Abstract
The main study objective was to compare different methods for assessing mold exposure in conjunction with an epidemiologic study on the development of children’s asthma. Homes of 184 children were assessed for mold by visual observations and dust sampling at child’s age 1 (Year 1). Similar assessment supplemented with air sampling was conducted in Year 7. Samples were analyzed for endotoxin, (1–3)-β-D-glucan, and fungal spores. The Mold Specific Quantitative Polymerase Chain Reaction assay was used to analyze 36 mold species in dust samples, and the Environmental Relative Moldiness Index (ERMI) was calculated. Homes were categorized based on three criteria: 1) visible mold damage, 2) moldy odor, and 3) ERMI. Even for homes where families had not moved, Year 7 endotoxin and (1–3)-β-D-glucan exposures were significantly higher than those in Year 1 (p<0.001), whereas no difference was seen for ERMI (p=0.78). Microbial concentrations were not consistently associated with visible mold damage categories, but were consistently higher in homes with moldy odor and in homes that had high ERMI. Low correlations between results in air and dust samples indicate different types or durations of potential microbial exposures from dust vs. air. Future analysis will indicate which, if any, of the assessment methods is associated with the development of asthma.
Keywords: endotoxin, (1–3)-β-D-glucan, fungi, polymerase chain reaction, house dust, air sampling
1. INTRODUCTION
Recent reviews and meta-analyses have concluded that sufficient epidemiological evidence is available from over 100 studies conducted in different countries and under different climatic conditions to show that the occupants of damp or moldy buildings are at increased risk of respiratory symptoms, respiratory infections, and exacerbation of asthma (IOM 2004; Fisk et al. 2007; Antova et al., 2008; WHO Europe, 2009). However, associations between health outcomes and concentrations of microorganisms (fungal spores, bacteria) or microbial components (endotoxin, (1–3)-β-D-glucan, extracellular fungal polysaccharides) are inconsistent. Some studies have shown that increased concentrations of fungi (total or specific species) are associated with increased risk of respiratory health outcomes (Dharmage et al., 2002; Gent et al., 2002; Müller et al., 2002; Stark et al., 2003; Pettigrew et al., 2004; Matheson et al., 2005; Stark et al., 2005; Dales et al., 2006; Salo et al., 2006; Rosenbaum et al., 2009) and some studies did not find such associations (Dales et al., 1999; Haverinen-Shaughnessy et al., 2007). Additionally, previous investigations have revealed increased risk of adverse respiratory health outcomes associated with increased concentrations of (1–3)-β-D-glucan (Thorn and Rylander, 1998) or endotoxin (Park et al., 2001; 2006; Dales et al., 2006), but a protective effect has also been demonstrated for endotoxin and extracellular fungal polysaccharides (Douwes et al., 2006a).
We have shown similar patterns of contradictory results between health outcomes and different exposure variables in our currently ongoing Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS). The study was designed to assess the effects of diesel particles and environmental exposures to animal allergens, mold and pollen allergens on the development of children’s atopy and asthma in a birth cohort of children of atopic parents (LeMasters et al., 2006). Exposure to visually observed mold contamination in Year 1 (at children’s age one) was associated with increased recurrent wheezing and rhinitis at age one (Biagini et al., 2006; Cho et al., 2006a; Iossifova et al., 2007) as well as with increased recurrent wheezing and increased risk of having a positive asthma predictive index at age three (Iossifova et al., 2009). In contrast, early exposure to high level of fungal (1–3)-β-D-glucan and high endotoxin exposure in the presence of multiple dogs were associated with decreased risk of recurrent wheeze, allergen sensitization, and positive asthma predictive index (Campo et al., 2006; Iossifova et al., 2007; 2009). Only visual observation of mold and water damage and dust sampling were included in the Year 1 home evaluations. Air sampling was conducted in homes of a subgroup of children as part of a nested matched case-control study (atopic children and controls matched on birth date within one month) at child’s average age of 1.7 years (Osborne et al., 2006). Some fungal types were associated with increased allergen sensitization and rhinitis while some with decreased allergen sensitization. Another set of homes (n=271) was included from CCAAPS in a study that resulted in the development of an environmental relative moldiness index (ERMI) based on the mold specific quantitative polymerase chain reaction assay (MQPCR) of 36 mold species (Vesper et al., 2007a). Higher ERMI-values were associated with increased risk of wheeze and rhinitis at children’s age one (Vesper et al., 2007a).
As different microbial components may affect the health outcomes in opposite directions and the association may also depend on the timing of the exposure, it is important to understand temporal variations and associations between different microbial exposures. The present study was conducted in a CCAAPS subgroup of 184 children at the child’s age of seven (henceforth referred to as Year 7 evaluation). The study objective was to investigate the associations between visually observed mold damage and several quantitatively assessed exposure variables. In addition to the variables included in the Year 1 assessment (visually observed mold damage, ERMI, and concentrations of endotoxin and (1–3)-β-D-glucan in dust samples) airborne concentrations of endotoxin, (1–3)-β-D-glucan and fungal spores were also measured.
2. Material and Methods
2.1 Recruitment of homes
Initial home evaluation in Year 1was completed in 777 dwellings of infants participating in the CCAAPS, a birth cohort study. Infants born in Cincinnati and Northern Kentucky between 2001 and 2003 were recruited using birth certificate data (LeMasters et al., 2006). Eligibility for the study required that at least one parent was atopic, which was determined by positive skin prick test (SPT) response (wheal ≥ 3 mm) to at least one of 15 common aeroallergens. In addition, all birth record addresses of participating children must have been within 400 m of a major road or greater than 1500 m of a major road. Children enrolled in the CCAAPS cohort completed clinical evaluations at ages one, two, three, four, and seven.
Homes for the Year 7 home evaluation were selected among 577 dwellings that had a home assessment completed at age one and child’s clinical evaluation completed at age 4. The age four clinical evaluation was selected as this was completed at time of study initiation, and therefore compliance and participation rate was known through this period. The group of 577 children was divided into eight 3-month subgroups, based on the child’s date of birth in order to match the time of home evaluation with child’s age 7 clinic visit. A balanced selection was then conducted for every 3-month subgroup based on the exposure criterion and the race of the child. For every home that contained some form of damage (visible mold, moldy odor, water damage or history of water damage), we randomly selected a home with no reported mold or water damage within the same age group. Altogether 220 recruitment letters were sent to families. Home evaluation was completed in 185 (84%) homes; one home had an incomplete set of environmental samples, eight families refused, six had moved out of the area, and we were unable to contact 21 families despite multiple attempts. The children were on average 6.9 ± 0.3 years old at the time of the home evaluation. Parents signed an informed consent form approved by the Institutional Review Board at the University of Cincinnati.
2.2 Home visit and sample collection
Questionnaire survey, visual assessment, and dust sampling were conducted both at Year 1 and Year 7 visits. During the home evaluation, a questionnaire was administered to a parent and included questions about water damage history, presence of pets, number of people living in the home, and past address history noting dates the family had moved. Following a checklist, each room including the basement, was visually inspected for signs of mold or water damage. Location of damage, changes in the color and integrity of the surface material and the size of damaged surface were recorded. For each room, a moldy odor observation was also recorded (Cho et al., 2006b).
The home visits were conducted by trained two-person teams and the moldy odor was assessed mainly by five different team members. In order to compare the sense of smell between the five team members, a commercially available test for odor threshold was conducted (OLFACT-RL, Osmic Enterprices, Inc., Cincinnati, OH). The test consists of comparing blank (no odor) with thirteen concentrations of an industry standard odorant, butanol. The odor threshold of team members varied from 9.0 to 9.8 on a scale from 0 (highest concentration) to 12 (lowest concentration). Compared to the national average of 8.0 for the age range of 18–59 years (Hastings and Bailey, 2006), the team members had a sensitive sense of smell.
The homes had previously been classified into three categories based on visual inspections in Year 1: Visible Damage Category 0 (no visible mold or moldy odor), Visible Damage Category 1 (low mold: reported history or observed water damage, observed moldy odor, or visible mold area ≤0.2 m2), and Visible Damage Category 2 (high mold: visible mold area >0.2 m2) (Cho et al., 2006b). Similar categorization was done based on the visual assessment in Year 7. In addition, a new two-level categorization (moldy odor yes/no) was developed based on the analysis of exposure data using the Principal Component analysis as described below.
House dust samples were collected from the child’s primary activity room as described earlier (Cho et al., 2006b). Briefly, a 2 m2 area of floor surface was vacuumed using a Filter Queen Majestic vacuum cleaner (Health-Mor; HMI Industries Inc., Seven Hills, OH) at a rate of 2 min/m2. Samples were desiccated, filtered through a 355-µm screens (W.S. Tyler, Mentor, OH), which were thoroughly cleaned. The screens were first rinsed with distilled water, then with 5% (vol.) bleach solution and wiped with 70% ethanol. Bleach treatment was included to inactivate any remaining microbial DNA. Cleaned screens were heated in dry air oven at 180°C for 4 hours to inactive any remaining endotoxin and (1–3)-β-D-glucan contamination. The mass of fine dust was measured and the fine dust was aliquoted, and stored at −20°C.
Air samples were collected during Year 7 assessment using Button Inhalable Aerosol Samplers (SKC, Inc., Eighty-Four, PA) at a flow rate of 4.0 ± 0.2 L min−1. The air flow was calibrated using a DryCal DC-Lite calibrator (Bios International Corp., Butler, NJ). The stationary air sampling was conducted for 24 hours on the day immediately before the dust sampling in the child’s primary activity room. The sampler collected inhalable particles onto a 25 mm polycarbonate membrane filter with a pore size of 3 µm (GE Osmonics, Inc., Minnetonka, MN). After sampling, particles were extracted from filters in pyrogen-free water (Associates of Cape Cod Inc., East Falmouth, MA) containing 0.05% Tween 80 by vortexing for 1 minute and keeping in an ultrasonic bath for 15 minutes. Suspensions were aliquoted and stored at −20°C until analysis.
Before the air sampling, the Button samplers and filters were thoroughly cleaned. All parts of the Button sampler, except the o-rings, were cleaned similarly as the screens used for sieving the dust. As the O-rings do not withstand high heat, their heat treatment was replaced with rinsing with pyrogen free water. As initial testing showed inconsistent background endotoxin and (1−3)-β-D-glucan contamination in the air sampling filters, the filters were cleaned following the same protocol that was used for the filter extraction, including 1-min vortexing and 15-min agitation in an ultrasonic bath. After this, the filter was rinsed twice with pyrogen free water and air-dried. All the handling of cleaned items, including the loading of the filter was done inside a class II biosafety hood.
2.3 Sample analysis
The air and dust samples were analyzed for endotoxin and (1–3)-β-D-glucan using the Limulus Amebocyte Lysate assay; Pyrochrome for endotoxin and Glucatell for (1–3)-β-D-glucan (LAL; Associates of Cape Cod Inc, Falmouth, MA) as described earlier (Campo et al., 2006; Iossifova et al., 2007). An aliquot of 0.5 mL of the air sample extract and an aliquot of 25 mg of sieved dust were used for each analysis. The samples were spiked with endotoxin standard of 0.50 EU/ml and (1–3)-β-D-glucan standard of 50 pg/ml to assure that there was no inhibition or enhancement between the extract and the reagents.
Endotoxin concentrations in dust were expressed as endotoxin units per mg of dust (EU/mg); similarly, (1–3)-β-D-glucan concentrations in dust were expressed as µg/g. Airborne endotoxin concentrations were expressed as EU/m3, whereas airborne (1–3)-β-D-glucan concentrations were expressed as ng/m3. The lower detection limit (LOD) for endotoxin was 0.053 EU/ml, which corresponded to detection limit of 0.002 EU/mg for dust samples and 0.046 EU/m3 for air samples. The respective LODs for (1–3)-β-D-glucan were 2.53 pg/ml, 0.0001 µg/g, and 0.004 ng/m3. The concentrations in all measured dust samples were above the LODs. Airborne endotoxin and (1–3)-β-D-glucan concentrations were below the LOD in five and two samples, respectively. Half of the detection limit was used for these samples in the data analysis. Among Year 1 dust samples, three did not have sufficient amount of dust to complete all the analysis.
For the analysis of fungal spores, 2 ml of air sample extract was filtered through a 13 mm diameter membrane of mixed cellulose ester (MCE) filter and made transparent by treating with acetone vapor as described by Adhikari et al. (2003). Fungal spores were counted using a bright light microscope (Labophot 2, Nikon Corp., Japan) at a magnification of 400× and the results were expressed as spores/m3. The detection limit was 6 spores/m3. Three samples had a value that was below the LOD and a value of 3 spores/m3 was used for these homes. One fungal spore sample had excessive amount of other particles and the microscopic spore count could not be obtained.
The MQPCR was used to analyze 36 mold species in 5 mg aliquots of fine dust as previously described (Haugland et al., 2004; Meklin et al., 2004; Vesper et al., 2007b). The detection limit for MQPCR analysis was 1 cell equivalent/5 mg of dust. The concentrations in all dust samples were above the detection limit. The ERMI-value was calculated for each home, and homes were classified into two groups based on the ERMI: ≤5 = low and >5 = high mold burden (Vesper et al., 2007b).
Media blanks analyzed in parallel with dust and air samples were below the detection limits of respective assays.
2.4 Statistical analysis
Data analysis was conducted using S-Plus (TIBCO Corp., Palo Alto, CA) and SAS for Windows, Version 9.2 (SAS Institute, Cary, NC). Histograms and quantile-quantile plots showed that the distribution of ERMI values approximated normality; 5% trimmed means were tested for normality by the Kolmogorov-Smirnov test, and the p-values were 0.08 and 0.15 for years 1 and 7, respectively. Distributions of (1–3)-β-D-glucan, endotoxin, and fungal spore levels were log-normal. Thus, these data were log-transformed for analysis. Descriptive statistics including geometric means and 95% confidence intervals were calculated for (1–3)-β-D-glucan, endotoxin, and fungal spore concentrations, and arithmetic means and standard deviations were calculated for ERMI. The agreement between the Visible Damage Category of a subject’s home at Years 1 and 7 was assessed by the weighted kappa statistic, using all subjects’ homes, and only homes of subjects who had not moved between assessments. Differences between Years 1 and 7 in the mean concentrations dust endotoxin, dust (1–3)-β-D-glucan, and ERMI as well as in the number of occupants and dogs were tested by paired t-tests. Pearson product moment correlations were performed to evaluate the associations between Year 1 and 7 values of dust endotoxin, (1–3)-β-D-glucan, and ERMI. Analysis of variance (ANOVA) and post-hoc t-tests were performed to examine differences in concentrations of microbial contaminants between pairs of mold categories.
Principal component analysis (PCA) and analysis of variance (ANOVA) were employed to combine the exposure information described by the levels of dust and air in home samples at Year 7 and the corresponding home mold category at Year 7. The ANOVA analyzed the values of first principal component (First PC) scores for three sets of exposure variables. The First PC score is a projection of the multidimensional coordinates of all exposure variables into one dimension, and explains the largest percent of the variability of the higher dimensional data set, than any other linear combination of exposure variables. For each set of exposure variables, the loadings which describe the First PC were multiplied by the values of exposure variables of each home to determine the First PC Score for the home. Least squares mean values and 95% confidence intervals were obtained from the ANOVA of First PC scores, classified by visible mold categories of homes. A mold category with a higher mean has a higher average First PC score, which translates to a higher average exposure level, compared to other categories for the same set of exposure variables. Three sets of exposure variables were analyzed: (i) dust endotoxin, dust (1–3)-β-D-glucan, and ERMI; (ii) air endotoxin, air beta (1–3)-β-D-glucan, and airborne fungal spores; (iii) both combined.
3. RESULTS
Tables 1 and 2 summarize mold exposure data for the 184 homes that were included in both Year 1 and Year 7 home assessments. The Visible Mold Damage Categories in Year 1 reflect good agreement with the target recruitment, which was 50% homes with no mold or water damage (Visible Damage Category 0) and 50% homes with some damage (Visible Damage Category 1 or 2) (Table 1). Even though 60% of the cohort had moved since the first evaluation, the number of homes in Visible Damage Category 2 at Year 1 (n=20) was about the same at Year 7 (n=19). There was, however, approximately twice the number of homes in Category 1 at Year 7 (n=127) than at Year 1 (n=65). The percents of homes that were classified in the same category at years 1 and 7 were 45% (82/184) for all homes and 38% (28/73) for homes of children that did not move. Weighted Kappa statistics showed slight agreement for classifications for all homes (Kappa= 0.14). Since the lower endpoint of the 95% confidence interval for kappa= 0.09 extended below zero, the data for homes of children who did not move are compatible with no agreement (Landis and Koch, 1977).
Table 1.
Evaluation of Visible Damage Categories of Homes at Years One and Seven. Kappa Statistics, 95% Confidence Interval, Interpretation of Kappa(a)
| Homes of all children (n=184) |
Homes of children that did not move (n=73) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year 7 | Year 7 | ||||||||
| 0 (no mold) |
1 (low mold) |
2 (high mold) |
0 (no mold) |
1 (low mold) |
2 (high mold) |
||||
| Year 1 | 0 (no mold) |
28 | 64 | 7 | 99 (53.8%) |
10 | 30 | 4 | 44 (60.3%) |
| 1 (low mold) |
7 | 50 | 8 | 65 (35.3%) |
1 | 17 | 6 | 24 (32.9%) |
|
| 2 (high mold) |
3 | 13 | 4 | 20 (10.9%) |
2 | 2 | 1 | 5 (6.8%) |
|
| 38 (20.7%) |
127 (69.0%) |
19 (10.3%) |
184 | 13 (17.8%) |
49 (67.1%) |
11 (15.1%) |
73 | ||
Kappa, 95% Confidence Interval, Interpretation of Kappa
Homes of All Children=0.14, [0.05, 0.24], Slight agreement
Homes of Children did not move=0.09, [−0.04, 0.22], no agreement
Table 2.
Geometric mean (GM), 95% Confidence Interval (95% CI) of results obtained for dust and air samples in Year 1 and Year 7 in all homes (N = 184).
| Exposure variable | Year 1 | Year 7 | P-values 1 testing the difference between means at Years 1 and 7 |
|||||
|---|---|---|---|---|---|---|---|---|
| n | GM | 95%CI | n | GM | 95%CI | All homes (n=184) |
Homes of children that did not move (n=74) |
|
| Dust Endotoxin (EU/mg) |
183 | 65.8 | (55.5, 78.1) | 184 | 161.3 | (134.5, 188.0) | <0.001 | <0.001 |
| Dust (1–3)-β-D- Glucan (µg/g) |
183 | 42.1 | (34.6, 51.1) | 184 | 146.2 | (124.2, 172.2) | <0.001 |
<0.001 |
| Dust ERMI2 | 181 | 2.6 | (1.5, 3.7) | 184 | 2.1 | (1.2, 3.1) | 0.53 | 0.78 |
| Air Endotoxin (EU/m3) |
N/A | 184 | 4.2 | (3.2, 5.3) | N/A | |||
| Air (1–3)-β-D-glucan (ng/m3) |
N/A | 184 | 1.5 | (1.2, 1.9) | N/A | |||
| Air Fungal Spores (#/m3) |
N/A | 183 | 330.8 | (269.1, 406.8) | N/A | |||
Statistically significant results are bolded
arithmetic mean
N/A not measured in Year 1
Table 2 presents geometric mean concentrations and 95% confidence intervals of dust and air samples at Years 1 and 7. Significantly higher levels were observed for dust endotoxin and dust (1–3)-β-D-glucan at Year 7 than Year 1, but there was no significant difference between ERMI mean values. Both the number of occupants and the number of dogs were significantly higher in Year 7 than in Year 1 (p < 0.001 for both).
When all the homes were included in the correlation analysis, statistically significant correlations between exposure values obtained in Year 1 and Year 7 were found for dust (1–3)-β-D-glucan and dust ERMI (Table 3). The respective correlation coefficients, however, were low: 0.16, and 0.28. When only homes where families had not moved (40%) were included, the correlation was stronger for ERMI (r=0.34). Although the rvalue remained about the same for dust (1–3)-β-D-glucan, the correlations lost significance due to smaller number of observations. No correlation was found for dust endotoxin values measured in Year 1 and Year 7.
Table 3.
Correlation coefficients and p-values1 (in parentheses) between data obtained in Year 1 and Year 7 homes for dust endotoxin, dust (1–3)-β-D-glucan and ERMI (total of 184 homes).
| Correlation between Year 1 and Year 7 | ||
|---|---|---|
| Exposure variable | All homes (n=184) | Homes of children that did not move (n=74) |
| Dust Endotoxin (EU/mg) |
0.05 (0.50) |
−0.07 (0.58) |
| Dust (1–3)-β-D-glucan (µg/g) |
0.16 (0.03) |
0.15 (0.21) |
| Dust ERMI |
0.28 (<0.001) |
0.34 (<0.01) |
Statistically significant results are bolded
Tables 4 and 5 present the correlations between the different microbial exposures measured in Year 1 and 7, respectively. In Year 1, no significant correlations were observed (Table 4), whereas in Year 7, significant correlations were found between all the other pairs, except the one between airborne endotoxin and dust (1–3)-β-D-glucan (Table 5). Strongest correlations were found between airborne endotoxin and airborne (1–3)-β-D-glucan (r = 0.50), between airborne (1–3)-β-D-glucan and airborne fungal spores (r = 0.46), and between airborne endotoxin and airborne fungal spores (r=0.42). Interestingly, the correlations between dust and air results for endotoxin and (1–3)-β-D-glucan, although statistically significant, was only moderate (r=0.34 for endotoxin 0.18 for (1–3)-β-D-glucan).
Table 4.
Correlation coefficients and p-values (in parentheses) for Year 1 exposure data for 184 homes.
| Exposure variable | Dust Endotoxin (EU/mg) |
Dust (1–3)-β-D-glucan (µg/g) |
Dust ERMI |
|---|---|---|---|
| Dust Endotoxin (EU/mg) |
1 | ||
| Dust (1–3)-β-D-glucan (µg/g) |
0.07 (0.35) |
1 | |
| Dust ERMI | 0.11 (0.15) |
−0.01 (0.87) |
1 |
Table 5.
Correlation coefficients and p-values1 (in parentheses) for Year 7 exposure data for 184 homes.
| Dust Endotoxin (EU/mg) |
Dust (1–3)-β-D- glucan (µg/g) |
Dust ERMI | Air Endotoxin (EU/m3) |
Air (1–3)-β-D- glucan (ng/m3) |
Air Fungal Spores (#/m3) |
|
|---|---|---|---|---|---|---|
| Dust Endotoxin (EU/mg) |
1 | |||||
| Dust (1–3)-β-D- glucan (µg/g) |
0.17 (0.02) |
1 |
||||
| Dust ERMI |
0.16 (0.03) |
0.22 (<0.01) |
1 | |||
| Air Endotoxin (EU/m3) |
0.34 (<0.001) |
0.02 (0.74) |
0.17 (0.02) |
1 | ||
| Air (1–3)-β-D-glucan (ng/m3) |
0.23 (<0.01) |
0.18 (0.01) |
0.16 (0.03) |
0.50 (<0.001) |
1 | |
| Air Fungal Spores (#/m3) |
0.21 (<0.01) |
0.27 (<0.001) |
0.31 (<0.001) |
0.42 (<0.001) |
0.46 (<0.001) |
1 |
Statistically significant results are bolded
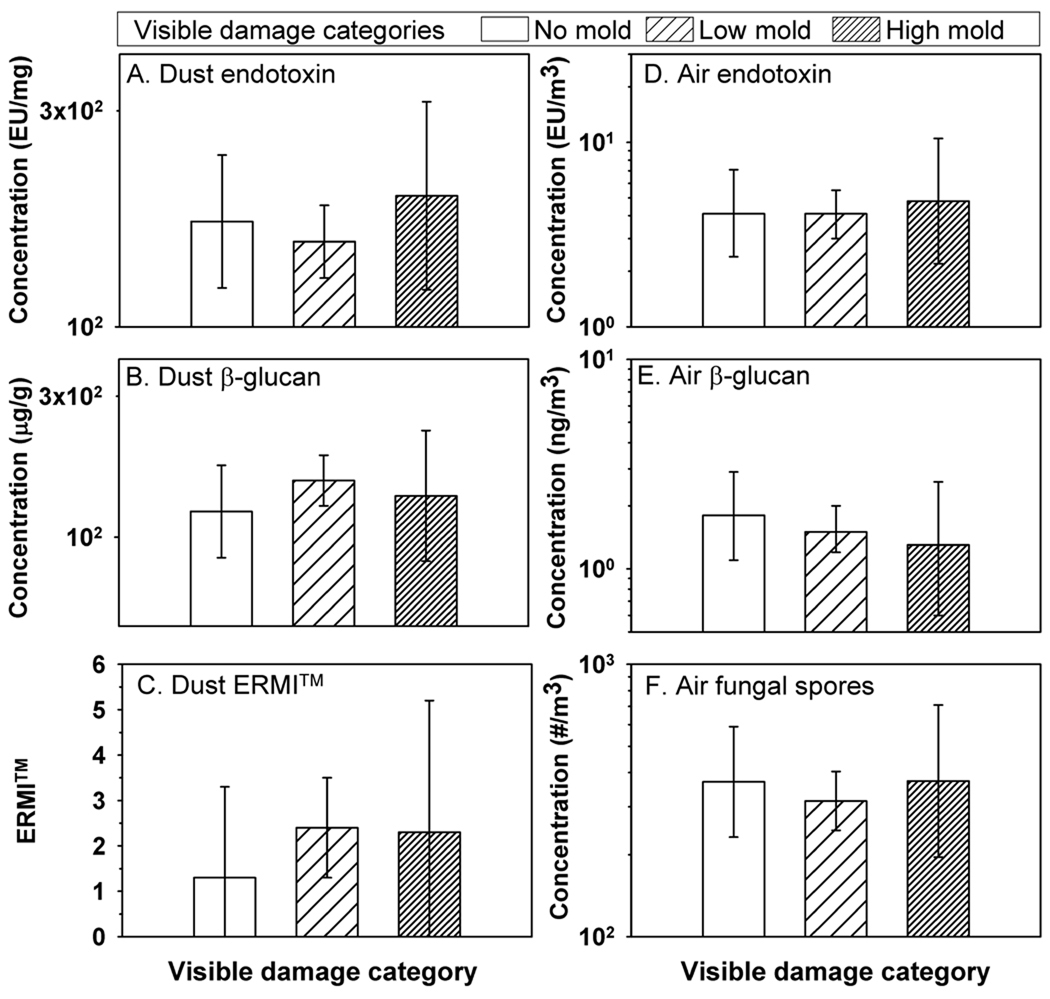
Year 7 data were investigated further by comparing the visual observation with the quantitative exposure values. Data based on the visual inspection showed that 10% of the homes were in the high mold category (Visible Mold Category 2) (Figure 1). No statistically significant differences or clear trends in the concentrations between the three Visible Mold Categories were found.
Figure 1.
Dust borne endotoxin, dust borne (1–3)-β-D-glucan, ERMI, airborne endotoxin, airborne (1–3)-β-D-glucan, and airborne fungal spores in homes divided into three levels of visible mold damage: no mold (n=38), low mold (n=127) and high mold n=19). Histograms show geometric means, except arithmetic mean for ERMI, and error bars show 95% confidence intervals.
Most of the homes (69%) belonged to Visible Mold Category 1. This category was divided into five subcategories: water damage history (n=27), water damage observed (n=42), low visible mold without moldy odor (n=39), moldy odor with low visible mold (n=6), and moldy odor without visible mold (n=13). It was found that all the microbial exposures were highest in homes that had moldy odor, but this difference was not significant when tested for the seven mold categories (Visible Damage Categories 0, 2 and five levels of Category 1 specified above). From the PCA analyses (Table 6), all of the three different combinations of exposure variables (both air and dust variables, dust only, air only) showed highest mean values for the two mold categories that included moldy odor. This finding indicates that homes that had moldy odor also had the highest concentrations of the measured microbial contaminants. The percents of variation explained by the First PC of exposure variables were 39%, 46% and 63% for air and dust, only dust, and only air variables, respectively. The highest % of explained variability was for the PCA that included air variables only, indicating that moldy odor may be more strongly associated with the concentrations of airborne contaminants than with the concentrations measured in dust samples. Table 7 shows the loadings associated with each exposure variable in sets of exposure variables included in the PCA analyses.
Table 6.
Means (95% confidence intervals) of First Principal Component Scores (first PC scores) for each subject’s home by mold level of home at year seven.
| Mold level | Visible Damage Category |
Homes per Mold Level (Total=184) |
Sets of Exposure Variables Combined to Obtain PC Score for Each Subject (1) |
||
|---|---|---|---|---|---|
| All variables2 | Dust variables3 | Air variables4 | |||
| Water Damage Observed |
1 | 42 | −0.13 (−0.59, 0.33) |
+0.16 (−0.20, 0.51) |
−0.26 (−0.67, 0.16) |
| Water Damage History | 1 | 27 | −0.28 (−0.86, 0.29) |
−0.31 (−0.75, 0.14) |
−0.09 (−0.61, 0.43) |
| No mold | 0 | 38 | −0.10 (−0.59, 0.39) |
−0.14 (−0.51, 0.24) |
−0.06 (−0.50, 0.39) |
| High mold | 2 | 19 | +0.09 (−0.59, 0.78) |
+0.08 (−0.44, 0.61) |
+0.04 (−0.58, 0.66) |
| Visible Only | 1 | 39 | −0.05 (−0.53, 0.42) |
−0.11 (−0.47, 0.26) |
+0.01 (−0.42, 0.44) |
| Moldy odor Only | 1 | 13 | +0.72 (−0.10, 1.55) |
+0.56 (−0.08, 1.19) |
+0.46 (−0.29, 1.21) |
| Low Visible Mold and Moldy Odor |
1 | 6 | +1.31 (0.09, 2.52) |
+0.36 (−0.58, 1.30) |
+1.37 (0.27, 2.47) |
| First PC % | 39% | 46% | 63% | ||
The First PC transforms sets of exposure variables into a single score. The First PC score is a one-dimensional summary of the variability in the set of exposure measurements, which is greater than any other transformation of exposure measurements. First PC scores were calculated for sets of exposure variables measured in each subject’s home, Mean values of PC scores were obtained by ANOVA, where the dependent variables were First PC Scores, and mold levels were the categories of analysis. A mold category with a higher mean has a higher average PC score, which translates to a higher average exposure level, compared to other categories in the same analysis.
Loge Dust Endotoxin, Loge Dust Beta-glucan, Loge Air Endotoxin, Loge Air Beta-glucan, Loge Airborne Spores and ERMI
Loge Dust Endotoxin, Loge Dust Beta-glucan, and ERMI
Loge Air Endotoxin, Loge Air Beta-glucan, Loge Airborne Spores
Table 7.
First Principal Component Score (First PC Score) 1 Loadings Corresponding to Exposure Variables in Each Set
| Sets of Exposure Variables Combined to Obtain First PC Score |
Loadings | ||
|---|---|---|---|
| All variables | Dust variables | Air variables | |
| Loge Dust Endotoxin | 0.35 | 0.53 | |
| Loge Dust Beta-Glucan | 0.28 | 0.61 | |
| Loge Air Endotoxin | 0.47 | 0.58 | |
| Loge Air Beta-Glucan | 0.48 | 0.60 | |
| Loge Airborne Spores | 0.49 | 0.56 | |
| ERMI Yr 7 | 0.33 | 0.59 | |
| First PC %2 | 39% | 46% | 63% |
The First PC Score is a transformation of sets of exposure variables into a single score. It is a projection of the multidimensional coordinates of the set of all exposure variables into one dimension. For each set of exposure variables, the loadings (shown above) may be multiplied by the values of exposure variables of each home to determine the First PC Score for the home. These values may be averaged for homes, classified by visible mold categories.
The First PC Score explains the largest percent of the variability of the higher dimensional data set, than any other linear combination of the exposure variables. The percents of variability explained by subsequent PC Scores decline, with the total variability explained by all PC Scores equaling 100%.
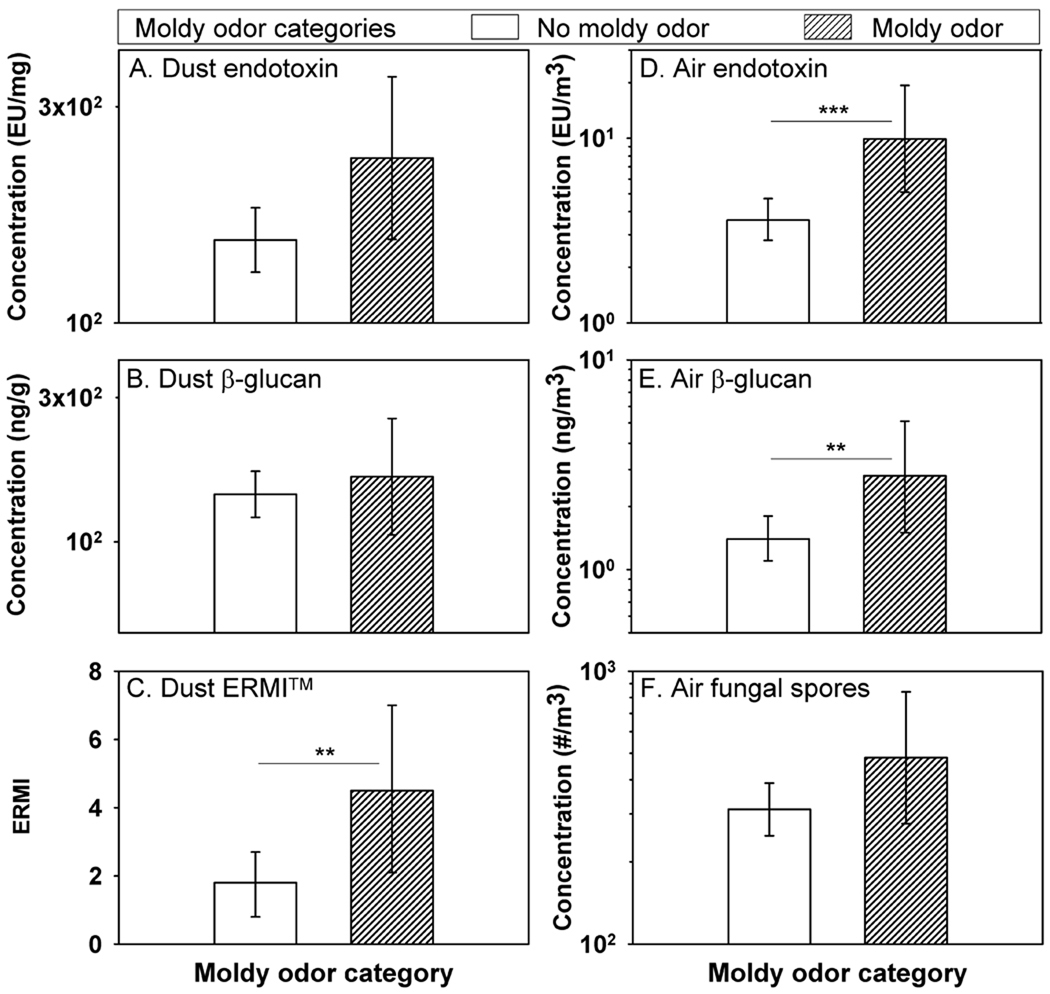
There were six homes in the original Visible Damage Category 2 that also had moldy odor. Thus, altogether 25 homes (14%) had moldy odor (with or without visible mold and water damage). When the homes were re-categorized into two groups based on moldy odor only (Moldy Odor Yes or No), all the results on dust and air sampling were higher in the homes that had moldy odor than in homes without moldy odor (Figure 2). This difference was statistically significant for ERMI-values and the airborne concentrations of endotoxin and (1–3)-β-D-glucan.
Figure 2.
Dust borne endotoxin, dust borne (1–3)-β-D-glucan, ERMI, airborne endotoxin, airborne (1–3)-β-D-glucan, and airborne fungal spores in homes divided into with two levels of moldy odor: with (n=25) and without moldy odor (n=159). Histograms show geometric means, except arithmetic mean for ERMI, and error bars show 95% confidence intervals. *** p < 0.001; ** p<0.01
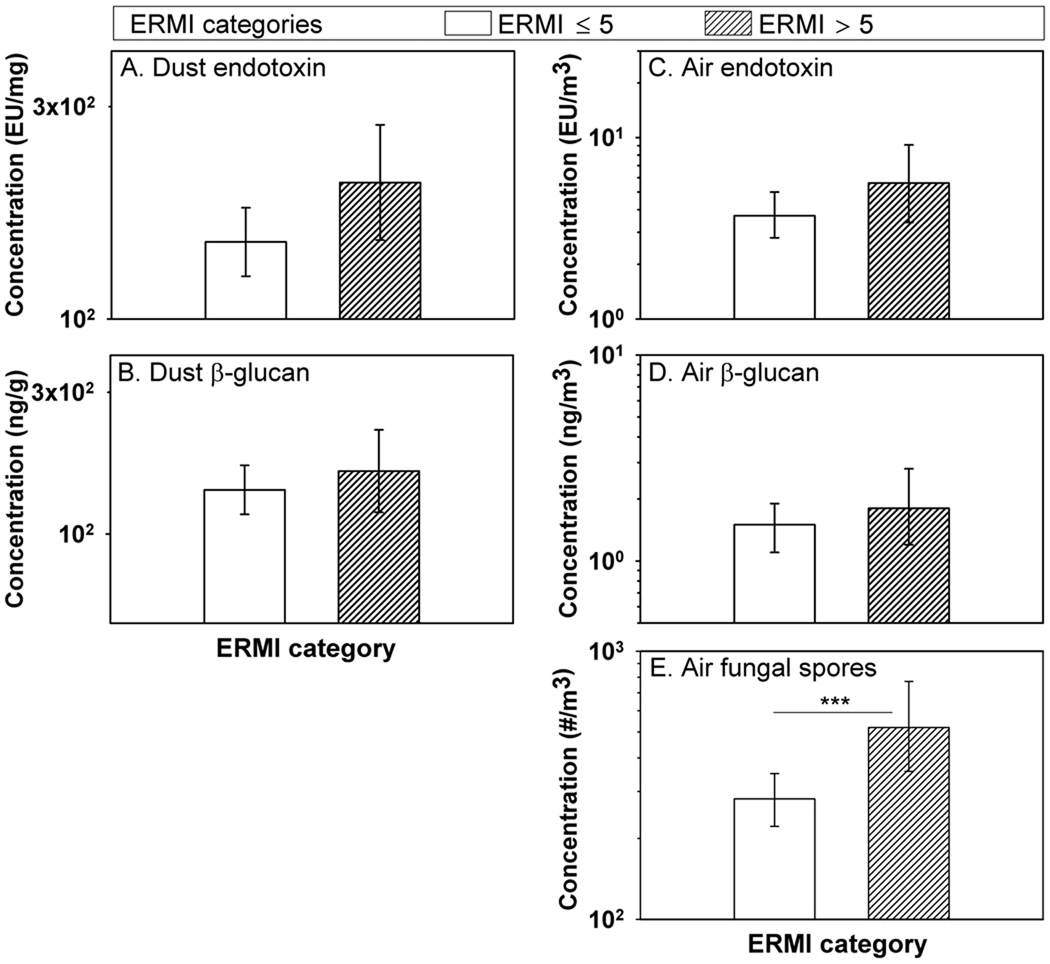
When an ERMI-index above five was used as the criterion, 26% of the homes belonged to the category of high mold burden (Figure 3). All the results from dust and air sampling were higher in the high ERMI category than in the low ERMI category, and this difference was statistically significant for airborne fungal spores.
Figure 3.
Dust borne endotoxin, dust borne (1–3)-β-D-glucan, airborne endotoxin, airborne (1–3)-β-D-glucan, and airborne fungal spores in homes divided into two levels of mold burden based on ERMI index: low (ERMI ≤5; n=135) and high (ERMI >5; n=48). Histograms show geometric means and error bars show 95% confidence intervals.
*** p < 0.001
4. DISCUSSION
Microbial concentrations measured in this study can be compared with those we have obtained in three previous studies using the same sampling and analysis methods (Reponen et al., 2007; Crawford et al., 2009; Adhikari et al., 2010). The geometric mean concentrations of endotoxin and (1–3)-β-D-glucan in the current study were about the same as those measured by our group in the three earlier studies. Fungal spore concentrations in the current study and in the study by Crawford et al. (2009) were clearly lower than in the studies which included severely mold contaminated homes (Reponen et al., 2007; Adhikari et al., 2010). Comparison to studies conducted by other research groups on endotoxin and (1–3)-β-D-glucan and fungal spore concentrations in dust and air samples is difficult due to differing extraction and analytical methods.
Exposure measured in Year 7 appears to be very different from the exposure in Year 1, even for families that did not move. The most stable exposures were measured for ERMI, which did not differ significantly between Years 1 and 7 and had the highest correlations between the results in Year 1 and 7. To our knowledge, the temporal variation of multiple microbial exposures has previously been investigated only in a few studies. Chew et al. (2001) measured fungal extracellular polysaccharides, (1–3)-β-D-glucans and culturable fungi in house dust in 23 Dutch homes six times during an eight-month period. They reported that the results from the first sampling were not significantly different from the other five for any of the measured fungal agents. Our results are in line with those reported by Matheson et al. (2003 and 2005) who did not find any correlation between ergosterol concentrations in dust or between concentrations of airborne fungal spores measured two years apart in 360 Australian homes.
Concentrations of dust endotoxin and (1–3)-β-D-glucan were found to be significantly higher in Year 7 than in Year 1, whereas ERMI did not differ between these two time points. Endotoxin is a measure of Gram-negative bacterial exposure, so its concentration in the dust is not necessarily associated with mold exposures. (1–3)-β-D-glucan is a cell wall component of fungi, but its content varies widely between different fungal species (Iossifova et al., 2008). ERMI is an index based on concentrations of 36 fungal species and is calculated by deducting the concentrations of common outdoor fungi from the concentrations of fungi that are common in water-damaged buildings (Vesper et al. 2007b). The increase in endotoxin and (1–3)-β-D-glucan may partially be explained by the higher number of occupants and dogs in Year 7 compared to Year 1. Presence of pets and increased number of occupants have been shown to increase dust endotoxin and (1–3)-β-D-glucan concentrations, partially because pets and people are one of the original sources for endotoxin in homes and partially because their activities can increase the outdoor-indoor transport of microbial contaminants originating from soil (Gehring et al., 2001; Bishof et al., 2002; Thorne et al., 2009). ERMI is designed so that the effect of common outdoor fungi is minimized. Therefore, it is not surprising that ERMI behaves differently than endotoxin and β-glucan.
When ERMI was used as the mold classification criterion, a larger fraction of homes belonged to the high mold category (26%), as opposed to the original Visible Mold Damage Criterion (11%) or the new 2-level criterion based on moldy odor (14%). This supports the previous findings that some of the mold contamination may be missed by the mere visual inspection (Haverinen-Shaughnessy et al., 2008). Various technical monitoring tools, such as moisture meters, and opening of structures can give additional information on the status of the moisture damage in a building (Haverinen-Shaughnessy et al., 2008). This finding is consistent with results of a national study of US homes which demonstrated that about 50% of the time neither the home occupants suspected nor the mold inspector detected the mold problem in high ERMI ( >5) homes (Vesper et al., 2009). Concentrations of endotoxin, (1–3)-β-D-glucan, and fungal spores showed inconsistent trends with the original Visible Mold Damage Categories; however, these concentrations were consistently higher in the high ERMI category compared to low ERMI. Furthermore, the PCA revealed that homes that had moldy odor also had the highest concentrations of the measured microbial contaminants. Even though moldy odor is common in water damaged buildings only a few studies have reported results on moldy odor. Roussel et al. (2008) reported that dampness odor was associated with increased concentration of airborne Aspergillus, Penicillium, and Cladosporium fungi. Cai et al. (2009) found higher total fungal DNA in dust in rooms that had reported dampness and odor, but did not find any association with observed dampness. Hägerhed-Engman et al. (2009) reported that odor (moldy/earthly/unpleasant/ stuffy odor) in the home was associated with asthma, rhinitis and eczema symptoms in children. Thus, moldy odor appears to be an important indicator for indoor air quality in homes.
Major moisture damage, such as the flooding in New Orleans after hurricanes Katrina and Rita has been shown to increase microbial concentrations in indoor air and dust samples (Solomon et al., 2006, Chew et al., 2006; Rao et al., 2007). This association is unclear in less severely damaged homes. Some studies have reported associations between visible mold damage and the concentration of airborne viable fungi and bacteria (Miller et al., 2000; Gent et al., 2002; Hyvärinen et al., 2006) and the concentrations of several microbial species in house dust determined by PCR (Lingnell et al., 2008). Borderline significant association have been reported between visible damage and the concentrations of (1–3)-β-D-glucan, endotoxin and 3-hydroxy fatty acids (chemical marker for gram-negative bacteria) in house dust (Douwes et al., 2006b; Hyvärinen et al. 2006; Bischof et al., 2002). Several studies, however, have found no association between visible mold and airborne or dustborne fungal levels (Ren et al., 2001; Müller et al., 2002; Chew et al. 2003; Stark et al., 2003). This lack of association partially explains why visible mold damage has been more clearly associated with adverse respiratory health effects than microbial concentrations and indicates that many traditional methods of measuring molds and other microorganisms are inadequate to detect mold contamination (WHO Europe, 2009).
Previous studies have shown varying levels of correlations between various microbial concentrations. Correlations have been reported between dust endotoxin, dust (1–3)-β-D-glucan, and culturable fungi in dust (Gehring et al., 2001; Chew et al., 2001; Douwes et al., 2006a,b; Park et al., 2006). Using the same method of long-term air sampling as in the current study, Lee et al. (2006) found a positive correlation (r=0.53) between airborne (1–3)-β-D-glucan and airborne fungal spore count. Similarly, Adhikari et al. (2010) reported positive correlations between endotoxin and (1–3)-β-D-glucan in dust (r=0.55) and in long-term air samples (r = 0.70). In the current study, among the correlations between the six different measures of microbial contamination, strongest were between the three airborne contaminants – endotoxin, (1–3)-β-D-glucan, and fungal spores – with correlation coefficients ranging from 0.42 to 0.50. The correlations between the three dust contaminants – endotoxin, (1–3)-β-D-glucan, and ERMI – were below 0.22. Weak correlations were also found between dust and air results for endotoxin and (1–3)-β-D-glucan (r < 0.34). Similar to our results, Park et al. (2000) and Chew et al., (2003) found only weak correlations between airborne and dustborne microbial concentrations. These results indicate that dust and air samples give different exposure information. A large portion of contaminants in dust are likely brought into the home through floor trafficking, and may never become aerosolized. On the other hand, dust sample may better reflect long-term exposure than the 24-hour air sampling. The strong correlation between the three different air contaminants observed in the current study is likely due to common factors that affect the aerosolization of these contaminants. For example human activity has been shown to increase fungal spore concentrations (Buttner and Stetzenbach, 1993) and is expected to re-suspend also endotoxin and (1–3)-β-D-glucan.
5. CONCLUSIONS
Our findings will aid in the future analysis on the association between asthma development in children and mold exposure obtained through the different assessment methods. This study shows very different levels of microbial exposures in Year 7 compared to Year 1. Therefore, it seems appropriate to differentiate the associations between the health outcomes and microbial exposures in early versus later in life. Moldy odor appears to be an important home characteristic that should be included in the health outcome assessment. Among the three different mold categorization methods (visible damage, moldy odor, ERMI), ERMI showed highest number of homes in the high mold category. This supports the previous findings that some of the mold contamination may be missed by mere inspection. Low correlation between results in air and dust samples indicates different types of potential microbial exposures from these two sources. On the other hand, the three measured airborne contaminants were highly correlated suggesting that common factors affect aerosolization. Future analysis will indicate which, if any, of the exposure values obtained with the different assessment methods are associated with the development of asthma or atopy.
ACKNOWLEDGEMENT
This study is supported by the US Department of Housing and Urban Development grant #OHLHH0162-07. The CCAAPS birth cohort study is supported by National Institute of Environmental Health Sciences (NIEHS) grant ES11170. We thank Osmic Enterprises, Inc. (Cincinnati, OH) for providing the OLFACT-RL and testing of the home assessment team.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adhikari A, Martuzevicius D, Reponen T, Grinshpun SA, Cho S, Sivasubramani S, et al. Performance of the Button Personal Inhalable Sampler for the measurement of outdoor aeroallergens. Atmos Environ. 2003;37:4723–4733. [Google Scholar]
- Adhikari A, Lewis JS, Reponen T, DeGrasse EC, Grimsley FF, Chew GL, et al. Exposure matrices of endotoxin, (1→3)-β-D-glucan, fungi, and dust mite allergens in flood-affected homes of New Orleans. Sci Total Environ. 2010 doi: 10.1016/j.scitotenv.2010.07.087. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antova T, Pattenden S, Brunekreef B, Heinrich J, Rudnai P, Forastiere F, et al. Exposure to indoor mold and children’s respiratory health in the PATY study. J Epidemiol Community Health. 2008;62:197–204. doi: 10.1136/jech.2007.065896. [DOI] [PubMed] [Google Scholar]
- Biagini JM, LeMasters G, Ryan PH, Levin L, Reponen T, Burkle J, Lockey J. Allergic rhinitis in early infancy. Is it environmental tobacco smoke or mold? Pediatr Allergy & Immunol. 2006;17:278–284. doi: 10.1111/j.1399-3038.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof W, Koch A, Gehring U, Fahlbusch B, Wichmann HE, Heinrich J. Predictors of high endotoxin concentrations in the settled dust of german homes. Indoor Air. 2002;12:2–9. doi: 10.1034/j.1600-0668.2002.120102.x. [DOI] [PubMed] [Google Scholar]
- Buttner MP, Stetzenbach LD. Monitoring airborne fungal spores in an experimental indoor environment to evaluate sampling methods and the effects of human activity on air sampling. Appl Environ Microbiol. 1993;59:219–226. doi: 10.1128/aem.59.1.219-226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo P, Kalra H, Levin L, Reponen T, Olds R, Lummus ZL, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol. 2006;118:1271–1278. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G-H, Bröms K, Mälarstig B, Zhao Z-H, Kim JL, Svärsdsudd K, et al. Quantitative PCR analysis of fungal DNA in Swedish day care centers and comparison with building charcateritcis and allergen levels. Indoor Air. 2009;19:392–400. doi: 10.1111/j.1600-0668.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- Campo P, Kalra HK, Levin L, Reponen T, Olds R, Lummus ZL, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy & Clin Immunol. 2006;118:1271–1278. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S-H, Reponen T, LeMasters G, Levin L, Huang J, Meklin T, et al. Mold damage in homes and wheezing in infants. Ann Allergy Asthma Immunol. 2006a;97:539–545. doi: 10.1016/S1081-1206(10)60947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S-H, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, et al. The effect of home characteristics on dust antigen concentrations and loads in homes. Sci Total Environ. 2006b;371:31–43. doi: 10.1016/j.scitotenv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew G, Douwes J, Doekes G, Higgins K, Strien R, Spithoven J, Brunekreef B. Fungal extracellular polysaccharides, β(1->3)-glucans and culturable fungi in repeated sampling of house dust. Indoor Air. 2001;11:171–178. doi: 10.1034/j.1600-0668.2001.011003171.x. [DOI] [PubMed] [Google Scholar]
- Chew GL, Rogers C, Burge HA, Muilenberg ML, Gold DR. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy. 2003;58:13–20. doi: 10.1034/j.1398-9995.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Chew GL, Wilson J, Rabito FA, Grimsley F, Iqbal S, Reponen T, et al. Mold and endotoxin levels in the aftermath of Hurricane Katrina: a pilot project of homes in New Orleans undergoing renovation. Environ Health Perspect. 2006;114:1883–1889. doi: 10.1289/ehp.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C, Reponen T, Lee T, Iossifova Y, Levin L, Adhikari A, Grinshpun SA. Temporal and spatial variation of indoor and outdoor airborne fungal spores, pollen, and (1→3)-β-D-glucan. Aerobiologia. 2009;25:147–158. [Google Scholar]
- Dales RE, Miller D. Residential fungal contamination and health: microbial cohabitants as covariates. Environ Health Perspect. 1999;107:481–483. doi: 10.1289/ehp.99107s3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales R, Miller D, Ruest K, Guay M, Judek S. Airborne endotoxin is associated with respiratory illness in the first 2 years of life. Environ Health Perspect. 2006;114:610–614. doi: 10.1289/ehp.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmage S, Bailey M, Raven J, Mitakakis T, Cheng A, Guest D, et al. Current Indoor Allergen Levels of Fungi and Cats, But Not House Dust Mites, Influence Allergy and Asthma in Adults with High Dust Mite Exposure. Am J Respir & Critical Care Med. 2001;164:65–71. doi: 10.1164/ajrccm.164.1.9911066. [DOI] [PubMed] [Google Scholar]
- Douwes J, van Strien R, Doekes G, Smit J, Kerkhof M, Gerritsen J, et al. Does early indoor microbial exposure reduce the risk of asthma? The Prevention and Incidence of Asthma and Mite Allergy birth cohort study. J Allergy Clin Immunol. 2006a;117:1067–1073. doi: 10.1016/j.jaci.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Douwes J, Siebers R, Wouters IM, Doekes G, Fitzharris P, Crane J. Endotoxin, (1→3)-β-d-glucans and fungal extra-cellular polysaccharides in New Zealand homes: a pilot study. Ann Agric Environ Med. 2006b;13:361–365. [PubMed] [Google Scholar]
- Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analyses of the assocation of respiratory health effects with dampness and mold in homes. Indoor Air. 2007;17:284–296. doi: 10.1111/j.1600-0668.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- Gehring U, Douwes J, Doekes G, Koch A, Bischof W, Fahlbusch B, et al. β(1→3)-Glucan in house dust of German homes: Housing characteristics, occupant behavior, and relations with endotoxins, allergens, and molds. Environ Health Perspect. 2001;109:139–144. doi: 10.1289/ehp.01109139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, Leaderer BP. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect. 2002;110:A781–A786. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings L, Bailey JM. Development of the OLFACT-RL; 28th Annual Meeting of the Association for Chemoreception Sciences; April 26–30, 2006; Sarasota, Florida. [Google Scholar]
- Haugland RA, Varma M, Wymer L, Vesper S. Quantitative PCR analysis of selected Aspergillus, Penicillium, and Paecilomyces species. Syst Appl Microbiol. 2004;27:198–210. doi: 10.1078/072320204322881826. [DOI] [PubMed] [Google Scholar]
- Haverinen-Shaughnessy U, Toivola M, Alm S, Putus T, Nevalainen A. Personal and microenvironmental concentrations of particles and microbial aerosol in relation to health symptoms among teachers. J Exp Sci & Environ Epidemiol. 2007;17:182–190. doi: 10.1038/sj.jes.7500494. [DOI] [PubMed] [Google Scholar]
- Haverinen-Shaughnessy U, Hyvärinen A, Putus T, Nevalainen A. Assessment of success of remediation: seven case studies of moisture damaged buildings. Sci Total Environ. 2008;399:19–27. doi: 10.1016/j.scitotenv.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Sebastian A, Pekkanen J, Larsson L, Korppi M, Putus T, Nevalainen A. Characterizing microbial exposure with ergosterol, 3-hydroxy fatty acids, and viable microbes in house dust: determinants and association with childhood asthma. Arch Environ & Occup Health. 2006;61:149–157. doi: 10.3200/AEOH.61.4.149-157. [DOI] [PubMed] [Google Scholar]
- Hägerhed-Engman L, Larsson M, Sundell J, Bornehag CG. Can we trust cross sectional studies when studying the risk of dampness for asthma and allergy; Proceedings of Healthy Buildings; Sept. 13–17, 2009; Syracuse NY. Paper 608. [Google Scholar]
- IOM (Institute of Medicine) Damp Indoor Spaces and Health. Washington, D.C: National Academic Press; 2004. [Google Scholar]
- Iossifova Y, Reponen T, Bernstein D, Levin L, Zeigler H, Kalra H, et al. House dust (1–3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62:504–513. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifova Y, Reponen T, Daines M, Levin L, Hershey GK. (2008) Comparison of two analytical methods for detecting (1–3)-β-D-glucan in pure fungal cultures and in home dust samples. Open Allergy J. 2008;1:26–34. [Google Scholar]
- Iossifova YY, Reponen T, Ryan PH, Levin L, Bernstein DI, Lockey JE, et al. Mold exposure during infancy as a strong predictor of potential asthma development. Ann Allergy, Asthma & Immunol. 2009;102:131–137. doi: 10.1016/S1081-1206(10)60243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lee T, Grinshpun SA, Kim K-Y, Iossifova Y, Adhikari A, Reponen T. Relationship between indoor and outdoor airborne fungal spores, pollen, and (1–3)-Β- D-glucan in homes without visible mold growth. Aerobiologia. 2006;22:227–236. [Google Scholar]
- LeMasters G, Wilson K, Levin L, Biagini J, Ryan P, Lockey J, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–511. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnell U, Meklin T, Rintala H, Hyvärinen A, Vepsälainen A, Pekkanen J, Nevalainen A. Evaluation of quantitative PCR and culture methods for detection of house dust fungi and streptomycetes in relation to moisture damage of the house. Lett App Microbiol. 2008;47:303–308. doi: 10.1111/j.1472-765x.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- Matheson MC, Dharmage SC, Forbes AB, Raven JM, Woods RK, Thieng FCK, et al. Residential characteristics predict changes in Der p 1, Fel d 1 and ergosterol but not fungi over time. Clin & Exp Allergy. 2003;33:1281–1288. doi: 10.1046/j.1365-2222.2003.01747.x. [DOI] [PubMed] [Google Scholar]
- Matheson MC, Abramson MJ, Dharmage SC, Forbes AB, Ravenz JM, Thienz FCK, Walters EH. Changes in indoor allergen and fungal levels predict changes in asthma activity among young adults. Clin & Exp Allergy. 2005;35:907–913. doi: 10.1111/j.1365-2222.2005.02272.x. [DOI] [PubMed] [Google Scholar]
- Meklin T, Haugland RA, Reponen T, Varma M, Lummus Z, Bernstein D, et al. Quantititive PCR analysis of house dust can reveal abnormal mold conditions. J Environ Monit. 2004;6:615–620. doi: 10.1039/b400250d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Haisley PD, Reinhardt JH. Air sampling results in relation to extent of fungal colonization of building materials in some water-damaged buildings. Indoor Air. 2000;10:146–151. doi: 10.1034/j.1600-0668.2000.010003146.x. [DOI] [PubMed] [Google Scholar]
- Müller A, Lehmann I, Seiffart A, Diez U, Wetzig H, Borte M, Herbarth O. Increased incidence of allergic sensitization and respiratory diseases due to mould exposure: Results of the Leipzig Allergy Risk children Study (LARS) Int J Hyg & Environ Health. 2002;204:363–365. doi: 10.1078/1438-4639-00110. [DOI] [PubMed] [Google Scholar]
- Osborne M, Reponen T, Adhikari A, Cho S-H, Grinshpun SA, Levin L, et al. Specific fungal exposures, allergic sensitization, and rhinitis in infants. Pediatric Allergy Immunol. 2006;17:450–457. doi: 10.1111/j.1399-3038.2006.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-H, Spiegelman DL, Burge HA, Gold DR, Chew GL, Milton DK. Longitudinal study of dust and airborne endotoxin in the home. Environ Health Perspect. 2000;108:1023–1028. doi: 10.1289/ehp.001081023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-H, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir & Crit Care Med. 2001;163:322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- Park J-H, Cox-Ganser J, Rao C, Kreiss K. Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air. 2006;16:193–203. doi: 10.1111/j.1600-0668.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- Pettigrew M, Gent JF, Triche EW, Belanger KD, Bracken MB, Leaderer BP. Association of early-onset otitis media in infants and exposure to household mould. Paediatr Perinat Epidemiol. 2004;18:441–447. doi: 10.1111/j.1365-3016.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- Rao CY, Riggs MA, Chew GL, Muilenberg ML, Thorne PS, Van Sickle D, et al. Characterization of airborne molds, endotoxins, and glucans in homes in New Orleans after Hurricanes Katrina and Rita. Appl Environ Microbiol. 2007;73:1630–1634. doi: 10.1128/AEM.01973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Jankun TM, Belanger K, Bracken MB, Leaderer BP. The relation between fungal propagules in indoor air and home characteristics. Allergy. 2001;56:419–424. doi: 10.1034/j.1398-9995.2001.056005419.x. [DOI] [PubMed] [Google Scholar]
- Reponen T, Seo S-C, Grimsley F, Lee T, Crawford C, Grinshpun SA. Fungal fragments in moldy houses: a field study in homes in New Orleans and Southern Ohio. Atmos Environ. 2007;41:8140–8149. doi: 10.1016/j.atmosenv.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum PF, Crawford JA, Anagnost SE, Wang CJK, Hunt A, Anbar RD. Indoor airborne fungi and wheeze in the first year of life among a cohort of infants at risk for asthma. J Exp Sci & Environ Epidemiol. 2009 doi: 10.1038/jes.2009.27. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Roussel S, Reboux G, Bellanger A-P, Sornin S, Grenouillet F, Dalphin JC, et al. Characteristics of dwellings contaminated by moulds. Environ Monit. 2008;10:724–729. doi: 10.1039/b718909e. [DOI] [PubMed] [Google Scholar]
- Salo PM, Arbes SJ, Jr, Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon GM, Hjelmroos-Koski M, Rotkin-Ellman M, Hammond SK. Airborne mold and endotoxin concentrations in New Orleans, Louisiana, after flooding, October through November 2005. Environ Health Perspect. 2006;114:1381–1386. doi: 10.1289/ehp.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark PC, Burge HA, Ryan LM, Milton DK, Gold DR. Fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am J Respirat & Crit Care Med. 2003;168:232–237. doi: 10.1164/rccm.200207-730OC. [DOI] [PubMed] [Google Scholar]
- Stark PC, Celedón JC, Chew GL, Ryan LM, Burge HA, Muilenberg ML, Gold DR. Fungal levels in the home and allergic rhinitis by 5 years of age. Environ Health Perspect. 2005;113:1405–1409. doi: 10.1289/ehp.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn J, Rylander R. Airways inflammation and glucan in a rowhouse area. Am J Crit Care Med. 1998;157:1798–1803. doi: 10.1164/ajrccm.157.6.9706081. [DOI] [PubMed] [Google Scholar]
- Thorne PS, Cohn RD, Mac D, Arbes SJ, Zeldin DC. Predictors of endotoxin in U.S. housing. Environ Health Perspect. 2009;117:763–771. doi: 10.1289/ehp.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper SJ, McKinstry C, Haugland RA, Iossifova Y, LeMasters G, Levin L, et al. EPA relative moldiness index© as predictor of childhood respiratory illness. J Exp Sci Environ Epidemiol. 2007a;17:88–94. doi: 10.1038/sj.jes.7500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper SJ, McKinstry C, Haugland RA, Wymer L, Ashley P, Cox D, et al. Development of an environmental relative moldiness index for homes in the U.S. J Occup and Environ Med. 2007b;49:829–833. doi: 10.1097/JOM.0b013e3181255e98. [DOI] [PubMed] [Google Scholar]
- Vesper S, McKinstry C, Cox D, Dewalt G. Correlation between ERMI values and other moisture and mold assessments of homes in the American Healthy Homes Survey. J Urban Health. 2009;86:850–860. doi: 10.1007/s11524-009-9384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Europe (World Health Organization Europe) Copenhagen, Denmark: World health Organization; WHO guidelines for indoor air quality: Dampness and mould. 2009 [PubMed]