Abstract
Nineteen male recipients of renal homografts were responsible for 23 pregnancies, resulting so far in 19 live births and one abortion; three additional wives have not yet been delivered of infants. Eighteen of the 19 infants were normal; the abnormal infant had a myelomeningocele and other anomalies. Eight female recipients have become pregnant ten times. Two of the pregnancies were terminated with therapeutic abortions, and two more are in progress. The other six resulted in live births. There were only two infants with a completely uncomplicated neonatal period. One premature baby died a few hours after birth from hyaline membrane disease. The other five survived, but one had pulmonary valvular stenosis, two had evidence of transient adrenocortical insufficiency plus lymphopenia, and one child had the respiratory distress syndrome. Renal function of three mothers underwent deterioration during pregnancy, but was restored after its termination
End-stage renal disease is often associated with loss of libido, amenorrhea in females, and impotence in males. A gratifying feature of successful renal transplantation is the restoration of sexual function and the opportunity of parenthood, should this be desired. The special questions to be asked under these circumstances are the following: What are the chances that the offspring of an immunosuppressed male or female parent will be normal? In the case of females, is there a risk to life or homograft viability with maternity?
Thus far, there have been very few reports of cases of parenthood in renal transplant recipients. In 1963, Murray et al1 described two successful pregnancies in a patient who had been given a kidney isograft from her identical twin sister. Three pregnancies occurred in the University of Virginia series, all in recipients of kidney homografts from related living donors. Of these, one ended in a spontaneous abortion.2,3 Further successful pregnancies were reported by Kaufman et al4 and by Merkatz and Stenzel.5 Caplan et al6 have reported delivery of a normal full-term infant by a cadaver renal recipient.
The present communication will attempt to expand this experience by describing 33 pregnancies involving eight female and 19 male renal transplant recipients treated at the Colorado General Hospital and the Denver Veterans Administration Hospital. Twenty-five of these pregnancies have already resulted in live births, five more are still in the gestational process, and the other three have ended in abortions (two elective and one spontaneous).
Methods
Between March 1962 and March 1970, a total of 292 patients were treated with renal homotransplantation, with the use of well-established surgical and immunosuppressive techniques.7,8 In almost all instances, the diseased native kidneys as well as the spleen were removed at the time of transplantation. The most common indications for operation were glomerulonephritis and pyelonephritis; these diseases accounted for approximately 90% and 5%, respectively, of the total case load. Two hundred of the recipients were male, and 92 were female. Excluding 69 patients who died within six months of transplantation, 31 children who are still prepubertal or young teen-agers, two postmenopausal women, and one woman who had tubal ligation, there was a total of 189 patients theoretically capable of having children (129 male and 60 female).
Because of uncertainties about their life expectancy, continuing renal function, and the prospects of having normal children, many patients sought advice about family planning. Unless there was a strong desire to have children, they were encouraged to practice contraception. This general policy probably reduced the number of babies that would have been produced without such conseling. In spite of the high incidence of family planning, pregnancy was totally unforeseen in a few instances, since some of the patients were under the mistaken impression that they could not have children.
Eventually, pregnancies were contributed to by 27 recipients: 19 male and eight female. Twelve of these 27 patients received their kidneys from their parents, 11 from siblings, one from an identical twin brother, one from an aunt, and two from unrelated donors (one volunteer and one cadaver). In each instance, the homograft was placed extraperitoneally in the iliac fossa. In the women, five of the transplants were on the right side and three were on the left; urinary reconstruction of three was with ureteroneocystostomy and that of five was with ureteroostomy.9
Except for the recipient who received a graft from an identical twin, all patients had immunosuppression with azathioprine and prednisone. Fifteen of the recipients had received additional treatment with heterologous antilymphocyte globulin which had been discontinued after the first four months, from 4.5 to 23 months before conception. In the female recipients, no significant changes in immunosuppression were made during pregnancy or post partum, except that each patient received supplemental hydrocortisone intravenously during labor or at the time of therapeutic abortion.
Standard renal function tests, including determinations of blood urea nitrogen levels, creatinine levels, and creatinine clearance, were performed frequently. The urine was analyzed for specific gravity, 24-hour protein content, and electrolyte levels, and in addition, microscopic examinations were performed and cultures were obtained. The patients’ blood pressure and body weight were also recorded at regular intervals.
At the time four of the female recipients were delivered, the cord blood, maternal blood, colostrum, breast milk were examined for 6-mercaptopurine, a pharmacologically active metabolite of azathioprine. The isotope dilution technique used was capable of detecting levels as low as 0.5 μg/ml.
Results
Male Recipients
Nineteen of 129 male recipients were responsible for 23 pregnancies (Table 1). These resulted in live births in 19 cases. In three more cases, the wives have not yet been delivered. There was one spontaneous abortion.
Table 1.
Parenthood in Kidney Homograft Recipients
| No. of Parents | No. of Pregnancies | No. of Abortions | No. of Current Pregnancies | No. of Live Births | No. of Children With Congenital Defects | |
|---|---|---|---|---|---|---|
| Fathers | 19 | 23 | 1 | 3 | 19 | 1 |
| Mothers | 8 | 10 | 2* | 2 | 6 | 1 |
| Total | 27 | 33 | 3 | 5 | 25 | 2 |
Both abortions were therapeutic.
Female Recipients
Eight of the 60 females had ten pregnancies (Tables 1 and 2). Six of these pregnancies ended in live births. Two patients are currently pregnant, and two others underwent therapeutic abortions. All patients were nulliparous, although one had had a spontaneous abortion two years prior to receipt of the kidney (case 5). Before transplantation, the patients were either amenorrheic for intervals of up to two years or had infrequent episodes of vaginal bleeding every three to six months. Following operation, menses reappeared after 3 to 12 months (mean, 6 months). The ten pregnancies occurred from 8.5 to 59 months after transplantation, with a mean of 30.8 months. The daily prednisone doses at the onset of and throughout pregnancy were 0 to 27.5 mg. The follow-up period after the termination of each pregnancy is now two weeks to 30 months (mean, 14 months)
Table 2.
Female Patients Who Became Pregnant After Renal Transplantation*
| No. of Case | Age, yr, at Pregnancy | Renal Donor | Onset of Pregnancy After Transplantation, mo | Prednisone Dose During Pregnancy, mg/Day† | Duration of Pregnancy, Weeks |
|---|---|---|---|---|---|
| 1 | 21 | Father | 31 | 10 | 39 |
| 2a | 20 | Mother | 23 | 10 | 37 |
| b | 23 | 59 | 10 | 8 (still pregnant) | |
| 3 | 24 | Cadaver | 8.5 | 27.5 | 12 (therapeutic abortion) |
| 4a | 23 | Brother | 19.5 | 10 | 33 |
| b | 24 | 32 | 15 | 34 | |
| 5 | 23 | Mother | 28 | 15 | 29 |
| 6 | 23 | Mother | 46.5 | 0 | 41 |
| 7 | 25 | Sister | 48 | 5 | 20 (still pregnant) |
| 8 | 29 | Mother | 12 | 17.5 | 8 (therapeutic abortion |
The original renal disease was chronic glomerulonephritis in all but case 2, in which the diagnosis was left renal aplasia and right pyelonephritis.
Patient weights at onset of pregnancies were 40.1 to 76.5 kg (88.4 to 168.6 Ib).
Conduct of Labor
Premature rupture of the membranes in one patient (case 4b) was managed by induction of labor with oxytocin. The other five labors were quite uncomplicated; normal vaginal deliveries were performed with the patients under local anesthesia, with or without low forceps assistance.
Pyelographic Studies
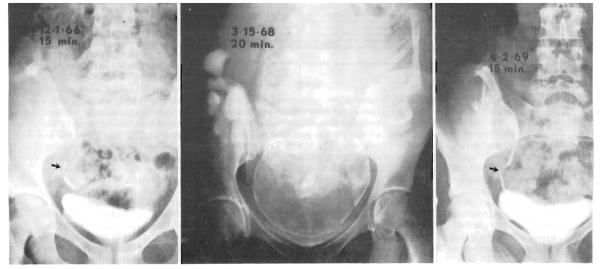
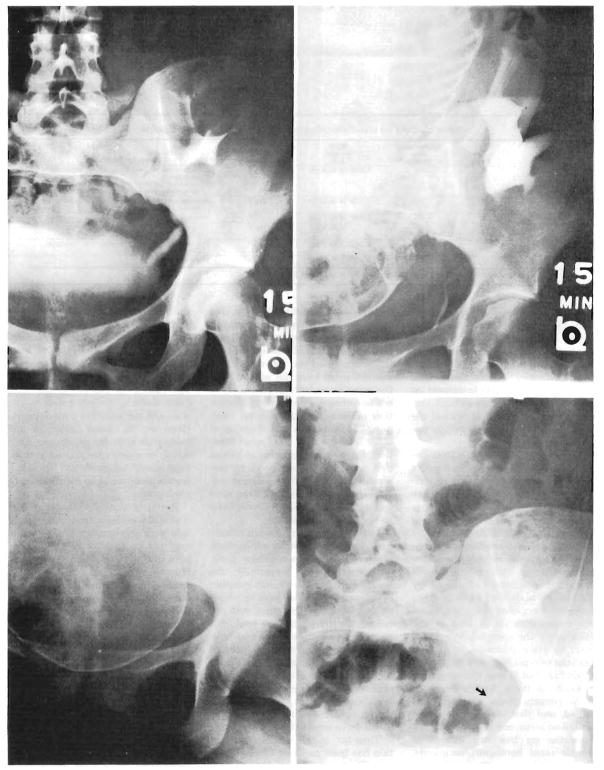
During five of the six pregnancies that resulted in live births, intravenous pyelograms were obtained. In two patients who were coincidentally being studied with pelvimetry, pyelography was performed during labor. Late in the pregnancies, pyelocaliectasis was seen, similar to that expected during the gestation of normal mothers (Fig 1 and 2). Compression of the pelvic ureter by the infant head was not evident during labor after urinary tract reconstruction with either ureteroureterostomy (Fig 1) or ureteroneocystostomy (Fig 2). Following delivery, the pyelocaliectasis regressed to the preexisting state.
Fig. 1.
Intravenous pyelograms. Left, Seventeen months after renal homotransplantation and seven months before pregnancy. Note ureteroureterostomy (arrow). Center, During labor. Pyelogram was obtained in the course of pelvimetry. Right, Thirteen months post partum. Appearance is now similar to before pregnancy (left).
Fig. 2.
Intravenous pyelograms. Upper left, Three years after transplantation. Urinary reconstruction was with ureteroneocystostomy. Upper right, Four and a half years after transplantation, 3½ weeks before childbirth. Lower left, During labor. Lower right, Fourteen months post partum. Because of peristalsis, distal ureter (arrow) appears smaller than before pregnancy (upper left).
Renal Function
The function of the renal homografts before the ten pregnancies in eight women ranged from essentially normal to markedly impaired (Table 3). The most severe preexisting renal functional defects were in the patient in case 5; this patient had mild azotemia and a creatinine clearance of only 24 ml/min prior to conception.
Table 3.
Laboratory Data Before, During, and After Pregnancy in Female Renal Homograft Recipients
| No. of Case | Studies* | Data |
Length of Follow-Up | ||
|---|---|---|---|---|---|
| Before | During | After | |||
| 1 | BUN | 24 | 20 | 27 | 6 mo after delivery |
| Creatinine Clearance | 77 | 88 | 72 | ||
| Albuminuria | trace | trace | trace | ||
| Blood Pressure | 114/80 | 112/82 | 98/50 | ||
| 2a | BUN | 9 | 9 | 12 | 30 mo after first delivery |
| Creatinine Clearance | 60 | 71 | 64 | ||
| Albuminuria | trace | trace | trace | ||
| Blood Pressure | 116/78 | 128/80 | 110/82 | ||
| 2b | BUN | 12 | 10 | (2 mo pregnant) | |
| Creatinine Clearance | 64 | 82 | |||
| Albuminuria | trace | trace | |||
| Blood Pressure | 110/82 | 120/68 | |||
| 3 | BUN | 26 | 16 | 11 | 4 ½ mo after abortion |
| Creatinine Clearance | 109 | 108 | 125 | ||
| Albuminuria | 1.4 | >6.0 | 1.5 | ||
| Blood Pressure | 116/92 | 138/110 | 136/108 (122/90) | ||
| 4a | BUN | 9 | 9 | 11 | 19 mo after first delivery |
| Creatinine Clearance | 97 | 114 | 85 | ||
| Albuminuria | trace | trace | trace | ||
| Blood Pressure | 104/82 | 110/78 | 110/80 | ||
| 4b | BUN | 11 | 9 | 12 | 5 mo after second delivery |
| Creatinine Clearance | 85 | 97 | 83 | ||
| Albuminuria | trace | trace | trace | ||
| Blood Pressure | 110/80 | 108/74 | 100/70 | ||
| 5 | BUN | 44 | 37 | 51 | 20 mo after delivery |
| Creatinine Clearance | 24 | 25 | 20 | ||
| Albuminuria | 1.4 | 10.8 | 2.4 | ||
| Blood Pressure | 110/78 | 140/90 | 142/100 (130/82) | ||
| 6 | BUN | 15 | 16 | 17 | 27 mo after delivery |
| Creatinine Clearance | 92 | 102 | 87 | ||
| Albuminuria | 0.4 | 0.6 | trace | ||
| Blood Pressure | 134/96 | 142/108 | 128/100 (120/84) | ||
| 7 | BUN | 20 | 16 | (5 mo pregnant) | |
| Creatinine Clearance | 66 | 64 | |||
| Albuminuria | trace | trace | |||
| Blood Pressure | 122/96 | 120/70 | |||
| 8 | BUN | 39 | 33 | (too recent) | 2 weeks after abortion |
| Creatinine Clearance | 49 | 41 | |||
| Albuminuria | 3.2 | 3.9 | |||
| Blood Pressure | 140/100 | 122/90 | |||
The BUN and creatinine clearance figures are average values for six months before pregnancy, during pregnancy, and in entire postpartum course. The albuminuria and blood pressure figures are maximum values for respective intervals. Included in parentheses are current blood pressure readings of those patients who were initially hypertensive post partum. BUN is given in mg/100 ml; creatinine clearance, ml/min; albuminuria, gm/24 hr; and blood pressure, mm Hg.
The course during pregnancy was variable. Five of the eight patients did not have any significant alterations in either renal function or blood pressure in the gestational period, nor did such changes evolve after termination of their pregnancies. In contrast, the other three developed hypertension severe enough to treat with medications (cases 3, 5, and 6) and in two of these three patients, there was significant albuminuria (cases 3 and 5). One of the latter pregnancies (case 3) was terminated with a therapeutic abortion. The other two women (cases 5 and 6) were delivered of live babies, although the child in case 5 was premature and did not survive. After abortion or delivery, the abnormalities receded (Table 3).
The primary indication for the two abortions was the desire of the patients to avoid childbearing. The decisions to proceed were made easier by the presence of abnormalities of renal function that either preceded pregnancy or developed with it (cases 3 and 8), as well as by the emotional instability of both women.
The lnfants
Paternity
Eighteen of the 19 babies were normal. The abnormal baby was born with a large myelomeningocele, bilateral dislocated hips, and bilateral talipes equinovarus. The mother of the infant apparently had an uncomplicated pregnancy and had not taken any medications. This case has been reported by Tallent et al.10
Maternity
Only two of the six live-born offspring (Table 4) had a completely uncomplicated neonatal period (cases 4a and 6). One of the other four (case 5) was markedly premature and died a few hours after delivery of pulmonary hyaline membrane disease. An additional infant (case 4b) also had pneumonitis and respiratory distress after birth, but recovered.
Table 4.
Data on Live-Born Offspring From Female Kidney Recipients
| No. of Case | Sex | Gestational Age, Weeks | Birth Weight, gm | Classification* | Neonatal Problems |
|---|---|---|---|---|---|
| 1 | M | 39 | 2,870 (6 lb 5 oz) | AGA | Adrenocortical insufficiency, lymphopenia; recovered. Congenital pulmonary artery stenosis; surgically corrected at 4 months. |
| 2 | F | 37 | 2,100 (4 lb 10 oz) | SGA | Adrenocortical insufficiency, lymphopenia, shock; recovered. |
| 4a | M | 33 | 1,920 (4 lb 4 oz) | AGA | Normal |
| 4b | M | 34 | 1,910 (4 lb 4 oz) | AGA | Respiratory distress syndrome and pneumonia; recovered. |
| 5 | F | 29 | 1,040 (2 lb 5 oz) | AGA | Prematurity, hyaline membrane disease; died at 8 hours. |
| 6 | F | 41 | 3,500 (7 lb 15 oz) | AGA | Normal |
AGA signifies size for gestational age; SGA, small for gestational age.
Both of the other infants had evidence of adrenocortical insufficiency and lymphopenia. The most extreme example was in case 2. At birth on March 15, 1968, the baby was small for her gestational age, but otherwise vigorous and apparently healthy. The white blood cell count (WBC) was 9,500/cu mm, but with a lymphocyte count of 760 cells per cubic millimeter. The serum sodium and potassium concentrations were 127 and 6.0 mEq/liter, respectively, approximately 60 hours later. The significance of these findings was not appreciated until March 20, when the baby became lethargic, mottled, and hypotensive. Repeated WBC was 8,100/cu mm, with a lymphocyte count of 558/cu mm. An x-ray film was interpreted as not showing a thymic shadow. It was suspected that the infant had a state analogous to that of congenital thymic aplasia. Therapy was instituted with hydrocortisone, gamma globulin, antibiotics, and the appropriate electrolyte solutions. There was marked improvement, and one, two, and ten days after the crisis, the total WBCs were 6,600, 6,600, and 10,000 per cubic millimeter, respectively, with lymphocyte counts of 2,600, 3,100, and 4,100 per cubic millimeter, respectively.
The baby in case 1 developed a similar syndrome, including the same electrolyte and white blood cell abnormalities. Treatment was instituted within the first day of life, and there was prompt recovery. A high-grade pulmonary valvular stenosis was also diagnosed in this child and corrected surgically four months later.
Special Studies
Gross and microscopic examinations of the placentas showed no abnormalities. Study of the cord blood, maternal blood, colostrum, and milk showed no detectable levels of 6-mercaptopurine. On the basis of the latter findings, it is planned to permit future mothers to breast-feed their infants.
Comment
There are special implications of parenthood by immunosuppressed graft recipients. One which probably applies to both paternity and maternity is the possibility that the agents used to prevent rejection may contribute to fetal defects. At our institution, the studies by Githens and Rosenkrantz and their associa tes11,12 showed that a high incidence of skeletal and central nervous system anomalies could be produced by giving large doses (4 to 30 mg/kg of body weight) of azathioprine to female mice during early pregnancy. Because this agent causes chromosome aberrations,13 it might be assumed that sperm could be affected and that males under treatment could, therefore, also contribute to fetal abnormalities, even though Rosenkrantz et al could not confirm the hypothesis.12 By the same logic, it would be reasonable to suspect an adverse role of adrenocortical steroids, since they lead in animal experiments to fetal anomalies14 by unknown mechanisms.
In spite of the formidable theoretical reasons to fear complications of parenthood, there is now enough evidence to indicate that the risks are not exorbitant, particularly for paternity. In our own experience with 19 pregnancies brought to term by wives of male transplantation patients, there has been only one anomalous offspring. That child is the one described in a report of a case by Tallent et al.10 The authors quite properly speculated about the role of the transplantation setting in the etiology of the complication. However, to place this case in a more realistic perspective, it is important to realize that it was the only such example in the Colorado series of male parents from which it was culled, and that, as such, it represented an incidence of 5.3%.
In the six live-born infants of female renal homograft recipients, there was also an example of a congenital anomaly; this was a pulmonary valve stenosis that required surgical correction at four months. Although the other five babies did not have such structural defects, this did not assure newborn vigor or even viability, since there was one death in the group. It is of interest that the infant that died was premature and was delivered from the woman with the worst homograft. Prematurity is not uncommon with nontransplantation patients with poor renal function.15 However, in the offspring of other mothers with good renal function, there were examples of babies that were small or subject to respiratory complications. These unfavorable conditions have been seen in infants whose mothers have been receiving steroid therapy.16
In addition, two of the six infants had a complication to which both azathioprine and prednisone probably contributed. Neonatally, the babies had few peripheral lymphocytes, and in the one case in which an x-ray film of the chest was obtained, the thymic shadow could not be seen. In addition, there was both clinical and laboratory evidence of adrenocortical insufficiency. Fortunately, the features of this syndrome regressed spontaneously within a few days. However, failure to take the proper therapeutic measures might have resulted in death.
Thus far, in this comment, attention has been confined to the infant risk. There is probably also a higher-than-normal hazard to prospective mothers whose good health is dependent upon continuing function of their transplants. Conceivably, the heterotopic location of the homograft could be responsible for mechanical dystocia, but only under conditions that have not yet been encountered. With the standard technique for renal transplantation, the kidney is placed in the iliac fossa7 outside the true pelvis, where it should not interfere with delivery. However, in some instances the homograft may lie within the birth canal, in which case special precautions would be necessary. This dangerous state could be identified with pyelograms.
It may be conceded that maternal morbidity is slight from mechanical injury to the homograft. The same may not be true for other kinds of homograft deterioration. Moore and Hume3 and Caplan et al6 recorded cases in which homograft function became impaired during late gestation and did not recover afterward. It could be argued that the renal damage in these patients was the consequence of coincidental chronic rejection, transmission glomerulonephritis, or other factors having nothing to do with the pregnancy. In our own observations, this explanation did not seem to pertain. There were three women who had worsening of function during pregnancy with return of function to the preexisting state after termination of pregnancy, suggesting that the gestational process was in some way responsible for the initial adverse changes.
In caring for a large number of long-term survivors after renal homotransplantation, we are often asked to provide opinions about the advisability of having children. On the basis of the studies of both males and females described in the present communication, some practical guidelines have evolved. Men are told that the prospects of having normal children are excellent and that the major consideration should be that their own long-term prognosis and consequent prospects of raising the offspring are still not known. Women are told approximately the same thing concerning the problems of anomalies and of their own life expectancy. However, it is also emphasized that risks in neonatal life are greater to the child and that, furthermore, there may be a slight intrinsic hazard to the homograft during gestation.
Acknowledgments
This investigation was supported by Public Health Service grants AM-12148. AI-AM-08898, AM-07772, AI-04152, RR-00051, RR-00069, and HE-09110.
Gertrude B. Elion, PhD, performed the 6-mercaptopurine studies.
Footnotes
Nonproprietary and Trade Names of Drugs
Azathioprine–Imuran.
Oxytocin–Pitocin, Syntocinon, Uteracon.
References
- 1.Murray JE, Reid DE, Harrison JH, et al. Successful pregnancies after human renal transplantation. New Eng J Med. 1963;269:341–343. doi: 10.1056/NEJM196308152690704. [DOI] [PubMed] [Google Scholar]
- 2.Board JA, Lee HM, Draper DA, et al. Pregnancy following kidney homotransplantation from a non-twin: Report of a case with concurrent administration of azathioprine and prednisone. Obstet Gynec. 1967;29:318–322. [PubMed] [Google Scholar]
- 3.Moore TC, Hume DM. The period and nature of hazard in clinical renal transplantation: 2. The hazard to transplant kidney function. Ann Surg. 1969;170:12–24. doi: 10.1097/00000658-196907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman JJ, Dignam W, Goodwin WE, et al. Successful normal childbirth after kidney homotransplantation. JAMA. 1967;200:338–341. [PubMed] [Google Scholar]
- 5.Merkatz IR, Stenzel KH. Obstetrical and gynecological management of renal transplant patients, abstracted. Int J Gynaec Obstet. 1970;8 [Google Scholar]
- 6.Caplan RM, Dossetor JB, Maughan GB. Pregnancy following cadaver kidney homotransplantation. Amer J Obstet Gynec. 1970;106:644–648. doi: 10.1016/0002-9378(70)90382-0. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE. Experience in Renal Transplantation. Philadelphia: WB Saunders Co; 1964. pp. 84–110.pp. 126–141. [Google Scholar]
- 8.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: WB Saunders Co; 1969. pp. 242–276. [Google Scholar]
- 9.Starzl TE, Groth CG, Putnam CW, et al. Urological complications in 216 human recipients of renal transplants. Ann Surg. 1970;172:1–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Tallent MB, Simmons RL, Najarian JS. Birth defects in child of male recipient of kidney transplant. JAMA. 1970;211:1854. [PubMed] [Google Scholar]
- 11.Githens JH, Rosenkrantz JG, Tunnock SM. Teratogenic effects of azathioprine (Imuran) J Pediat. 1965;66:959–961. doi: 10.1016/s0022-3476(65)80071-3. [DOI] [PubMed] [Google Scholar]
- 12.Rosenkrantz JG, Githens JH, Cox SM, et al. Azathioprine (Imuran) and pregnancy. Amer J Obstet Gynec. 1967;97:387–394. doi: 10.1016/0002-9378(67)90503-0. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MK. Chromosome studies in patients treated with azathioprine and Amethopterin. Acta Med Scand. 1967;182:445–451. doi: 10.1111/j.0954-6820.1967.tb10869.x. [DOI] [PubMed] [Google Scholar]
- 14.Bongiovanni AM, McPadden AJ. Steroids during pregnancy and possible fetal consequences. Fertil Steril. 1960;11:181–186. doi: 10.1016/s0015-0282(16)33723-2. [DOI] [PubMed] [Google Scholar]
- 15.Felding CF. Obstetric aspects in women with histories of renal disease. Acta Obstet Gynec Scand. 1969;48 (suppl 2):1–43. doi: 10.3109/00016346909156679. [DOI] [PubMed] [Google Scholar]
- 16.Warrell DW, Taylor R. Outcome for the foetus of mothers receiving prednisone during pregnancy. Lancet. 1968;1:117–118. doi: 10.1016/s0140-6736(68)92723-2. [DOI] [PubMed] [Google Scholar]