Abstract
Posterior capsule opacification (PCO) or secondary cataract formation, following intraocular lens implantation, is a significant complication affecting an estimated 28% of cataract patients. Matrix metalloproteinases (MMPs) have been demonstrated to play a role in the formation of anterior subcapsular cataracts and it has been shown that the presence of MMP inhibitors (MMPI) decreases subcapsular cataract formation ex vivo. Since the mechanisms responsible for anterior subcapsular cataract formation and posterior capsule opacification are similar, it is reasonable to suggest that MMP inhibitors may also mitigate PCO. One of the most effective ways of delivering the inhibitors may be from the implanted intraocular lens (IOL) material itself. In the current work, delivery of three different MMP inhibitors from silicone rubber as a model IOL material was examined. Loading methods were developed which allowed continuous release of active MMPI for periods of over 5 months in some cases. Reduced migration rates were observed in human lens epithelial cells in vitro, suggesting that an effect on PCO may be possible. While further studies are necessary to tune the systems to achieve the desired rates of release, this work demonstrates that delivery of MMPI from silicone IOL materials has the potential to decrease the incidence of PCO.
Keywords: Matrix metalloproteinase inhibitor, Polydimethylsiloxane, Posterior capsule opacification, Controlled drug release, Lens epithelial cells, Migration
1. Introduction
Intraocular lenses (IOLs) have been used successfully for over 50 years to replace the cataractous human lens. However, the frequent occurrence of secondary cataracts (posterior capsule opacification, PCO) requiring subsequent treatment, recently estimated at 28% within 5 years after the surgery, with rates as high as 70% and 78% reported in young (<40 years old) and pediatric patients respectively [1–3], suggests the need for improvements. Recent advances, including redesign of IOLs to incorporate square edges and special haptics [3–6], combined with improvements in surgical technique [7–10], have reduced the occurrence of PCO, but unacceptably high incidences remain. Secondary cataracts are relatively easily treated by Nd:YAG laser but this procedure, aside from the obvious financial burden to the health care system and inconvenience for the patient, can lead to such complications as recurrence of the opacity, cystoid macular edema, retinal detachment, and glaucoma [11–14]. Furthermore, PCO is of particular concern in pediatric patients, due to the high incidence and that fact that delay in diagnosis can cause irreparable amblyopia [2,15].
Secondary cataracts are the result of a variety of cellular changes that lead to excessive deposition of extracellular matrix components, particularly type I, III and IV collagen, as well as wrinkling of the capsular bag, linked to the presence of α-smooth actin (α-SMA) [8,16–18]. Lens epithelial cells (LECs) remaining in the equatorial region of the lens capsule following cataract surgery, migrate post-operatively due to a stress-induced phenotypical change known as epithelial to mesenchymal transition (EMT) [17,19]. The EMT process is thought to be initiated by the presence of transforming growth factor β2 (TGFβ2) [19,20] or epidermal growth factor [21] or which are released by these remaining lens cells as a wound healing response, following the mechanical stress of cataract removal and IOL implantation [8,16,17]. While the complete cascade of events that leads to EMT has yet to be elucidated, activation of the matrix metalloproteinases MMP-2 and MMP-9 in the lens has been observed as a result of the increased growth factor levels [16,20]. Treatment of anterior subcapsular cataracts, which have similar pathology to PCO with MMP inhibitors (MMPI) has been shown to minimize secondary cataract formation [20]. Therefore it is hypothesized that delivery of MMPI to any remaining LECs after cataract surgery might have therapeutic potential improving the long-term success of IOL devices.
Silicones with indices of refraction varying between 1.382 and 1.600 are commonly used in the manufacture of IOL materials. This wide range of refraction is advantageous for drug release, as the thickness of the IOL can be adjusted to incorporate variable amounts of drug [22]. Furthermore, silicone IOLs are preferred by some surgeons for their rapid unfolding times, relative to acrylic materials [1,23]. While some studies have demonstrated significantly lower PCO rates with silicone IOLs than with their acrylic counterparts [1,6,24] there is no consensus in the literature about which of the two materials has superior performance. Release of drugs from IOL materials, particularly acrylic IOL materials, has been previously examined as a means of reducing post-operative infection and inflammation [25–29]. Controlled delivery of agents such as daunorubicin [30], indomethacin [30–32], diclofenac sodium, tranilast, mitomycin C, colchicines, ethylene diamine tetraacetic acid [33], 5-fluorouracil [33,34], flurbiprofen [28], gatifloxacin and levofloxacin [29] and fibroblast growth factor 2-saporin [35,36] has been proposed as a method of reducing PCO in canine, rabbit or bovine eyes, although success to date has been limited. In addition to their widespread use as IOL materials, silicones were selected in the current work due to their previous extensive use in controlled drug delivery applications, in patches or coatings, with successful outcomes for both protein and low molecular weight drugs [29,37–39].
In this study, release of three MMPIs, a general inhibitor, GM6001 [40], and two specific inhibitors, MMP 2/9 Inhibitor I and MMP 2/9 Inhibitor II [41] from silicone rubber (poly(dimethyl)siloxane (PDMS)), as a model lens material, was investigated. Previous studies have demonstrated that treatment of lens epithelial cells with GM6001 may reduce both MMP-2 and MMP-9 levels, and the ability of lens epithelial cells to migrate [42–44]. The latter is important as some researchers suggest that inhibiting cell migration in the first month post-surgery may be sufficient to prevent PCO, since after this time, the formation of a mechanical barrier known as the capsular bend is thought to minimize access of the LECs to the posterior capsule [45,46]. Therefore, the current work focuses on investigating whether the MMPIs can be delivered in active form over periods of one month or more and whether these released inhibitors have the potential to impact lens epithelial migration. Since the released inhibitors may diffuse to other ocular tissues, the effect of MMPIs on neighboring ocular cells was also examined.
2. Materials and methods
2.1. Sample preparation
All reagents, unless otherwise specified, were purchased from Sigma Aldrich (Oakville, ON). PDMS was prepared from Sylgard 184 from Dow Corning (Midland, MI), according to the manufacturer's instructions, using 10:1 ratio of elastomer base to curing agent. Disks with a diameter of approximately 0.6 cm and a thickness of approximately 0.5 mm were punched from the PDMS films. The MMPIs, GM6001, MMP 2/9 Inhibitor I and MMP 2/9 Inhibitor II (Calbiochem, San Diego, CA) were dissolved in either dimethyl formamide (DMF) or ethanol. The inhibitors are of similar molecular weight, but differ in functional groups (see Table 1). The appropriate inhibitor solution was mixed with the PDMS elastomer base prior to the addition of curing agent. Following addition of the curing agent, drug-loaded films were cured for approximately 48 h at 37 °C.
Table 1.
Physical properties of the MMP inhibitors used in the study and amounts loaded in each batch.
| Inhibitor | Chemical structure | Molecular weight (Da) | Batch | Amount per disk (nmols) | Solvent | Release buffer |
|---|---|---|---|---|---|---|
| GM6001 |
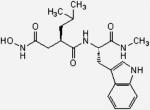
|
388.47 | HC | 55.0 | DMF | PBS |
| LC | 6.4 | DMF | PBS | |||
| LC | 6.4 | DMF | TBS | |||
| SOAK | Unknown | Ethanol | PBS | |||
| MMP 2/9 Inhibitor II |
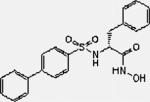
|
396.5 | HC | 54.0 | DMF | PBS |
| LC | 6.3 | Ethanol | PBS | |||
| LC | 6.3 | Ethanol | TBS | |||
| SOAK | Unknown | Ethanol | PBS | |||
| MMP 2/9 Inhibitor I |
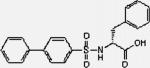
|
396.5 | HC | 86.0 | DMF | PBS |
On average, the disks with high concentration of drugs, (HC) contained approximately 55 nmols of inhibitor per disk (GM6001 and MMP 2/9 Inhibitor II) and approximately 86 nmols per disk for MMP 2/9 Inhibitor I. Approximately 6.4 nmols of inhibitor GM6001 and MMP 2/9 Inhibitor II were loaded per disk for the lower concentration samples (LC). These loadings were determined based on the solubility of the drug in solvent.
An alternative loading method involved soaking PDMS disks in solutions of inhibitors in ethanol (250 αm for GM6001 and 150 μm for MMP 2/9 Inhibitor II) for 4 days with mild shaking, followed by evaporation of the solvent for 48 h in the fume hood. Disks prepared in this way are denoted as SOAK.
2.2. Refractive index and light transmittance measurements
A digital hand-held pocket refractometer, (Atago, Bellevue, WA) was used to measure refractive index of the cured disks. The optical properties of the material were also determined by measuring transmittance at 405 nm, 540 nm, 595 nm and 630 nm with a BioRad plate reader. All measurements were done in triplicate.
2.3. Inhibitor release
Release studies were performed in triplicate, using 6 disks, produced from the same sheet of PDMS, or soaked in the same inhibitor solution. Each inhibitor-loaded silicone disk was incubated in a known volume (400–600 μl depending on release time) of phosphate buffered saline (PBS, pH 7.4) at 37 °C with mild agitation. This relatively small release volume was selected to mimic the relatively low volume of fluid that would be expected in the lens capsule. At regular time intervals, the disks were transferred to a known volume of fresh buffer solution, and the releasates were analyzed. The concentration of the drug was measured spectrophotometrically based on a standard curve.
2.4. MMPI activity assay
Activity of the released MMPIs from disks prepared with high concentration (HC) solutions of inhibitors was measured using a modified version of the assay for MMP-2 activity [41]. Briefly, human active recombinant MMP-9 enzyme (Calbiochem, San Diego, CA) was diluted to 200 ng/ml in PBS; 2 ng was used per reaction. MMP Substrate III Fluorogenic (Calbiochem, San Diego, CA) was diluted to 12.5 mm in PBS. The reactions were set in 50 mm TrisHCl, 150 mm NaCl, 5 mm CaCl2, 1 μm ZnCl, 0.01% Brij35. A 250 μl volume of sample was placed into a 96-well plate and the substrate and enzyme added. The change in the relative fluorescence units (ΔRFU) was measured using an excitation wavelength of 340 nm and an emission wavelength of 485 nm. The initial reading was made immediately following addition of the substrate, and the final reading was performed after 18 h. Calibration and quantification of the results was based on fresh inhibitors of known concentration.
Due to the lower concentrations of inhibitor present in the solution, activity of the released MMPIs from LC and SOAK samples was measured using a modified version of the Invitrogen EnzCheck gelatinase activity kit (Invitrogen, Burlington, ON), using human active recombinant MMP-9 enzyme (Calbiochem, San Diego, CA), and DQ-gelatin substrate (Invitrogen, Burlington, ON). The enzyme was diluted to 75 ng/ml in reaction buffer, and 10 μl was used per reaction. The DQ-gelatin substrate was diluted to 250 μg/ml in reaction buffer, and 20 μl were used per reaction. The reactions were performed in 50 mm TrisHCl, 150 mm NaCl, 5 mm CaCl2, and 1 μm ZnCl. An 80 μl volume of sample was placed into a 96-well plate and the enzyme and substrate added. The change in the relative fluorescence units (ΔRFU) was measured using an excitation wavelength of 495 nm and an emission wavelength of 518 nm. As above, the initial reading was made immediately after adding the substrate, while in this case, the final reading was performed after 2 h. Calibration of the results was based on fresh inhibitors of known concentration.
2.5. Cell toxicity assay
To examine potential effects of released MMPI on ocular cells, cell toxicity studies were performed. All cell media and supplements were purchased from Invitrogen (Burlington ON) unless otherwise stated. Human corneal epithelial cells were grown in keratinocyte serum-free media with complete supplement. Human corneal stromal fibroblasts were grown in M199 media with 1% ITS supplements (Becton Dickinson, Mississauga, ON). Human retinal pigment epithelial (RPE) cells were grown in DMEM:F12 media, with 5% FBS, 1% l-glutamine and 0.8% sodium bicarbonate. The human lens epithelial cell line, FHL 124, was grown in MEM media, with 1% l-glutamine, 10% FBS and 50 μg/ml gentamycin. The human lens epithelial cell line B3 was grown in MEM:F15 media (Sigma, Oakville, ON), with 1% l-glutamine, 0.8% sodium bicarbonate, 20% FBS, 1% sodium pyruvate. All media preparations also contained 1% penicillin–streptomycin solution. Cells were seeded in 24-well plates at a density of 30,000 cells per well. They were incubated in the appropriate growth medium for 3 days at which time the medium was replaced with fresh medium containing 90 nm of either GM6001 or MMP 2/9 Inhibitor II. The concentrations were chosen based on the average concentration of released drug in the first 3 days and based on results obtained previously in the West-Mays lab. The cells were incubated for 1 day and 5 days. On days 1 and 5 following addition of the inhibitor, the cells were detached using Tryple-Express (Invitrogen, Burlington, ON) and were counted using a Beckman Coulter cell counter. Untreated cells were considered 100%, and the treated cells were represented as percentages compared to untreated controls. Significance between treatment groups and untreated controls was assessed by one way ANOVA tests.
Cell viability was also tested using the MTT assay. Briefly, cells were grown in appropriate medium in 96-well plates for 1 or 5 days, in presence or absence of inhibitors (90 nm for GM6001, 0.1 mm for MMP 2/9 Inhibitor I and 900 nm for MMP 2/9 Inhibitor II). MTT reagent was added and cells were incubated for a further 16 h period. At the end, the medium was removed and the purple formazan precipitate was dissolved in 100 μl DMSO. The concentration was measured using a BioRad 96-well plate reader at 570 nm, with a reference filter at 630 nm. Viability was assessed by comparison to the untreated controls. Significance between treatment groups and untreated controls was assessed by one way ANOVA tests.
2.6. Cell migration
While a variety of markers can be used for PCO, migration was selected as the primary method of assessing the effect of the released inhibitors on lens cells since migration is critical to PCO. Following a previously published protocol [48], FHL 124 were grown to confluence, under the conditions described above. The cell monolayer was scratched with a pipet tip and the gap was monitored microscopically using a 20× magnification on a Zeiss Axiovert 200 microscope. The cells were treated with an 800 μm aphidicolin solution, to inhibit mitosis [49]. TGFβ2 levels in the media were measured using an ELSA kit (R&D Systems, Minneapolis, MN). The distance migrated by the cells was measured after 48 h and analyzed using the AxioVision 3.1 software. Significance between treatment groups and untreated controls was assessed by one way ANOVA tests.
2.7. Protein quantification
Collagen presence was detected by staining with a 1% Direct Red 80 solution in water, based on a protocol adapted from Taskiran [47]. Briefly, B3 human lens epithelial cells were exposed to 90 nm solution of inhibitor and 10 ng/ml TGFβ2 (Calbiochem, San Diego, CA) for 48 h. Cells were then either fixed with 10% formalin solution (Sigma, Burlington, ON), or detached and counted on a Beckmann Coulter cell counter. Fixed cells were incubated with a 1% Direct Red 80 solution for 1 h, at 22 °C, with mild agitation. The dye was then quantified by absorbance at 630 nm on a BioRad plate reader. Untreated controls were set as reference. Results were normalized to reflect collagen amounts/cell number.
Immunostaining experiments were done to assess the expression of fibronectin. Cells were fixed (using 4% formalin) and blocked with 5% goat serum in PBS for 30 min. This was followed by incubation with a 1:300 dilution of rabbit anti-fibronectin primary antibody (Abcam, Cambridge, MA) for 4 h at room temperature. Anti-rabbit secondary antibody (1:100 dilution) was subsequently added, and incubated for 2 h at room temperature with mild shaking. The relative concentration of fibronectin on the was determined fluorimetrically using the Perkin–Elmer Victor 3 fluorometer and the stained surfaces were observed using fluorescence microscopy at 20× and 40× magnification. Fluorescence readings were set for λex 485 nm; λem 520 nm. Alpha-smooth muscle actin (α-SMA) was detected fluorometrically using an FITC-labeled primary antibody (Sigma, Oakvile, ON). The relative amount of bound antibody was calculated based on the change in fluorescence units (ΔRFU). Significance between treatment groups and untreated controls was assessed by one way ANOVA tests.
3. Results
3.1. Refractive index and light transmittance measurements
The drug-loaded disks have refractive properties similar to those of PDMS, with values ranging between 1.4107 and 1.3640. All disks were within the refractive index limit for IOLs [22], with the exception of MMP 2/9 Inhibitor II LC samples. There was no significant difference between the GM6001 samples in terms of refractive index, regardless of solvent or amount of drug loaded (p < 0.05). However, MMP 2/9 Inhibitor II disks revealed a significant difference in refractive index (p < 0.05) when different solvents were used in the preparation of the samples, even when lower amounts of drugs were present (Table 2). Transparency of the materials was not visibly altered in most cases. However, the GM6001 HC disks had significantly lower light transmittance than plain PDMS, however, the disks were still transmitting more light than the normal adult lens [50] (Fig. 1).
Table 2.
Refractive index measurements of drug-loaded IOL materials.
| Drug | Solvent | Average ± sd | |
|---|---|---|---|
| None | DMF | Control | 1.4001 ± 0.007 |
| GM6001 | DMF | HC | 1.4102 ± 0.0001 |
| GM6001 | DMF | LC | 1.4107 ± 0.001 |
| GM6001 | Ethanol | LC | 1.4107 ± 0.002 |
| MMP 2/9 Inhibitor II | Ethanol | LC | 1.3640 ± 0.007 |
| MMP 2/9 Inhibitor II | DMF | HC | 1.3722 ± 0.0016 |
Fig. 1.
Light transmittance through PDMS materials at wavelengths within visual range. The values for transmittance are very high similar to those of the crystalline lens of a 4–5 year old child [50]. This indicates material is suitable as an IOL device.
3.2. Inhibitor release
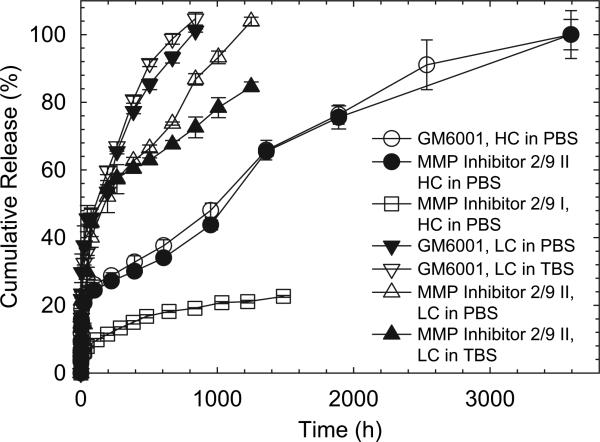
In order to determine the potential of lens materials for the delivery of MMP inhibitors, release from PDMS, as a model material, was monitored over periods of approximately 5 months for HC samples, 6 weeks for LC samples, and 2 weeks for SOAK samples. The release profile for GM6001, a general MMP inhibitor, from HC samples, (Fig. 2a) shows an initial burst of 12–15% of the total amount loaded in the first 24 h. After 6 days, the drug release occurred at a nearly constant rate of approximately 61 pmols/cm2/h, and continued for another 144 days (see Table 3). For LC, the profile remained similar but the steady state release rate dropped to 8–11 pmols/cm2/h, a value which is somewhat dependant on the release buffer as shown in Table 3. The steady state release rate could be altered to some extent by changing the concentration of MMPI in the solvent for PDMS loading, as it can be observed in the difference between the curves of HC and LC disks (Fig. 2a). Not unexpectedly, the disks loaded by soaking released the inhibitor at a much faster rate over a shorter time, reaching a relatively constant release rate of approximately 71 pmols/cm2/h after 72 h of release, and continuing for only another 32 days (Fig. 3).
Fig. 2.
Release profiles of MMP inhibitors from PDMS. GM6001 release profiles reveal that HC and SOAK formulations have a similar high burst and constant release, while no visible difference is noted for the LC batch, when released in either TBS or PBS (A). In contrast, panel B shows that MMP 2/9 Inhibitor II shows a high burst only in the HC formulation, while the SOAK batch is releasing similarly to the LC formulation.
Table 3.
Release details for all inhibitors. Notable differences are seen between loading methods and release buffers for the first two inhibitors.
| Batch | GM6001 |
MMP 2/9 Inhibitor II |
MMP 2/9 Inhibitor I |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HC | LC PBS | LC TBS | SOAK | HC | LC PBS | LC TBS | SOAK | HC | |
| Constant rate of release (pmol/cm2/h) | 61.0 | 8.4 | 10.8 | 71.4 | 56.0 | 10.7 | 3.0 | 3.5 | 88.2 |
| Initial burst (nmols/cm2 released in first 5–6 h) | 23.3 | 3.8 | 2.4 | 17.1 | 17.6 | 2.1 | 1.1 | 6.3 | 15.4 |
| Percentage released after 24 h | 24.9 | 45.6 | 32.5 | n/a | 21.1 | 23.2 | 14.4 | n/a | 4.7 |
| Percentage released after 7 days | 31.3 | 63.5 | 59.8 | n/a | 27.8 | 52.1 | 58.1 | n/a | 9.4 |
| Percentage released after 4 weeks | 40.9 | 97.2 | 98.7 | n/a | 34.8 | 63.2 | 69.2 | n/a | 13.4 |
Fig. 3.
Percentage release profiles of MMP 2/9 Inhibitor I, MMP 2/9 Inhibitor II and GM6001 outline the possible longevity of the drug-releasing IOL device. The HC formulations show the longest constant release, lasting over 145 days, while the LC formulations release all drug in 35–52 days. Notably, only the release profile of MMP 2/9 Inhibitor II LC is visibly affected by the release buffer, showing a potential longer release in TBS, compared to PBS. Also of interest, the MMP 2/9 Inhibitor I release profile has the longest expected lifetime, however, the current loading capacity of the device might not be sufficient, as this drug is needed in higher concentrations to have the same effect as its counterparts on the target enzymes.
Similarly, the release of the specific MMP 2/9 Inhibitor II from the HC samples shows a slightly lower initial burst of approximately 8–10% of the total amount in the first 24 h (Fig. 2b). After 6 days, the specific MMP 2/9 Inhibitor II was released at a nearly constant rate of 56 pmols/cm2/h, a rate relatively similar to that observed with the general inhibitor. The drugs continued to release at this steady rate for another 140 days (Fig. 2b). Likewise, as expected, the steady state release rate could be altered to some extent by changing the concentration of MMPI in the solvent for PDMS loading. For LC disks the constant rate was somewhat more variable but decreased to between 3 and 11 pmols/cm2/h, in TBS and PBS respectively (Table 3), and continues to release for 45 days. The disks loaded by soaking released the inhibitor at a constant release rate of approximately 3 pmols/cm2/h after 48 h of release, similar to the LC samples; however these samples only continued release for an additional 14 days (Fig. 3).
Of note, with the LC samples, there is a marked difference in the release of MMP 2/9 Inhibitor II in the two different buffers, a difference that is not observed for the GM6001 inhibitor. Similarly for SOAK, MMP 2/9 Inhibitor II is released much slower, with less of a burst in comparison with GM6001 in the PBS relative to the TBS. The reason for this is as of yet unknown. These buffers were selected to mimic biological fluids and are widely used in drug release studies.
The other specific inhibitor tested, MMP 2/9 Inhibitor I, behaved in a slightly different way, with an initial burst of 25% in the first 24 h. Release of this inhibitor reached a steady rate of approximately 88 pmols/cm2/h after two weeks and continues to release at approximately this rate for another 48 days (Fig. 3). Other formulations of this drug were not tested, as the release rate obtained with the HC loading conditions was not believed to be sufficient to efficiently inhibit the MMP-2 and MMP-9 naturally present in the lens, since this particular inhibitor has an IC50 between 240 and 310 nm [41] and these concentrations may not be easily maintained in active form (data not shown).
3.3. MMP inhibitor activity
To demonstrate that the inhibitors retain functional activity following release from PDMS, an MMP activity assay was developed based on a commercially available assay and performed on random samples of the released GM6001 and MMP 2/9 Inhibitor II. Activity was assessed based on cleavage of a fluorescent substrate. Table 4 summarizes relative activity results based on MMP-9 in the presence of fresh and released inhibitor from different samples. The assay was performed on the release samples after various time periods. Controls were inhibitors in PBS samples for appropriate times. Blank samples incubated in the presence of PDMS disks containing no drug were also tested. No effect on enzyme activity was observed (results not shown).
Table 4.
Activity profiles of the released inhibitors.
| MMP 2/9 Inhibitor II |
GM6001 |
||||
|---|---|---|---|---|---|
| % Activity | Time (h)a | Δt (h)b | % Activity | Time (h)a | Δt (h)b |
| HC in PBS | |||||
| 22.0% ± 0.8% | 1359.8 | 408.5 | |||
| 29.4% ± 3.3% | 1890.1 | 530.3 | 19.9% ± 3.7% | 2535.8 | 1176.0 |
| 3.9% ± 0.5% | 3592.8 | 1702.8 | 22.4% ± 0.7% | 3592.8 | 1057.0 |
| LC in PBS | |||||
| 40.4% ± 1.0% | 1.0 | 1.0 | |||
| 29.8% ± 1.6% | 47.3 | 22.8 | 85.4% ± 9.2% | 265.3 | 69.9 |
| 15.4% ± 0.2% | 195.3 | 119.3 | 58.2% ± 19.1% | 385.0 | 120.0 |
| LC in TBS | |||||
| 91.6% ± 3.4% | 4.2 | 1.7 | |||
| 57.9% ± 2.3% | 24.4 | 18.4 | 94.2% ± 13.5% | 76.0 | 28.8 |
| 45.6% ± 0.8% | 385.3 | 120.0 | 72.5% ± 11.0% | 195.3 | 119.3 |
| SOAK in PBS | |||||
| 97.8% ± 7.1% | 4.5 | 1.8 | 19.3% ± 1.8% | 2.8 | 1.8 |
| 34.7% ± 16.1% | 72.0 | 24.0 | 3.2% ± 0.4% | 72.0 | 24.0 |
| 25.6% ± 4.5% | 384.0 | 117.3 | |||
Time elapsed since the start of the release.
Time elapsed between samplings of releasate.
The potency of the freshly prepared solutions was greater than that of the released drug. Regardless, the released MMP inhibitors retained significant activity. The general inhibitor, GM6001, was found to retain approximately 85–95% activity for sampling times below 100 h where the increment of time between sample points was small; activity decreased to approximately 20% at times over 1000 h, where sampling increments were larger. Similarly, the MMP 2/9 Inhibitor II retained 91.6% activity at the initial sample points but the activity was found to decrease to between 20% and 30% with sample times greater than 400 h, and even lower to 4% when sampling times were over 1000 h. However, MMP 2/9 Inhibitor II activity, when released into PBS, was dramatically reduced, with an initial remaining activity of only 40.4% after 1 h, that drops to 30% after only 23 h. Unlike GM6001, the specific inhibitor maintained most of its activity in the first hours of release from SOAK samples, and still retained over 25% activity after a week of release, while GM6001 lost all activity after 24 h of release (Table 4).
3.4. Toxicity to neighboring ocular cell types
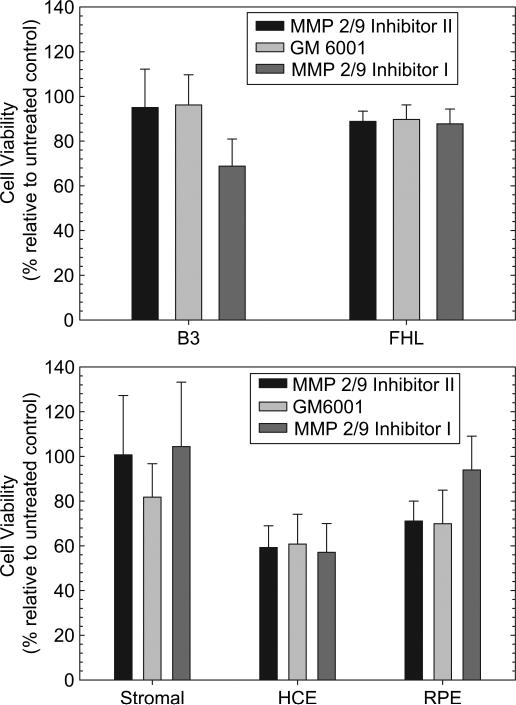
Since the released inhibitors could potentially diffuse out of the capsular bag, which is compromised during the surgical procedure, the potential effect of the inhibitors on neighboring ocular cell types was tested. Various human ocular cell lines were examined including corneal epithelial cells, corneal stromal fibroblasts, two lines of lens epithelial cells as well as retinal pigment epithelial cells. Response clearly differed depending on the cell type and inhibitor in question as shown in Fig. 4. Specifically, no response was observed for corneal epithelial and RPE cells with any of the three inhibitors at early times, B3 cells showed decreased viability in the presence of all three inhibitors (p < 0.05) while FHL 124 cells showed only a small response to GM6001 (data not shown). By 5 days a dramatic and significant (p < 0.001) decrease in cell viability of approximately 40% was observed with corneal epithelial and FHL 124 cells, in response to all three drugs while the RPE cells showed decreased viability in the presence of MMP 2/9 Inhibitor II and GM6001 (p < 0.0006) but not MMP 2/9 Inhibitor I. However, the B3 cells were found to be very sensitive to all of the molecules tested, showing a significant (p < 0.05) viability decrease in all cases. The corneal stromal cells remained unaffected in all cases.
Fig. 4.
MMP inhibitors show little or no toxic effect on neighboring ocular cell types. There were slight decreases in the viability of FHL 124 cells remains unchanged with inhibitor treatment while only MMP 2/9 Inhibitor had a significant effect on the viability of B3 cells. While slight but significant decreases in viability were observed in HCE and RPE cells with inhibitor treatment, the corneal stromal fibroblasts were unaffected by even the relatively high concentrations of inhibitor used. Error bars represent standard deviation.
The concentrations of the inhibitors used in this study were approximately 100 times greater than those reported in the literature to affect cell expression of posterior capsule opacification specific markers [17,20]. However, even at these high concentrations, the inhibitors failed to decrease either cell numbers or cell viability of the various ocular cells examined by 50% or more. Therefore, despite the significant decreases noted in this work in some cases, it is unlikely that the surrounding ocular cells would be significantly affected by the released MMPI.
3.5. Effect of MMPI on protein production
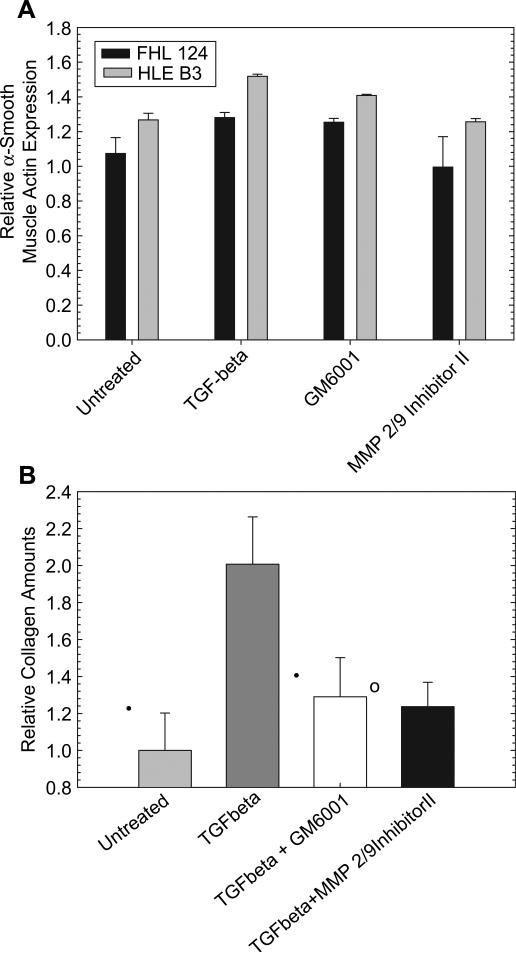
There was no significant change in fibronectin expression in either of the lens cells when treated with TGFβ2 with or without the various MMP inhibitors. With both lens cell lines, a significant increase in the expression of α-smooth muscle actin was observed with TGFβ2 treatment. There was no change in α-SMA expression with inhibitor treatment with the FHL cells but MMP 2/9 Inhibitor II decreased TGFβ2 induced α-smooth muscle actin production by a small but significant (p < 0.05) amount (Fig. 5a). Similarly, cells treated with TGFβ2 show an increase in collagen production (Fig. 5b). However, treatment of the TGFβ2 stimulated cells with a90 nm solution of the MMPI molecules GM6001 and MMP 2/9 Inhibitor II for periods of 48 h leads to a significant (p < 0.05) 10–15% decrease in collagen production in HLE B3 cells compared to TGFβ2 treatment alone. These results suggest that these molecules may potentially alter PCO markers in a desired fashion although the relatively high basal production of the markers masks the effects.
Fig. 5.
The MMP inhibitors have the potential to significantly alter the expression of fibrotic markers. A) Treatment of the cells with TGFβ2 results in a significant increase in the expression of α-smooth muscle actin. However, presumably due to the relatively high basal production of this marker, only MMP 2/9 Inhibitor II was found to decrease expression in FHL 124 cells. Both inhibitors tested resulted in a significant reduction in expression in the B3 cells. B) The inhibitors were also able to reduce the amount of collagen I/III produced by B3 HLE in vitro after 48 h exposure to both drugs and TGFβ2. While TGFβ2 is capable of increasing the amount of collagen production, as expected, co-treatment with MMPIs reduce the amount of collagen even in comparison to untreated controls. Error bars represent standard deviation. * Indicates p < 0.0008; ○ indicates p < 0.009; • indicates p < 0.05.
3.6. Cell migration
In addition to aberrant matrix deposition, cell migration is a key component of the process which leads to PCO; therefore, a migration assay was used to determine the effect of the inhibitors on lens epithelial cells in vitro. After scratching and in the absence of the inhibitor, the FHL 124 cells produce increased amounts of TGFβ2 (Table 5) and migrate into the opening. Of note, these cells reach a 2 ng/ml level of cytokine (the treatment level used in assays other than a scratch assay) by day 3.
Table 5.
Amounts of TGFβ2 (pg/ml) in the media following cell sheet scratch demonstrate that cells are capable of producing high levels of TGFβ2 after inducing mechanical stress.
| Day | Untreated | MMP 2/9 Inhibitor II, SOAK | GM6001, SOAK | MMP 2/9 Inhibitor II, HC |
|---|---|---|---|---|
| 0 | 49.4 ± 2.5 | 49.4 ± 2.5 | 49.4 ± 2.5 | 49.4 ± 2.5 |
| 2 | 877.3 ± 43.9 | 917.5 ± 45.9 | 383.3 ± 19.2 | 1203.7 ± 60.2 |
| 3 | 1807.5 ± 90.4 | n/a | n/a | n/a |
| 7 | 4414.3 ± 220.7 | n/a | n/a | n/a |
| 9 | 4312.1 ± 215.6 | 3672.5 ± 183.6 | 4859.9 ± 243.0 | 3461.7 ± 173.1 |
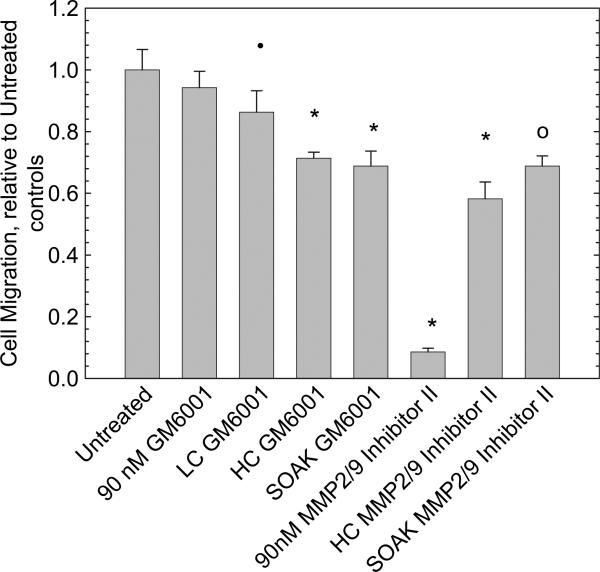
Treatment with a 90 nm solution of the GM6001 general inhibitor did not significantly reduce migration as shown in Fig. 6. However, treatment with a 90 nm solution of MMP 2/9 Inhibitor II, resulted in a significant 82% reduction in migration. When the cells were exposed to GM6001 containing disks, migration was reduced by 14% for LC disks, 29% for HC and 31% for SOAK disks, a significant reduction relative to controls (p < 0.0005). When exposed to MMP 2/9 Inhibitor II disks, migration was significantly reduced by 42% for HC disks and 33% for SOAK disks (p < 0.005). Therefore, delivery of the inhibitors from the disks in all cases led to a significant reduction in migration in the first 48 h following creation of the scratch.
Fig. 6.
FHL 124 cell migration is significantly reduced with all prepared disk treatments. A 90 nm solution of GM6001 inhibitor does not significantly reduce migration, while a 90 nm solution of MMP 2/9 Inhibitor II has a very pronounced effect. Error bars represent standard deviation. * Indicates p < 0.0008; ○ indicates p < 0.009; • indicates p < 0.05.
4. Discussion
In this work, MMP inhibitors were demonstrated to reduce LEC migration rate and increase in collagen I/III after TGFβ2 stimulation, both characteristic features of PCO suggesting that delivery of these molecules may have the potential to minimize PCO. While the potential of IOLs for delivering drugs to the eye has been previously demonstrated, the use of drug-releasing IOL materials for mitigating posterior capsule opacification, the main complication of intraocular lens surgery [8,16], has not been widely investigated. A potentially efficient method of delivering MMP inhibitors directly to the migrating lens cells was hypothesized to be using the IOL itself. To assess the potential of this as a treatment methodology, the release of three MMP inhibitors, GM6001, a general inhibitor, and two MMP 2/9 specific inhibitors, from silicone rubber (PDMS) as a model IOL material was examined.
Some research has suggested that MMP-9 activation and production precedes MMP-2 activation and production in TGFβ2 stimulated cells, in ASC studies [51]. As it remains unclear whether MMP-2 or MMP-9 becomes activated first in the process that leads to PCO, the effect of both inhibitors is of interest as they elicit differential preferences for MMPs, with MMP 2/9 Inhibitor II having a stronger influence on MMP-2 and GM6001 having a stronger influence on MMP-9. Of note, the current study included only release profiles and toxicity data for MMP 2/9 Inhibitor I, as the data demonstrate that this compound is much less efficient in inhibiting the target enzymes and would therefore need to be delivered at relatively high rates for efficacy.
For the most part, the loaded materials maintain sufficient transparency to be useful in ophthalmic applications as shown in Fig. 3 and Table 3. From previous studies done in our lab (unpublished data) and from the data presented in Fig. 1 and Table 2, it is clear that loading drugs in combination with solvents can influence the structure, refractive index and transparency of the PDMS network. Therefore, it was felt that the addition of sufficient MMP 2/9 Inhibitor I to affect cellular response would negatively affect the materials properties and make the system unusable in the intended application.
The release profiles presented in Figs. 2 and 3, demonstrate that, following an initial burst, the various inhibitors can be delivered at a relatively constant rate over a period of up to 5 months depending on such factors as loading amounts, conditions of loading and inhibitor properties. For example, the LC sample releasing GM6001 showed virtually complete release after a period of one month, while under similar conditions, the MMP 2/9 Inhibitor II LC samples were found to release over a period of 2 months. Desired duration of release is not known at this time. There is evidence that the wound healing response after IOL implantation in rabbit eyes is approximately 8 weeks in length [52] although there are reports of MMP enzyme and inhibitor activity in human lens explants for periods of up to 18 months [53]. It has also been shown that the production of MMP enzymes is initially decreased in the presence of GM6001 inhibitor, with slow restoration after 2 weeks [43]. Regardless, the variation in the release profiles suggests that efficacious delivery of the inhibitor may be possible by tuning the system and that delivery over a sufficiently long period of time might be feasible.
As expected, release profiles (Fig. 2) show a large initial burst of inhibitors in the first 24 h; the amounts released during this burst period may actually be desirable as they would presumably be sufficient to inhibit the higher amounts of MMP present following cataract surgery. Ultimately, however, more detailed studies will be necessary to determine for example if a relatively short period of release immediately following implantation is sufficient to inhibit EMT. As presented in Fig. 5, both inhibitors have the capacity to reduce collagen I/III production and to a lesser extent α-smooth muscle actin in presence of TGFβ2, after 48 h.
A hallmark of TGFβ2 stimulated lens cells is their capability to migrate following EMT [54]. MMP levels, especially MMP-2 and MMP-9 protein and mRNA levels, also increase following EMT [55,56]. It was thus hypothesized that MMP inhibitors would play a major role in inhibiting the cell migration process. Research has shown that in vivo, a period of 7–11 days of migration is necessary for the remaining lens epithelial cells in the anterior capsule to reach the posterior [57–59]. With a square-edged IOL, the best PCO prevention method to date [3,60], the capsular bend, formed in 2–4 weeks [57,58,61], creates a physical barrier preventing the LECs from reaching the posterior [61,62]. It is thus speculated that it is crucial to aggressively reduce migration and LEC viability during the first 2–4 weeks post-surgery, in order to prevent the cells from reaching the posterior prior to capsular bend formation.
The released inhibitors were able to significantly (p < 0.005) decrease LEC (FHL 124) migration in the first 48 h, in all formulations (Fig. 6). The results represent only cell migration, as the cells have been treated with aphidicolin, a mitosis inhibitor that allows for mRNA production and protein synthesis, but blocks DNA gyrase [49]. Preliminary studies demonstrated that a 800 μm concentration of aphidicolin was sufficient to block incorporation of labeled nucleotides into LEC DNA, as tested with the Click-IT Edu kit (Invitrogen, Burlington, ON). The most efficient formulation proved to be the MMP 2/9 Inhibitor II HC disks, which reduced migration by 42% (p < 0.005). However, of note, SOAK formulations of both inhibitors were also able to reduce migration by approximately 30%. This result is of particular interest, since manufacturing of such IOL materials does not have to interfere with current fabrication processes, and it may therefore be possible to incorporate the inhibitors as a final step in IOL preparation. While these results are promising, additional studies are needed to determine the impact on migration over longer periods of time. As well, studies on human capsule explants and in vivo testing will also be necessary to further validate these preliminary results.
A significant advantage to using MMP inhibitors for mitigating PCO is that the effects of these compounds are mainly on cellular transformation and therefore cellular toxicity is not expected to be significant. To test this hypothesis, the effect of the active MMP inhibitors on various ocular cells was examined (Fig. 4). The general inhibitor, GM6001 had the greatest effect on the cell populations tested, as expected, since this molecule can affect several pathways by inhibiting a large number of enzymes. However, even at high concentrations, this potent inhibitor did not reduce cell numbers by more than 30%, with the most affected being the corneal stromal fibroblast line. The MTT viability assay demonstrated both slower growth and reduced mitochondrial function in some cases. Slower growth is a more desirable side effect as cells in the eye are mostly in a fully differentiated state, and are not actively growing. Immediate effects of drugs, after one day exposure were observed and exposure for 5 days was found to cause significant decreases in viability in most cell lines, as expected. In all cases, the concentrations of drugs tested in the viability assay were high based on the total amounts loaded and released; accumulation in ocular compartments other than the lens capsular bag is not anticipated. Therefore, the relatively low levels of toxicity that were observed with the very high concentrations of MMP inhibitors examined suggest that delivery of the inhibitors from the IOL has potential to affect cellular function of the remaining lens epithelial cells without significantly adversely affecting other cell types in the eye.
It is clear that both release duration and amount of inhibitor released can be altered by changing relatively simple key loading parameters. Furthermore, as shown in Table 4, it is clear that the inhibitors can be released in active form although in most cases, some activity was lost, particularly when the inhibitors were released over much longer durations. However, this loss of activity was thought to be at least in part due to hydrolysis which occurred during the long incremental time periods between samplings [41,63]. Together with the released inhibitor capacity to reduce collagen I/III production and LEC migration rates, this research demonstrates that the delivery of MMP inhibitors from IOL materials has great potential to mitigate PCO.
5. Conclusions
In the current work release of MMP inhibitors from silicones as model lens materials was demonstrated. Release durations of more than 5 months were possible. Inhibitors were active and resulted in cellular changes consistent with decreased EMT. While further investigations are needed to demonstrate the potential of these released inhibitors in ablating PCO in vivo, these results suggest that MMP inhibitors can be released from IOL materials at concentrations appropriate for inhibition of MMP-2 and MMP-9 activity in the human lens capsule, which may mitigate anterior subcapsular cataract formation in vitro. Furthermore, these molecules at high concentrations were found to have only a relatively small effect on other ocular cell types, presumably slowing growth. The disks produced in this experiment were able to significantly reduce both collagen levels, and lens epithelial cell migration after 48 h of exposure in vitro. Further work will focus on examining the effect of the released inhibitors on lens cells, specifically related to the inhibition of EMT and long-term LEC migration. Therefore, delivery of MMPI drugs directly to the LECs from the IOL may represent a very promising solution to reduce the incidence of secondary cataract formation.
Acknowledgments
NSERC is acknowledged for funding.
References
- 1.Cheng JW, Wei RL, Cai JP, Xi GL, Zhu H, Li Y, et al. Efficacy of different intraocular lens materials and optic edge designs in preventing posterior capsular opacification: a meta-analysis. Am J Ophthalmol. 2007;143:428–36. doi: 10.1016/j.ajo.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 2.Hosal BM, Biglan AW. Risk factors for secondary membrane formation after removal of pediatric cataract. J Cataract Refract Surg. 2002;28:302–9. doi: 10.1016/s0886-3350(01)01028-8. [DOI] [PubMed] [Google Scholar]
- 3.Matsushima H, Iwamoto H, Mukai K, Katsuki Y, Nagata M, Senoo T. Preventing secondary cataract and anterior capsule contraction by modification of intraocular lenses. Expert Rev Med Dev. 2008;5:197–207. doi: 10.1586/17434440.5.2.197. [DOI] [PubMed] [Google Scholar]
- 4.Sacu S, Menapace R, Findl O, Georgopoulos M, Buehl W, Kriechbaum K, et al. Influence of optic edge design and anterior capsule polishing on posterior capsule fibrosis. J Cataract Refract Surg. 2004;30:658–62. doi: 10.1016/j.jcrs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Buehl W, Findl O. Effect of intraocular lens design on posterior capsule opacification. J Cataract Refract Surg. 2008;34:1976–85. doi: 10.1016/j.jcrs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 6.Vock L, Crnej A, Findl O, Neumayer T, Buehl W, Sacu S, et al. Posterior capsule opacification in silicone and hydrophobic acrylic intraocular lenses with sharp-edge optics six years after surgery. Am J Ophthalmol. 2009;147:683–90. doi: 10.1016/j.ajo.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Chang MA, Congdon NG, Baker SK, Bloem MW, Savage H, Sommer A. The surgical management of cataract: barriers, best practices and outcomes. Int Ophthalmol. 2008;28:247–60. doi: 10.1007/s10792-007-9121-2. [DOI] [PubMed] [Google Scholar]
- 8.Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127:555–62. doi: 10.1001/archophthalmol.2009.3. [DOI] [PubMed] [Google Scholar]
- 9.Coppe AM, Lapucci G. Posterior vitreous detachment and retinal detachment following cataract extraction. Curr Opin Ophthalmol. 2008;19:239–42. doi: 10.1097/ICU.0b013e3282fc9c4a. [DOI] [PubMed] [Google Scholar]
- 10.Kelkar A, Kelkar J, Amuaku W, Kelkar U, Shaikh A. How to prevent endophthalmitis in cataract surgeries? Indian J Ophthalmol. 2008;56:403–7. doi: 10.4103/0301-4738.42418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burq MA, Taqui AM. Frequency of retinal detachment and other complications after neodymium:Yag laser capsulotomy. J Pak Med Assoc. 2008;58:550–2. [PubMed] [Google Scholar]
- 12.Werblin TP, Krider D. Another view of neodymium:YAG capsulotomy. J Cataract Refract Surg. 2006;32:373–4. doi: 10.1016/j.jcrs.2005.12.132. author reply 374. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S, Garg P. “String of pearls” following Nd:YAG laser posterior capsulotomy. Indian J Ophthalmol. 2002;50:140–2. [PubMed] [Google Scholar]
- 14.Trinavarat A, Atchaneeyasakul L, Udompunturak S. Neodymium:YAG laser damage threshold of foldable intraocular lenses. J Cataract Refract Surg. 2001;27:775–80. doi: 10.1016/s0886-3350(00)00855-5. [DOI] [PubMed] [Google Scholar]
- 15.Pandey SK, Apple DJ, Werner L, Maloof AJ, Milverton EJ. Posterior capsule opacification: a review of the aetiopathogenesis, experimental and clinical studies and factors for prevention. Indian J Ophthalmol. 2004;52:99–112. [PubMed] [Google Scholar]
- 16.Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Tamiya S, Wormstone IM, Marcantonio JM, Gavrilovic J, Duncan G. Induction of matrix metalloproteinases 2 and 9 following stress to the lens. Exp Eye Res. 2000;71:591–7. doi: 10.1006/exer.2000.0916. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi T, Hatae T, Inomata H. Collagen types in human posterior capsule opacification. J Cataract Refract Surg. 1994;20:643–6. doi: 10.1016/s0886-3350(13)80655-4. [DOI] [PubMed] [Google Scholar]
- 19.de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation. Cells Tissues Organs. 2005;179:43–55. doi: 10.1159/000084508. [DOI] [PubMed] [Google Scholar]
- 20.Dwivedi DJ, Pino G, Banh A, Nathu Z, Howchin D, Margetts P, et al. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am J Pathol. 2006;168:69–79. doi: 10.2353/ajpath.2006.041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Q, Zhou C, Bi Z, Wan Y. EGF-induced cell migration is mediated by ERK and PI3K/AKT pathways in cultured human lens epithelial cells. J Ocul Pharmacol Ther. 2006;22:93–102. doi: 10.1089/jop.2006.22.93. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd AW, Faragher RG, Denyer SP. Ocular biomaterials and implants. Biomaterials. 2001;22:769–85. doi: 10.1016/s0142-9612(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 23.Oh KT, Oh KT. Optimal folding axis for acrylic intraocular lenses. J Cataract Refract Surg. 1996;22:667–70. doi: 10.1016/s0886-3350(96)80299-9. [DOI] [PubMed] [Google Scholar]
- 24.Prosdocimo G, Tassinari G, Sala M, Di Biase A, Toschi PG, Gismondi M, et al. Posterior capsule opacification after phacoemulsification: silicone CeeOn Edge versus acrylate AcrySof intraocular lens. J Cataract Refract Surg. 2003;29:1551–5. doi: 10.1016/s0886-3350(02)02051-5. [DOI] [PubMed] [Google Scholar]
- 25.Kleinmann G, Apple DJ, Chew J, Hunter B, Stevens S, Larson S, et al. Hydrophilic acrylic intraocular lens as a drug-delivery system for fourth-generation fluoroquinolones. J Cataract Refract Surg. 2006;32:1717–21. doi: 10.1016/j.jcrs.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Kleinmann G, Apple DJ, Chew J, Stevens S, Hunter B, Larson S, et al. Hydrophilic acrylic intraocular lens as a drug-delivery system: pilot study. J Cataract Refract Surg. 2006;32:652–4. doi: 10.1016/j.jcrs.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Siqueira RC, Filho ER, Fialho SL, Lucena LR, Filho AM, Haddad A, et al. Pharmacokinetic and toxicity investigations of a new intraocular lens with a dexamethasone drug delivery system: a pilot study. Ophthalmologica. 2006;220:338–42. doi: 10.1159/000094626. [DOI] [PubMed] [Google Scholar]
- 28.Duarte AR, Simplicio AL, Vega-Gonzalez A, Subra-Paternault P, Coimbra P, Gil MH, et al. Impregnation of an intraocular lens for ophthalmic drug delivery. Curr Drug Deliv. 2008;5:102–7. doi: 10.2174/156720108783954851. [DOI] [PubMed] [Google Scholar]
- 29.Tsuchiya Y, Kobayakawa S, Tsuji A, Tochikubo T. Preventive effect against post-cataract endophthalmitis: drug delivery intraocular lens versus intracameral antibiotics. Curr Eye Res. 2008;33:868–75. doi: 10.1080/02713680802382971. [DOI] [PubMed] [Google Scholar]
- 30.Tetz MR, Ries MW, Lucas C, Stricker H, Volcker HE. Inhibition of posterior capsule opacification by an intraocular-lens-bound sustained drug delivery system: an experimental animal study and literature review. J Cataract Refract Surg. 1996;22:1070–8. doi: 10.1016/s0886-3350(96)80120-9. [DOI] [PubMed] [Google Scholar]
- 31.Nishi O, Nishi K, Fujiwara T, Shirasawa E. Effects of diclofenac sodium and indomethacin on proliferation and collagen synthesis of lens epithelial cells in vitro. J Cataract Refract Surg. 1995;21:461–5. doi: 10.1016/s0886-3350(13)80541-x. [DOI] [PubMed] [Google Scholar]
- 32.Nishi O, Nishi K, Morita T, Tada Y, Shirasawa E, Sakanishi K. Effect of intraocular sustained release of indomethacin on postoperative inflammation and posterior capsule opacification. J Cataract Refract Surg. 1996;22(Suppl. 1):806–10. doi: 10.1016/s0886-3350(96)80166-0. [DOI] [PubMed] [Google Scholar]
- 33.Matsushima H, Mukai K, Gotoo N, Yoshida S, Yoshida T, Sawano M, et al. The effects of drug delivery via hydrophilic acrylic (hydrogel) intraocular lens systems on the epithelial cells in culture. Ophthalmic Surg Laser Imag. 2005;36:386–92. [PubMed] [Google Scholar]
- 34.Cochener B, Pandey S, Apple D, Bougaran R, Colin J. Nonbiodegradable drug-sustained capsular ring for prevention of secondary cataract. Part II: in vivo evaluation. J Fr Ophtalmol. 2003;26:439–52. [PubMed] [Google Scholar]
- 35.Behar-Cohen FF, David T, Buechler Y, Nova MP, Houston LL, Pouliquen YM, et al. Cytotoxic effects of FGF2-saporin on bovine epithelial lens cells in vitro. Invest Ophthalmol Vis Sci. 1995;36:2425–33. [PubMed] [Google Scholar]
- 36.Behar-Cohen FF, David T, D'Hermies F, Pouliquen YM, Buechler Y, Nova MP, et al. In vivo inhibition of lens regrowth by fibroblast growth factor 2-saporin. Invest Ophthalmol Vis Sci. 1995;36:2434–48. [PubMed] [Google Scholar]
- 37.Brown RS, Akhtar P, Akerman J, Hampel L, Kozin IS, Villerius LA, et al. Partition controlled delivery of hydrophobic substances in toxicity tests using poly(-dimethylsiloxane) (PDMS) films. Environ Sci Technol. 2001;35:4097–102. doi: 10.1021/es010708t. [DOI] [PubMed] [Google Scholar]
- 38.Barbu E, Verestiuc L, Nevell TG, Tsibouklis J. Polymeric materials for ophthalmic drug delivery: trends and perspectives. J Mater Chem. 2006;16:3439–43. [Google Scholar]
- 39.Park JH, Cho YW, Cho YH, Choi JM, Shin HJ, Bae YH, et al. Norfloxacin-releasing urethral catheter for long-term catheterization. J Biomater Sci Polym Ed. 2003;14:951–62. doi: 10.1163/156856203322381438. [DOI] [PubMed] [Google Scholar]
- 40.Galardy RE, Grobelny D. N alpha-(diphenoxyphosphoryl)-l-alanyl-l-proline, N alpha-[bis (4-nitrophenoxy)phosphoryl]-l-alanyl-l-proline, and N alpha-[(2-phenylethyl)phenoxyphosphoryl]-l-alanyl-l-proline: releasers of potent inhibitors of angiotensin converting enzyme at physiological pH and temperature. J Med Chem. 1985;28:1422–7. doi: 10.1021/jm00148a008. [DOI] [PubMed] [Google Scholar]
- 41.Tamura Y, Watanabe F, Nakatani T, Yasui K, Fuji M, Komurasaki T, et al. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J Med Chem. 1998;41:640–9. doi: 10.1021/jm9707582. [DOI] [PubMed] [Google Scholar]
- 42.Awasthi N, Wagner BJ. Suppression of human lens epithelial cell proliferation by proteasome inhibition, a potential defense against posterior capsular opacification. Invest Ophthalmol Vis Sci. 2006;47:4482–9. doi: 10.1167/iovs.06-0139. [DOI] [PubMed] [Google Scholar]
- 43.Wong TT, Daniels JT, Crowston JG, Khaw PT. MMP inhibition prevents human lens epithelial cell migration and contraction of the lens capsule. Br J Ophthalmol. 2004;88:868–72. doi: 10.1136/bjo.2003.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JH, Chen FH, Wang NL. Study on inhibitory effects of matrix metal-loproteinase inhibitor on migration of cultured human lens epithelial cells. Zhonghua Yan Ke Za Zhi. 2008;44:315–20. [PubMed] [Google Scholar]
- 45.Nishi O, Yamamoto N, Nishi K, Nishi Y. Contact inhibition of migrating lens epithelial cells at the capsular bend created by a sharp-edged intraocular lens after cataract surgery. J Cataract Refract Surg. 2007;33:1065–70. doi: 10.1016/j.jcrs.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Menapace R, Sacu S, Georgopoulos M, Findl O, Rainer G, Nishi O. Efficacy and safety of capsular bending ring implantation to prevent posterior capsule opacification: three-year results of a randomized clinical trial. J Cataract Refract Surg. 2008;34:1318–28. doi: 10.1016/j.jcrs.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 47.Taskiran D, Taskiran E, Yercan H, Kutay F. Quantification of total collagen in rabbit tendon by the sirius red method. Turk J Med Sci. 1999;29:7. [Google Scholar]
- 48.Wormstone IM, Tamiya S, Eldred JA, Lazaridis K, Chantry A, Reddan JR, et al. Characterisation of TGF-beta2 signalling and function in a human lens cell line. Exp Eye Res. 2004;78:705–14. doi: 10.1016/j.exer.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Jackman J, O'Connor PM. Methods for synchronizing cells at specific stages of the cell cycle. Curr Protoc Cell Biol. 2001 doi: 10.1002/0471143030.cb0803s00. Chapter 8:Unit 8.3. [DOI] [PubMed] [Google Scholar]
- 50.van den Berg TJ, Spekreijse H. Near infrared light absorption in the human eye media. Vision Res. 1997;37:249–53. doi: 10.1016/s0042-6989(96)00120-4. [DOI] [PubMed] [Google Scholar]
- 51.Nathu Z, Dwivedi DJ, Reddan JR, Sheardown H, Margetts PJ, West-Mays JA. Temporal changes in MMP mRNA expression in the lens epithelium during anterior subcapsular cataract formation. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka S, Saika S, Ohmi S, Miyamoto T, Ishida I, Okada Y, et al. Cellular fibronectin, but not collagens, disappears in the central posterior capsules during healing after lens extraction and IOL implantation in rabbits. Jpn J Ophthalmol. 2002;46:147–52. doi: 10.1016/s0021-5155(01)00503-2. [DOI] [PubMed] [Google Scholar]
- 53.Kawashima Y, Saika S, Miyamoto T, Yamanaka O, Okada Y, Tanaka S, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases of fibrous humans lens capsules with intraocular lenses. Curr Eye Res. 2000;21:962–7. doi: 10.1076/ceyr.21.6.962.6989. [DOI] [PubMed] [Google Scholar]
- 54.Stump RJ, Lovicu FJ, Ang SL, Pandey SK, McAvoy JW. Lithium stabilizes the polarized lens epithelial phenotype and inhibits proliferation, migration, and epithelial mesenchymal transition. J Pathol. 2006;210:249–57. doi: 10.1002/path.2049. [DOI] [PubMed] [Google Scholar]
- 55.Hodgkinson LM, Duncan G, Wang L, Pennington CJ, Edwards DR, Wormstone IM. MMP and TIMP expression in quiescent, dividing, and differentiating human lens cells. Invest Ophthalmol Vis Sci. 2007;48:4192–9. doi: 10.1167/iovs.06-1371. [DOI] [PubMed] [Google Scholar]
- 56.Seomun Y, Kim J, Lee EH, Joo CK. Overexpression of matrix metalloproteinase-2 mediates phenotypic transformation of lens epithelial cells. Biochem J. 2001;358:41–8. doi: 10.1042/0264-6021:3580041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi H, Hayashi K, Nakao F, Hayashi F. Elapsed time for capsular apposition to intraocular lens after cataract surgery. Ophthalmology. 2002;109:1427–31. doi: 10.1016/s0161-6420(02)01112-0. [DOI] [PubMed] [Google Scholar]
- 58.Nishi O, Nishi K, Akura J. Speed of capsular bend formation at the optic edge of acrylic, silicone, and poly(methyl methacrylate) lenses. J Cataract Refract Surg. 2002;28:431–7. doi: 10.1016/s0886-3350(01)01094-x. [DOI] [PubMed] [Google Scholar]
- 59.Wormstone IM, Liu CS, Rakic JM, Marcantonio JM, Vrensen GF, Duncan G. Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci. 1997;38:396–404. [PubMed] [Google Scholar]
- 60.Cleary G, Spalton DJ, Koch DD. Effect of square-edged intraocular lenses on neodymium:YAG laser capsulotomy rates in the United States. J Cataract Refract Surg. 2007;33:1899–906. doi: 10.1016/j.jcrs.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 61.Sacu S, Findl O, Linnola RJ. Optical coherence tomography assessment of capsule closure after cataract surgery. J Cataract Refract Surg. 2005;31:330–6. doi: 10.1016/j.jcrs.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 62.Katayama Y, Kobayakawa S, Yanagawa H, Tochikubo T. The relationship between the adhesion characteristics of acrylic intraocular lens materials and posterior capsule opacification. Ophthalmic Res. 2007;39:276–81. doi: 10.1159/000108121. [DOI] [PubMed] [Google Scholar]
- 63.Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–4. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]