as the cause of bronchiolitis that results in respiratory compromise severe enough to require admission to hospital.20, 21 HRVs might have an important role in asthma because they are the most common viral respiratory infection in early life and can infect the lower airways.22
Many factors have led to a better understanding of the part HRVs can play in the development of asthma. First, molecular techniques are now available to detect HRV in respiratory secretions.23, 24 These techniques are highly sensitive and specific, and can use secretions obtained via non-invasive techniques, such as a nose blow into a plastic bag.25 Second, PCR methods can detect small amounts of virus that might not be detected by culture. Third, when HRV or other viruses are detected, the viral load can be measured; large viral loads will lead to a more symptomatic infection and a greater likelihood of asthma exacerbation compared with small viral loads.26 Finally, to differentiate between the many HRV strains has now become possible and, as will be discussed later, this ability has led to the discovery of a new species, which might be strongly associated with wheezing illnesses and asthma.25, 27, 28
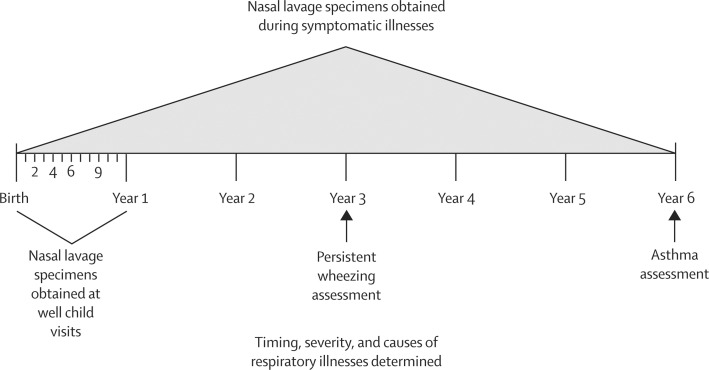
To more precisely study and identify the role of respiratory viruses in the development of asthma, Lemanske and colleagues29 established a birth cohort of children at high risk for asthma. These children had at least one parent with asthma or allergic disease. The Childhood Origins of Asthma (COAST) study was initiated in 1998, with 289 children enrolled between 1998 and 2000. Nasal lavage specimens were obtained from the children at well child visits during their first year of life—at 1, 4, 6, 9, and 12 months of age—and whenever they had a symptomatic respiratory infection. For the next 5 years, nasal lavage specimens were taken only when a child had symptomatic respiratory illnesses. When the children were 3 years of age, the investigators assessed the frequency of persistent wheezing.30 Then at age 6 years, the 259 children remaining in the study were tested for asthma. With this study design (figure 2 ), the investigators were able to determine associations between respiratory illnesses with early life wheezing and the eventual development of asthma. The diagnoses of asthma were determined independently by four clinician investigators, in a blinded fashion and on the basis of fulfilling several clinical criteria: diagnosis of asthma by a physician; frequent albuterol or asthma-controller medication use; a step-up plan during illness; with or without oral prednisone use for an asthma exacerbation.16 On the basis of these prestudy criteria, 28% (n=73) of the children were diagnosed with asthma at age 6 years.
Figure 2.
Childhood Origins of Asthma (COAST) study design
In the first 3 years of the COAST study, 454 wheezing respiratory illnesses were recorded and, in 97% of these episodes, nasal specimens were obtained. In 90% of specimens obtained, a viral cause was noted. HRV was recorded in 48% of the specimens and RSV in 21% of the specimens. Of 73 children identified with asthma, 48% (35) had intermittent asthma, 34% (25) had mild persistent asthma, and 18% (13) had moderate persistent asthma.
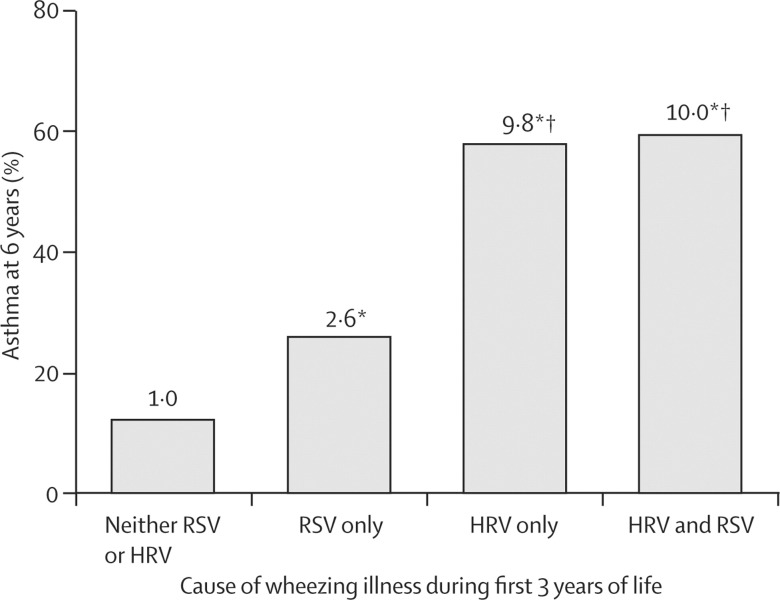
With this information about the detection of a respiratory virus with wheezing illnesses, Jackson and colleagues16 were able to establish the risk factors for wheezing illness (due to HRV or RSV) during the first 3 years of life and a diagnosis of asthma in later life. They reported that the risk of asthma at 6 years of age was substantially greater if a child had had a wheezing episode due to HRV or RSV infection at 3 years of age than if they had had wheezing episodes only in the first year of life. The odds ratio (OR) for asthma diagnosis at age 6 years if the child wheezed with an RSV infection during the first 3 years of life was 2·6 (95% CI 1·0–6·3). When wheezing during the first 3 years of life was due to HRV, the OR for asthma at 6 years of age increased to 9·8 (4·3–22·0). There was no additional risk when an individual had been infected with both viruses (figure 3 ).
Figure 3.
Risk of asthma at age 6 years in children who wheezed during the first 3 years of life
Number at top of bar=odds ratio. HRV=human rhinovirus. RSV=respiratory syncytial virus. *p<0·05 versus no RSV or HRV. †p<0·05 versus RSV only. Reprinted with permission from Jackson and colleagues.16
Jackson and colleagues16 also investigated the relation between allergic sensitisation, HRV-related wheezing, and development of asthma in the COAST study cohort (table ). Aeroallergen sensitisation without wheezing in the first year of life and wheezing without sensitisation were both associated with an increased risk for asthma at 6 years of age. The frequency of asthma was highest in infants with both sensitisation and HRV-related wheezing. Aeroallergen sensitisation did not increase the risk for asthma if an infant had wheezing in the first year of life. When these comparisons were made in the third year of life, HRV-related wheezing had a greater effect on asthma risk (25·6 [8·2–79·6]) than had aeroallergen sensitisation (3·4 [7·7–6·9]). Again, there was no evidence that aeroallergen sensitisation at age 3 years changed the association between HRV-related wheezing illnesses and the development of asthma at 6 years of age. Therefore, during the early years of life, HRV respiratory infections seem to be the dominant risk factor associated with the diagnosis of asthma at age 6 years.
Table.
Contribution of rhinovirus wheezing illnesses and aeroallergen sensitisation to risk of asthma at age 6 years
| N |
Asthma |
OR (95% CI) |
||||
|---|---|---|---|---|---|---|
| n | % | HRV wheezing | Aeroallergen sensitisation | |||
| First year of life | 2·8 (1·4–5·6) | 3·6 (1·7–7·7) | ||||
| No HRV wheezing/no aeroallergen sensitisation | 183 | 38 | 21 | |||
| No HRV wheezing/yes aeroallergen sensitisation | 27 | 12 | 45 | |||
| Yes HRV wheezing/no aeroallergen sensitisation | 38 | 15 | 39 | |||
| Yes HRV wheezing/yes aeroallergen sensitisation | 7 | 6 | 86 | |||
| Third year of life | 25·6 (8·2–79·6) | 3·4 (1·7–6·9) | ||||
| No HRV wheezing/no aeroallergen sensitisation | 150 | 21 | 14 | |||
| No HRV wheezing/yes aeroallergen sensitisation | 50 | 18 | 36 | |||
| Yes HRV wheezing/no aeroallergen sensitisation | 16 | 13 | 81 | |||
| Yes HRV wheezing/yes aeroallergen sensitisation | 14 | 13 | 93 | |||
OR=odds ratio. HRV=human rhinovirus. Reprinted with permission, Jackson and colleagues.16
Kusel and colleagues17 reported the risk of asthma to be nearly doubled at 6 years of age when sensitisation to common aeroallergens was recorded. The risk for asthma was increased by four times if more than two respiratory infections with wheezing were recorded during this time. When they assessed the effect of both allergic sensitisation and wheezing with a respiratory infection, the risk for asthma was increased by about nine times. Therefore, although wheezing episodes with a respiratory infection and allergic sensitisation are independent risk factors for asthma, their combined effect seemed to be synergistic.
Collectively, these studies implicate virus-related respiratory-tract wheezing illnesses in the initial clinical expression of persistent wheezing and, ultimately, in many cases of childhood asthma. HRV has been confirmed as the virus that contributes most to these developments, which raises the question: do HRVs cause asthma? Since HRV infections are so widespread in the general population, it seems unlikely that they do. There are several hypotheses that associate severe HRV-related illnesses with recurrent wheezing and asthma. First, asthma might be fundamentally associated with dysfunctional antiviral immune responses, such as deficient production of interferons.26, 31 Second, there might be environmental factors (eg, tobacco smoke exposure) that promote severe HRV-related illnesses, recurrent wheezing, and asthma. Third, specific viral strains could be more likely to cause asthma. These hypotheses are not necessarily mutually exclusive and will be discussed later in this Review.
The extent to which genetics determines the association between viral infections and the development of asthma is of much interest,32 although data are sparse, especially for the relation between HRV and the development of asthma.33, 34, 35, 36 This area of research, along with the identification of factors that regulate these interactions, is essential to inform our understanding of why some individuals have recurrent wheezing or asthma after viral respiratory infections. The interplay between host risk factors, respiratory viruses, and allergen sensitisation is an area of much research.
Role of respiratory viruses in asthma exacerbations
Asthma exacerbations are a major cause of morbidity for asthma patients of all ages. During the past 25 years, major advances in research have taken place, which are leading to an improved understanding of many aspects of asthma exacerbations—such as a more precise identification of infectious causes, the frequency at which viral respiratory infections lead to asthma exacerbation episodes, host-dependent risk factors, and an appreciation of the synergistic role of respiratory viruses and the atopic state.
For decades, respiratory infections were suspected to be the major cause of asthma exacerbations. Initially, bacterial infections were thought to cause asthma exacerbations, but there was little evidence to support this theory. Before the development of PCR technology, the cause of respiratory infections associated with exacerbations was established by cultures, which are difficult to do and do not often support HRV growth. Despite the recent evidence that HRVs are the main asthma-causing respiratory infection, less than 50% of asthma exacerbations were associated with respiratory infections, including HRV.37
The advent of PCR technology increased the number of respiratory viruses that could be detected; the methods were highly sensitive and specific but were not quantitative. In 1995, Johnston and colleagues6 recruited 108 children who were 9–11 years of age and had asthma. They were assessed for 13 months. At the onset of a respiratory tract infection, or when asthma worsened, respiratory tract samples were obtained and tested with PCR-based viral diagnostics. Viral respiratory tract infections were detected in association with 85% of the asthma exacerbations. Nearly two-thirds of the infections were due to HRV. Similar associations between asthma exacerbations and viral respiratory tract infections, especially those caused by HRV, have also been recorded in adults.9, 10 Thus, the development and application of PCR assessment has enabled a more precise identification of respiratory viruses associated with asthma exacerbation and focused research mainly on the role and contribution of HRV. This striking association was pivotal in bringing this virus to the forefront of asthma research.
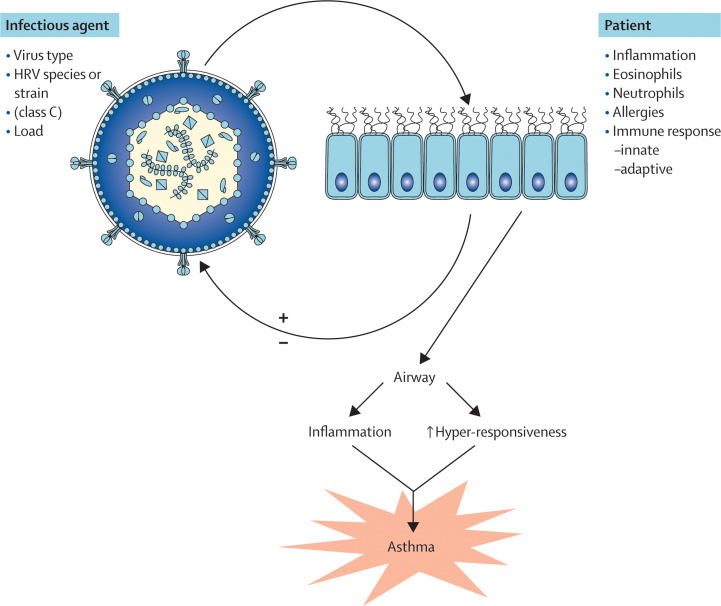
What factors contribute to an asthma exacerbation with respiratory infections? We do not fully understand how, and under what conditions, a respiratory infection can provoke asthma. The interaction between the host and the infectious agent is complex and determined by many factors that are components of the gene–environment interactions (figure 4 ).
Figure 4.
Factors that determine whether a respiratory infection provokes asthma
The interaction between the infectious agent and the patient are the purported determinants of the outcome of infection.
Additional strains of several respiratory viruses, including influenza and RSV, have been identified that might be more pathogenic, and further strains of HRV will also probably be identified. This identification will be challenging because of the large number of HRV strains. There are 99 serotypes of the virus that differ by growth in tissue culture and inhibition by specific antisera, which are classified into two groups (HRV-A and HRV-B) on the basis of genetic analysis and responses to some antivirals. Studies with molecular diagnostics have identified up to 50 additional strains of HRV.27, 38, 39, 40, 41, 42, 43, 44, 45, 46 Most of these newly identified strains belong to the newly discovered HRV-C group.26 Like several other newly identified respiratory viruses, viruses in this group do not grow easily in standard tissue culture and were discovered only after the application of molecular diagnostics. Analysis of the restricted number of full-length genomes suggests that HRV-C probably binds to unique cellular receptors.26 The development of tissue culture systems for these strains will enable researchers to obtain more definitive information about the HRV-C group.
The clinical importance of the new HRV-C strains is the subject of much study. These viruses are often detected in children with wheezing episodes, including those receiving treatment in hospital,27 and there is one report of a systemic infection with HRV-C.47 HRV epidemiology is complex because around 20 strains circulate through a community during one season. Furthermore, the prevailing strains change almost completely each season. In view of these complex epidemiological patterns and the large number of different strains, long-term population-based studies will be needed to determine the virulence of HRV-C. If more virulent strains of HRV are identified, the door could open to focused preventive efforts such as vaccination for children with asthma and other at-risk populations.
There is little evidence that asthma patients have more colds than have healthy individuals.48, 49 However, in a prospective study of cold transmission,50 couples were identified for whom one partner had asthma and the other did not. The investigators noted that colds caused an extended duration of illness and increased severity of lower respiratory symptoms in individuals with asthma. These findings suggest that response to infection, but perhaps not the frequency of infection, might differ between some patients with asthma. Olenec and colleagues25 sampled nasal secretions every week from a group of children with asthma and noted that the relation between infection and resulting illness varied with the presence of allergic sensitisation. Infection caused upper and lower respiratory tract symptoms that were of greater duration and severity in children with allergic asthma than in those with non-allergic asthma. Furthermore, viral infections were more likely to cause a loss of asthma control in children with allergic asthma than in those with non-allergic asthma. Finally, children with non-allergic asthma had twice the rate of asymptomatic infection. These findings suggest that allergic asthma is associated with more severe illness than non-allergic asthma after infection with respiratory viruses.
The possibility that individuals with asthma have abnormal antiviral activity has been assessed by several investigators who measured virus-induced cytokine responses of peripheral blood mononuclear cells. HRV-induced interferon-γ responses were reported to be inversely associated with viral shedding.51 There is evidence that mononuclear cell production of interferon-α and interferon-γ might be impaired in asthma,52 and that HRV-induced interferon-γ responses in individuals with asthma are positively associated with measures of pulmonary function.53 Furthermore, in airway sputum cells, an increased Th1 response (ratio of interferon-γ to interleukin-5 mRNA), was associated with mild colds and rapid clearance of the virus.54
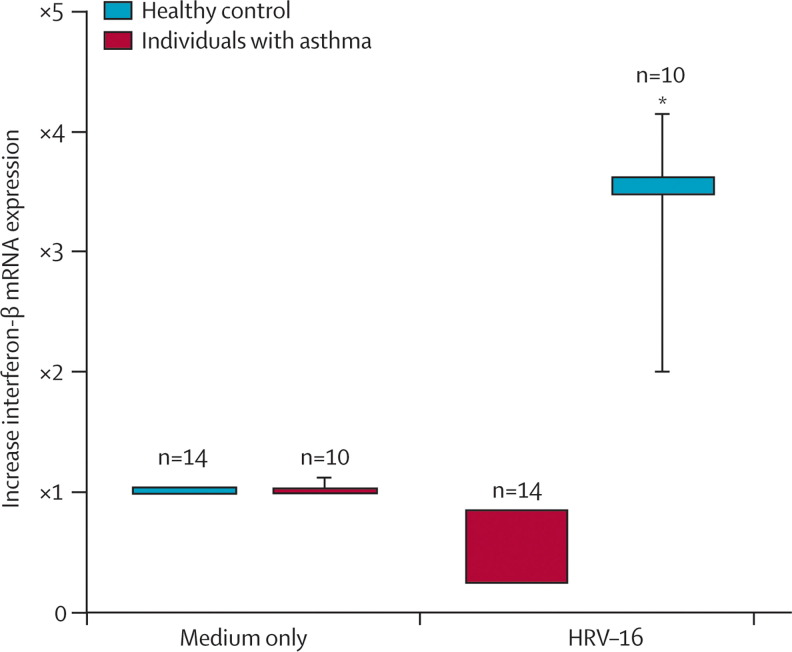
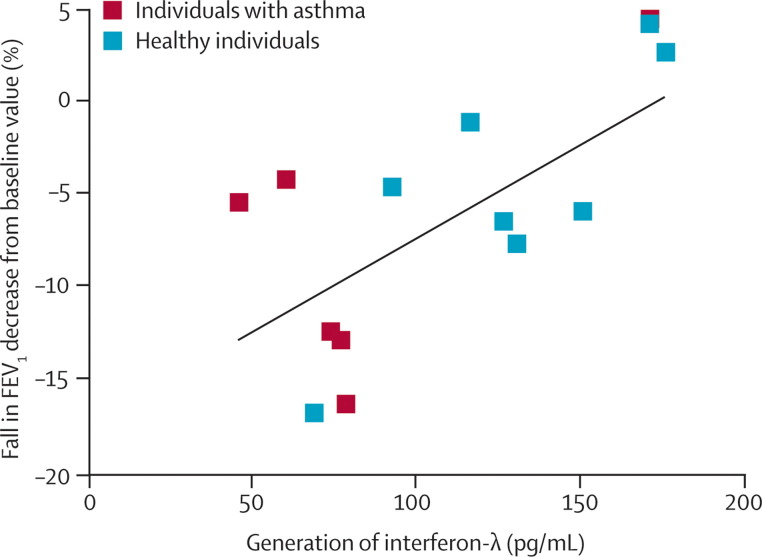
In addition to these differences in interferon responses of mononuclear and sputum cells, there is evidence that epithelial-cell interferon responses are diminished in asthma. Wark and colleagues30 used bronchoscopy to obtain bronchial epithelial cells for culture from individuals with and without asthma. These cells are highly relevant to an individual's susceptibility to infection because they are the primary site for an HRV infection in the lower airway. The investigators reported HRV-16 replication to be increased and interferon-β responses to this virus to be diminished in bronchial epithelial cells from asthmatic patients (figure 5 ). The addition of interferon-β to the HRV bronchial epithelial cell cultures from asthmatic individuals reduced virus replication. Similarly, Contoli and co-workers31 recorded decreased interferon-λ responses in bronchoalveolar airway cells from the same asthmatic individuals. Additionally, after inoculation with HRV-16, a fall in lung function (ie, forced expiratory volume in one second) was inversely proportional to interferon-λ generation previously noted (figure 6 ). These data suggest that airway cells in some asthma patients have defective antiviral responses, as evidenced by reduced generation of interferons to HRV-16.
Figure 5.
Interferon-β mRNA expression from cultured bronchial epithelial cell in response to human rhinovirus-16
Samples were obtained from healthy and asthmatic individuals and then infected in vitro with human rhinovirus (HRV)-16. Vertical bars=95% CI. *(p=004) patients versus controls. Reprinted with permission from Wark and colleagues.30
Figure 6.
Response to human rhinovirus infection
Bronchoalveolar lavage cells were obtained from healthy and asthmatic individuals. FEV1= forced expiratory volume in one second. Reprinted with permission from Contoli and colleagues.31
Not all investigations accord with this conclusion; two groups were unable to confirm that epithelial cell interferon responses were deficient in asthma.55, 56 Moreover, studies of volunteers inoculated with HRV have identified no pronounced differences in HRV shedding related to asthma.57, 58, 59 Many factors can contribute to these differences in observations, especially the severity of asthma in an individual under investigation. Consequently, additional studies of patients with asthma and naturally acquired colds will be needed to resolve these differences.
The mechanisms that contribute to changes in lower airway inflammation and altered pulmonary physiology with respiratory infections are also poorly understood. To address these issues, Message and colleagues59 assessed responses to HRV infection in asthmatic patients and healthy volunteers. Innoculation with HRV induced similar illness in both groups, but substantially increased lower respiratory symptoms, lung function impairment, bronchial hyper-reactivity, and eosinophilic lower airway inflammation in asthmatic individuals compared with healthy controls. In asthmatic, but not healthy, individuals, the virus load was strongly associated with lower respiratory symptoms, bronchial hyper-reactivity, and reductions in blood total and CD8+ lymphocytes; lung function impairment was strongly associated with neutrophilic and eosinophilic lower airway inflammation. The same virological and clinical outcomes were strongly related to deficient interferon-γ and interleukin-10 responses and to augmented interleukin-4, interleukin-5, and interleukin-13 responses. The augmented generation of the Th2 cytokines interleukin-4, interleukin-5, and interleukin-13 suggests a possible link, or risk factor, between allergic sensitisation and susceptibility to HRV infection.
One of the most consistent findings in clinical studies of asthma is that allergy and viral infections synergistically increase the risk for acute exacerbations. For example, Heymann and co-workers8 investigated the presence of viral respiratory tract infections in infants and children admitted for wheezing and the relation of the patient's atopic status to their asthma episode. In children aged 3–18 years, there was a distinct seasonal pattern, with the largest percentage of children admitted between September and November. For the children aged 3 years and older, HRV was the predominant respiratory virus detected in those admitted for wheezing (61% vs 21% in controls). Additionally, the wheezing children had substantially higher serum IgE values than did healthy controls (geometric means: 386 IU/mL vs 38 IU/mL), and 84% of the children with wheezing were sensitised to at least one aeroallergen in their test panel. These data suggest that allergic sensitisation, at least of children, is an important risk factor for wheezing with an HRV infection, and that IgE-dependent responses might also contribute to the development of wheezing with a cold. Additional studies in children and adults indicate that allergic individuals who are exposed to allergens have the highest risk for virus-induced wheezing and asthma exacerbations.60, 61
Johnston and Sears62, 63 have been instrumental in recognising and describing the September epidemics of asthma, which happen regularly in the northern hemisphere, and how these events might provide novel insights into both host and environmental factors that lead to asthma exacerbations. In Canada, the sharp increases in emergency department visits for acute asthma and admissions for childhood asthma have been predictable year-to-year. As these investigators point out, aeroallergens, air pollution, and interactions with climate can affect asthma exacerbations. To test the hypothesis that asthma exacerbations at the beginning of the school year were related to viral respiratory infections, these researchers assessed children presenting to an emergency department during 3 weeks in September along with community-recruited children with equally severe asthma who did not require acute care.62 For 62% (n=52) of the children with asthma visiting emergency departments, a respiratory virus was identified by PCR detection methods; respiratory viruses were identified in only 41% (n=150) of controls. Detection of respiratory viruses this frequently during September probably indicates a high prevalence of the virus at this time of the year. As the researchers note, the beginning of the school term is often associated with high stress levels and increased concentrations of aeroallergens in the environment. They conclude that although a viral infection can be the immediate trigger for an asthma exacerbation, these factors are likely to act together to cause the September epidemic.
These clinical observations have led to several theories attempting to explain how respiratory viruses interact with allergic inflammation to cause wheezing illnesses. Both viral infections and allergic inflammation can damage airway epithelium, which can exacerbate morbidity. On one hand, viral infections weaken the barrier function of the airway epithelium, which can lead to enhanced absorption of allergens and irritants across the airway wall and increase allergic inflammation.64 On the other hand, HRV replication in vitro is greater in damaged epithelium than in undamaged epithelium, which implies that allergen-induced damage to airway epithelium could promote viral replication and lead to severe clinical illness.65, 66 This notion could also apply to pollutants and explain why exposure to toxic agents (eg, tobacco smoke, nitrogen dioxide) also increases the risk of viral wheeze.67, 68 Additionally, airway epithelium in asthma has distinct features, including increased numbers of mucus-secreting goblet cells; there is laboratory evidence that HRV replication is enhanced in these cells.69
Recent mechanistic studies have provided evidence of an antagonistic relation between allergic inflammation and antiviral immunity. For example, allergic inflammation can inhibit innate immune interferon responses under specific conditions.70 Gill and colleagues71 suggest that cross linking or engagement of IgE receptors on peripheral blood dendritic cells—a major contributor to innate immune host defence pathways—leads to inhibition of virus-induced interferon-α secretion.71 Dendritic cells can also have a key role in allergic sensitisation, by directing the development of naive T cells into Th1 rather than Th2 cells; Th2 cells secrete cytokines that lead to IgE production (interleukin-4, interleukin-13) and eosinophilic inflammation (interleukin-5). Both HRV infection and allergic inflammation induce epithelial cells to secrete thymic stromal lymphopoietin, a cytokine that can act on a various types of cell, including dendritic cells, to promote Th2 differentiation, thereby enhancing allergic inflammation.72 Studies in animals suggest that biased innate immune responses to respiratory viruses encountered in early life could establish overproduction of key cytokines such as interleukin-13, leading to suboptimum antiviral responses, an increased risk for respiratory allergies, and changes in airway structure to promote asthma.73
Finally, in an elaborate set of studies, Subrata and coworkers74 assessed the hypothesis that underlying interactions between immunoinflammatory pathways related to aeroallergen exposure and respiratory viruses are implicated in determining and defining severe asthma exacerbations in children. 67 children were recruited at the time of emergency treatment for an acute asthma exacerbation. Postnasal aspirates were obtained along with blood samples for microarray assays of cells during the acute and convalescent period, and leucocyte phenotypic features were analysed by flow cytometry. 80% of the children had a respiratory virus detected, with HRV accounting for 95% of the infections. Circulating lymphocytes were decreased, whereas monocyte and dendritic cell populations had upregulated expression of the chemokine receptor CCR2 and an enhanced potential to provoke inflammation when recruited to the lung. These cells also had an upregulated expression of FcɛRIα (a high affinity receptor for IgE) with a concomitant gene expression signature that is representative of the interleukin-4/interleukin-13-dependent, alternatively activated phenotype.
Together with studies of respiratory virus infection in mice,73 these findings implicate specific components of the innate antiviral response, such as dendritic cells, alternatively activated macrophages, and natural-killer T cells, in the pathogenesis of virus-induced wheeze and asthma. Thus, underlying allergic disease seems to be either a risk factor for wheezing with a cold or might lay the foundations that enable the respiratory virus to interact with airway epithelium and start the processes leading to an asthma exacerbation. Determination of these interactive events promises to provide insight not only to mechanisms of asthma provoked by respiratory viruses, but also clues to more effective treatment.
Conclusions
There are strong, reproducible data that respiratory viruses, especially HRV, are associated with and probably important in the development and exacerbation of asthma. Over the past two decades we have developed our understanding and appreciation of the complexity of this association in terms of altered antiviral responses in asthma and the possibility that newly recognised HRV groups are important to this process. Many issues remain, including the patterns of inflammation that arise from respiratory viruses, the mechanisms of airway dysfunction, and the many genes or gene products that regulate these processes. Addressing these issues will be an important step forward towards a better understanding of asthma pathogenesis and more effective treatments.
Search strategy and selection criteria
We searched Medline for relevant articles using the search terms “asthma”, “viral respiratory infections”, “rhinovirus”, and “asthma exacerbations” from Jan 1, 2005 to May 31, 2010. For completeness of references we also checked our personal databases.
Acknowledgments
Acknowledgments
WWB received funding from NIAID/NIH grant HHSN272200900052C. RFL received grant support from NIH grants P01 HL070831, U10 HL064305, U10 HL074212, U10 HL098090, and R01 HL097134. JEG received research support from NIH grants U19 AI070503, P01 HL070831, NIAID/NIH HHSN272200900052C, and R01 HL097134.
Contributors
All authors contributed equally to the preparation of this Review.
Conflicts of interest
WWB has provided consultant and advisory board services to Altair, Amgen, Centocor, GlaxoSmithKline, Merck, Pfizer, Wyeth, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Novartis, and TEVA; honoraria from Merck; and grant support from Novartis, AstraZeneca, GlaxoSmithKline, MedImmune, and Ception. RFL has received consulting fees from MAP, Gray Consulting, Smith Research, Quintiles, Scienomics, R C Horowitz, Merck, AstraZeneca, Novartis; honoraria from Medicus Group, Merck, AstraZeneca; and grant support from Genentech and Pharmaxis. JEG has provided consultant and advisory board services to GlaxoSmithKline, Centocor, 3V Biosciences, Biota Inc, Synairgen, and Boehringer Ingelheim; has stock options for 3V BioSciences and EraGen Biosciences; and received grant support from Merck Inc and AstraZeneca.
References
- 1.Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther. 2008;117:313–353. doi: 10.1016/j.pharmthera.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen SO, Aaby P, Hall AJ, et al. Measles and atopy in Guinea-Bissau. Lancet. 1996;347:1792–1796. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 3.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(suppl 1):2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Mutius E, Radon K. Living on a farm: impact on asthma induction and clinical course. Immunol Allergy Clin North Am. 2008;28:631–647. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 10.Grissell TV, Powell H, Shafren DR, et al. IL-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;1724:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 11.Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, Szczeklik A. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med. 2008;177:1082–1089. doi: 10.1164/rccm.200607-973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life—The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 14.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 15.Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen SF, van der Sluis S, Stensballe LG, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 20.Calvo C, Garcia-Garcia ML, Blanco C, Pozo F, Flecha IC, Perez-Brena P. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr Infect Dis J. 2007;26:904–908. doi: 10.1097/INF.0b013e31812e52e6. [DOI] [PubMed] [Google Scholar]
- 21.Miller EK, Lu X, Erdman DD, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosser AG, Vrtis R, Burchell L, et al. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 23.Gama RE, Horsnell PR, Hughes PJ, et al. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989;28:73–77. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- 24.Lee WM, Grindle K, Pappas T, et al. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olenec JP, Kim WK, Lee WM, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 27.Palmenberg AC, Spiro D, Kuzmickas R, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324:55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 30.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175:108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 33.Ober C, Tan Z, Sun Y, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson EE, Pan L, Ostrovnaya I, et al. Integrin beta 3 genotype influences asthma and allergy phenotypes in the first 6 years of life. J Allergy Clin Immunol. 2007;119:1423–1429. doi: 10.1016/j.jaci.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Tan Z, Randall G, Fan J, et al. Allele-specific targeting of microRNAs to HLA-G and risk of asthma. Am J Hum Genet. 2007;81:829–834. doi: 10.1086/521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffjan S, Nicolae D, Ostrovnaya I, et al. Gene-environment interaction effects on the development of immune responses in the 1st year of life. Am J Hum Genet. 2005;76:696–704. doi: 10.1086/429418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minor TE, Dick EC, DeMeo AN, Ouellette JJ, Cohen M, Reed CE. Viruses as precipitants of asthmatic attacks in children. JAMA. 1974;227:292–298. [PubMed] [Google Scholar]
- 38.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kistler AL, Webster DR, Rouskin S, et al. Genome-wide diversity and selective pressure in the human rhinovirus. Virol J. 2007;4:40. doi: 10.1186/1743-422X-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–1402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kistler A, Avila PC, Rouskin S, et al. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McErlean P, Shackelton LA, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol. 2007;39:67–75. doi: 10.1016/j.jcv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McErlean P, Shackelton LA, Andrews E, et al. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS One. 2008;3:e1847. doi: 10.1371/journal.pone.0001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheuk DK, Tang IW, Chan KH, Woo PC, Peiris MJ, Chiu SS. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr Infect Dis J. 2007;26:995–1000. doi: 10.1097/INF.0b013e3181586b63. [DOI] [PubMed] [Google Scholar]
- 45.Jin Y, Yuan XH, Xie ZP, et al. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J Clin Microbiol. 2009;47:2895–2900. doi: 10.1128/JCM.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renwick N, Schweiger B, Kapoor V, et al. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J Infect Dis. 2007;196:1754–1760. doi: 10.1086/524312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tapparel C, L'Huillier AG, Rougemont AL, Beghetti M, Barazzone-Argiroffo C, Kaiser L. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J Clin Virol. 2009;45:157–160. doi: 10.1016/j.jcv.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pattemore PK, Johnston SL, Bardin PG. Viruses as precipitants of asthma symptoms—I: Epidemiology. Clin Exp Allergy. 1992;22:325–336. doi: 10.1111/j.1365-2222.1992.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horn ME, Gregg I. Role of viral infection and host factors in acute episodes of asthma and chronic bronchitis. Chest. 1973;63(suppl):44–48. doi: 10.1378/chest.63.4_supplement.44s-a. [DOI] [PubMed] [Google Scholar]
- 50.Corne JM, Marshall C, Smith S, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 51.Parry DE, Busse WW, Sukow KA, Dick CR, Swenson C, Gern JE. Rhinovirus-induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol. 2000;105:692–698. doi: 10.1067/mai.2000.104785. [DOI] [PubMed] [Google Scholar]
- 52.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin Exp Allergy. 2002;32:537–542. doi: 10.1046/j.0954-7894.2002.01313.x. [DOI] [PubMed] [Google Scholar]
- 53.Brooks GD, Buchta KA, Swenson CA, Gern JE, Busse WW. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003;168:1091–1094. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]
- 54.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Souza N, Favoreto S, Wong H, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009;123:1384–1390. doi: 10.1016/j.jaci.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeMore JP, Weisshaar EH, Vrtis RF, et al. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J Allergy Clin Immunol. 2009;124:245–252. doi: 10.1016/j.jaci.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleming HE, Little FF, Schnurr D, et al. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med. 1999;160:100–108. doi: 10.1164/ajrccm.160.1.9808074. [DOI] [PubMed] [Google Scholar]
- 59.Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray CS, Poletti G, Kebadze T, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376–382. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: school children as disease vectors. J Allergy Clin Immunol. 2006;117:557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 63.Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol. 2007;120:526–529. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakamoto M, Ida S, Takishima T. Effect of influenza virus infection on allergic sensitization to aerosolized ovalbumin in mice. J Immunol. 1984;132:2614–2617. [PubMed] [Google Scholar]
- 65.Lopez-Souza N, Dolganov G, Dubin R, et al. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am J Physiol Lung Cell Mol Physiol. 2004;286:373–381. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 66.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venarske DL, Busse WW, Griffin MR, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193:1536–1543. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chauhan AJ, Inskip HM, Linaker CH, et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361:1939–1944. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lachowicz-Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. Interleukin-13 induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2009-0244OC. published online Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tversky JR, Le TV, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via Toll-like receptor 9. Clin Exp Allergy. 2008;38:781–788. doi: 10.1111/j.1365-2222.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gill MA, Bajwa G, George TA, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benoit LA, Holtzman MJ. New immune pathways from chronic post-viral lung disease. Ann N Y Acad Sci. 2010;1183:195–210. doi: 10.1111/j.1749-6632.2009.05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subrata LS, Bizzintino J, Mamessier E, et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol. 2009;183:2793–2800. doi: 10.4049/jimmunol.0900695. [DOI] [PubMed] [Google Scholar]