Abstract
Ischemic mitral regurgitation (IMR) results from displacement of the papillary muscles due to ischemic ventricular distortion. Recurrent IMR is frequent after annuloplasty, particularly when left ventricular remodeling continues to progress. Our hypothesis is that repositioning of the papillary muscles can be achieved by injection of polyvinyl-alcohol (PVA) hydrogel polymer into the myocardium in chronic MR despite advanced left ventricular remodeling.
Methods
Nine sheep underwent ligation of circumflex branches to produce chronic ischemic MR over eight weeks. Once MR developed, PVA was injected into the myocardium underlying the infarcted PM. 2D and 3D echocardiograms and hemodynamic data were obtained pre infarct (baseline), pre PVA (Chronic MR) and post PVA.
Results
One animal died early, one did not develop MR, and the remaining 7 developed moderate MR. PVA injection significantly decreased the MR from moderate to trace. This was associated with a decrease in infarcted papillary muscle-to-mitral annulus tethering distance (32.6 ± 4.4 to 27.6 ± 4.2 mm, P<0.05), tenting volume (2.1±0.3 to 1.6 ± 0.3 mm2 P<0.05) and leaflet closure area (9.3 ± 0.8 to 8.2 ± 0.7 mm2, P<0.04). PVA was not associated with significant decreases in LVEF (42 ± 3 % vs 40 ± 2 %, p=ns) or end-systolic elastance. Measures of left ventricular diastolic function, tau (99 ± 55 ms to 87 ± 36;) and left ventricular stiffness coefficient (0.04 ± 0.03 to 0.05 ± 0.03) did not increase post PVA.
Conclusions
PVA hydrogel injections improve coaptation and reduce remodeling in chronic MR without impairing LV systolic and diastolic function. This new approach offers a potential alternative for relieving ischemic mitral regurgitation by correcting papillary muscle position, thus relieving tethering that causes ischemic mitral regurgitation.
Key Words: Mitral regurgitation, left ventricular remodeling, coronary artery disease
Journal Subject Heads: CV surgery: valvular disease, Chronic ischemic heart disease, Animal models of human disease
Introduction
Ischemic mitral regurgitation (MR) is an important sequella of coronary artery disease that significantly increases late mortality.1, 2 Extensive evidence has shown that IMR results from remodeling of the ischemic left ventricle (LV), leading to displacement of the papillary muscle (PM), annular dilatation, and therefore tethering of the mitral leaflets.3–5
Therapy for ischemic mitral regurgitation remains difficult. Mitral ring annuloplasty, performed at the time of bypass surgery, is the current therapy for ischemic MR and reduces mitral annular area by reducing the anterior–posterior diameter. However, there is a variable and often significant recurrence rate of ischemic MR of up to 30%.6–9 Given the fundamental mechanism of mitral leaflet tethering, mitral ring annuloplasty does not directly address the problem of ischemic left ventricular distortion. LV dilation is progressive so that initial annular compensation for ventricular dilatation may not be durable. 10–11
Therapies which directly reverse the ischemic distortion of the LV wall and leaflet tethering, have included surgical plication of the infarcted LV, surgical relocation of the papillary muscle, placement of external patch or discs over the infarct and leaflet augmentation; all which remain relatively invasive.12–16 An alternative approach, which is potentially less disruptive to the LV wall employs the use of biomaterials injected directly into the myocardium, acting as tissue-bulking agents to reposition the papillary muscles. Biomaterial injections could have the effect of displacing the papillary muscle toward the mitral annulus, potentially buttressing the weakened muscle wall to limit outward bulging.
One type of biomaterial which has physical and mechanical properties for use as a tissue-bulking agent is polyvinyl alcohol-based hydrogel (PVA hydrogel). The base polymer, PVA, is biocompatible, biologically inert, and in current use in human applications 17–21. It can be formulated so that it is injectable but forms a stable gel at body temperature.
We have previously tested the injection of a PVA hydrogel in an acute model of ischemic MR 22. Injection of PVA in the acute model of ischemic MR decreased mitral regurgitation, providing proof of concept for PVA hydrogel injection therapy; however, acute ischemic mitral regurgitation is not commonly encountered in the clinical setting. Therefore, it is important to establish whether injecting PVA hydrogel remains effective in a model of chronic ischemic MR which represents the most clinically relevant and challenging situation
Our hypothesis is that injection of PVA hydrogel into the myocardium is effective in reducing mitral regurgitation in a chronic ischemic model.
Methods
As detailed by Llaneras et al 23 anesthesia was induced in Dorsett hybrid sheep with sodium thiopental (12.5 mg/kg IV), and the trachea intubated and ventilated at 15 ml/kg with a mixture of 2% isoflurane and oxygen. All animals received glycopyrrolate (0.4 mg IV) and vancomycin (0.5 gm IV) one hour before incision. The heart was exposed through a sterile left thoracotomy. After the pericardium was opened, baseline imaging was performed and the second and third circumflex obtuse marginal branches were ligated to infarct the inferoposterior wall. The chest was then closed and the animals recovered.
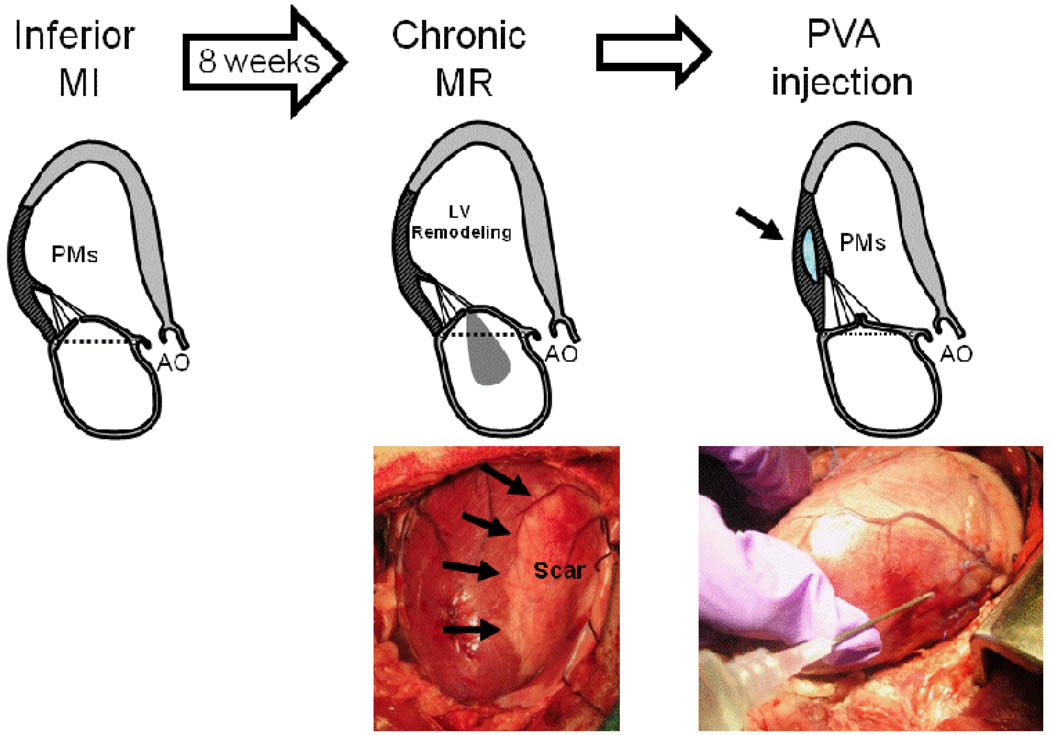
After eight weeks, each animal had a second thoracotomy under general anesthesia. Micromanometer-tipped Millar catheter was placed in the LV to measure heart rate, pressure, dP/dt, relaxation constant (tau) and stiffness coefficient. Echocardiographic imaging was performed at the following stages: Baseline, chronic MR and PVA hydrogel injection (post-PVA) (Figure 1).
Figure 1.
Experimental protocol using a sheep model of chronic ischemic mitral regurgitation. Echocardiography were performed at baseline, post Chronic mitral regurgitation (MR) and post PVA injection.
This study was reviewed and approved by our institutional Animal Care Committee.
Polymer injection
A 11% PVA hydrogel aqueous solution with 36% polyethylene glycol (PEG) (used as a gellant) (Cambridge Polymer Group, Inc, Boston, MA) was preformulated and stored in 10 cc syringes at room temperature (20 to 30 ° C) as a solid gel.24 The formulation of the PVA was modified from the acute studies to decrease viscosity, making it easier to inject into scar yet retain the same gelling profile. These syringes were packaged in heat-vacuum packed bags and terminally sterilized using 25 kGy gamma radiation (Steris Isomedix, Northboro, MA). The materials were tested for cytotoxicity and endotoxicity using conventional in vitro tests (LAL and Agarose overlay respectively). This PVA formulation was designed to gel at or near body temperature (below 45° C). The PVA hydrogel syringes were heated to over 90°C in a water bath to achieve liquid state and then allowed to cool. The cooled PVA formulation remains injectable for a reasonable period (5–8 minutes). The temperature of the polymer solution is monitored as it exits the needle with a thermometer attached to the needle exit point to ensure that injection occurs at 39°C to 40°C. Once injected into the myocardium, the PVA substantially solidifies over a 5–10 minute period. The PVA hydrogel was injected into the myocardium underlying the papillary muscles using a 18 gauge short bevel needle via a long tipped catheter. The location of the injections was guided by direct visualization of anatomic landmarks as well as by real-time echocardiography. Additional injections were performed as needed, guided by echo assessment of MR reduction. If there was no MR reduction following an initial injection, injections were repeated adjacent to the initial site to broaden the area of tissue bulking.
Data collection and analysis
LV pressure was recorded along with an ECG lead on a multichannel physiologic recorder (Sonometrics Inc, London, Ontario). 2D, Doppler, and 3D echo data were collected using an X3 matrix array transducer and iE33 Ultrasound machine (Philips Medical Systems, Andover, MA). Epicardial imaging was performed through an agarose standoff to minimize near field effects. For 3D reconstruction, the probe was positioned along the apex to align the LV apex through the center of the mitral valve. By adjusting the mitral valve to the center of the screen, sector settings were optimized for image and color resolution. 3D data sets were acquired using the full-volume mode over 4 to 7 sequential heart beats with ECG gating and suspended respiration. All images were stored on a DVD disk and transferred for offline analysis using QLAB Advanced Quantification Software, version 6.0 (Philips Medical Systems, Andover, MA). 3D datasets were exported from QLAB in a Cartesian-volumetric format and analyzed using customized software.25 MR was quantitated by measuring the vena contracta in a long-axis view perpendicular to the coaptation line, averaged in 3 cardiac cycles. Vena contracta width ≥5mm was considered moderate in degree.26–27 Polymer application was adjusted to reduce MR based on visual assessment of the proximal jet width.
LV and mitral valve measures
LV end-diastolic and end-systolic volumes (EDV, ESV) were obtained by 3D echo, using endocardial borders from 6 planes at equal angular intervals and using a surfacing algorithm.25 Mitral geometry was reconstructed from rotated images at mid-systole, when the leaflets most closely approach the annulus. Mitral annular area (MAA) was measured at mid-systole by manually tracing the annular points which were defined as the annular hinge points where the leaflets inserted. The PMs were traced to identify their tips by reviewing several adjacent images. The tethering length over which the mitral leaflets and chordae are stretched between the PMs and the relatively fixed fibrous portion of the annulus were measured from postero-medial PM tip to the medial trigone of the aortic valve (medial junction of aortic and mitral annuli).5,25 Leaflet closure surface area of leaflets defined as the area of the surface separating the LV and LA was calculated at mid-systole by measuring tracing leaflet surfaces using six imaging planes and applying a surface fitting algorithm. The tenting volume of the leaflets corresponds with the volume under the leaflets to the plane of mitral annulus at midsystole.28 LV volumes and contractile performance were assessed using 4 sonomicrometer crystals (Sonometrics) placed over the LV epicardium at the base and apex (long axis) and the anterior and posterior walls (short axis). Pressure–volume loops were constructed from continuous tracings of LV volume, calculated using a standard algorithm, and Millar micromanometer pressure. Sonomicrometry and pressure data were measured using CardioSOFT Pro 3.4 (Sonometrics). The slope (elastance) of the end systolic pressure–volume relationship as a relatively load-independent measure of LV contractility was obtained by transiently occluding the inferior vena cava with umbilical tape, thereby rapidly producing beats with varying systolic pressures and LV volumes. End systole was defined as the maximum ratio of LV pressure to LV volume and the end systolic points fitted to a linear equation; its slope (elastance) was taken as a measure of contractile state.29 End diastole was defined by the trough in the LV pressure tracing after atrial contraction. The end diastolic pressure–volume relationship data from caval occlusion were fitted to an exponential equation: LVEDP =D*exp(Kp*LV[EDV]) where D is a curve fitting variable and Kp is the stiffness coefficient, which is a measure of the compliance of the ventricle.(30) The relaxation time constant (tau) was calculated as the time for LV pressure to fall from peak negative dP/dt to half its value.
Histological studies
In a subset of animals, after sacrifice, hearts were excised for histologic examination of tissue architecture following injection. Injected myocardium was fixed in 10% formalin and histopathologic examination performed on 5µm sections stained with hematoxylin-eosin (H & E) and Masson’s trichrome stain for microscopic examination.
Statistical analysis
The efficacy of PVA hydrogel injection was tested by repeated measures analysis of variance (baseline, chronic-MR, post-PVA hydrogel). Significant differences were examined by paired t-test, using Fisher’s F-test criterion for multiple comparisons. A 2-tailed p-value of 0.05 was considered significant.
Conflicts of Interest Disclosures
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agreed to the manuscript as written. GJCB and JB are employees of and OKM has an equity interest in Cambridge Polymer Inc. The remaining authors have no conflicts to declare.
Results
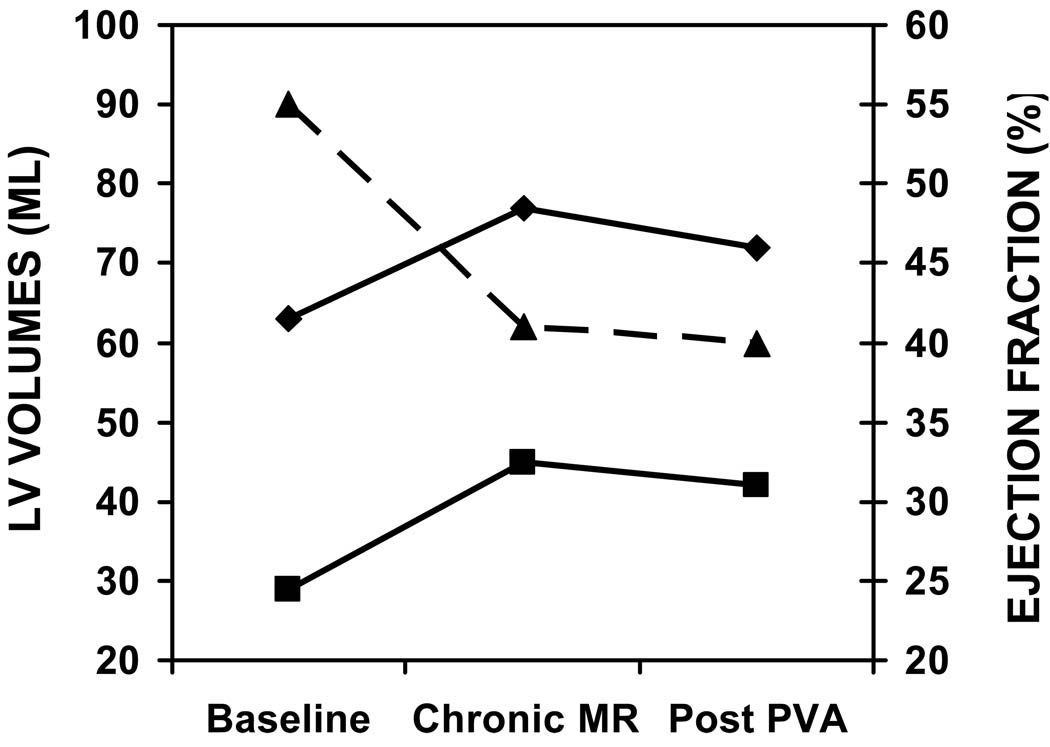
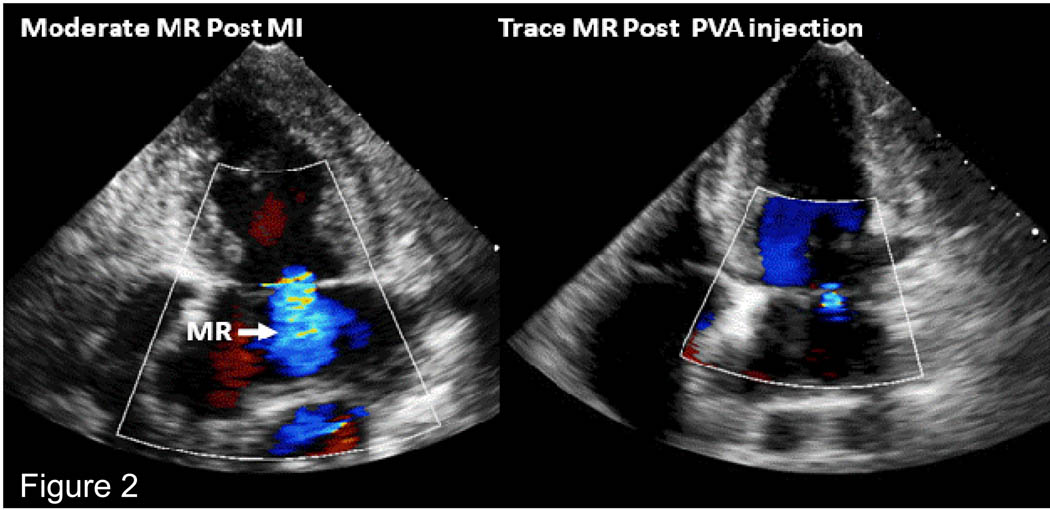
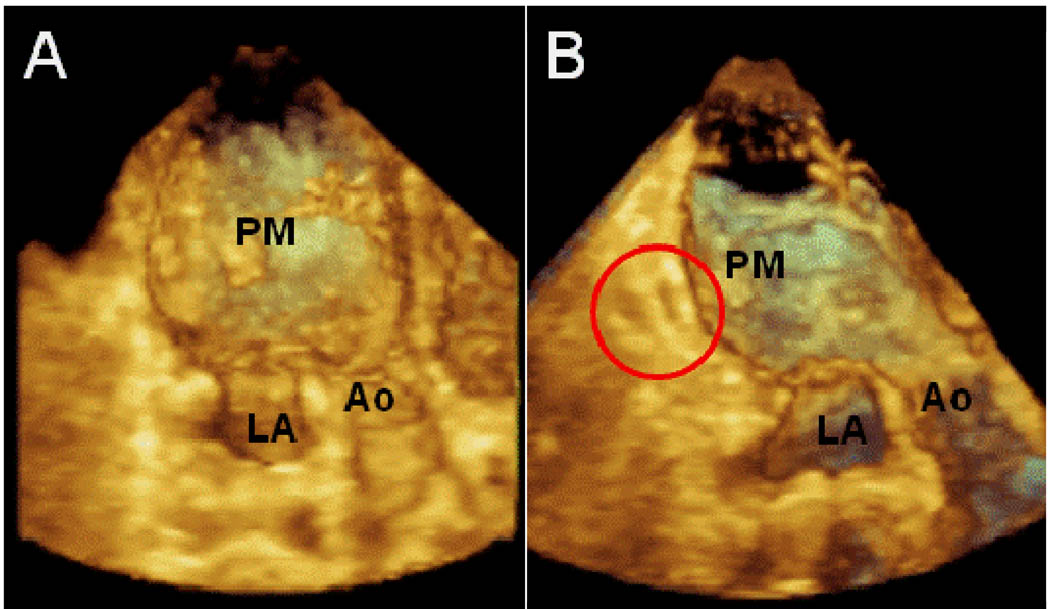
In 9 sheep, an infarction was created by ligation of left circumflex branches. One animal died following ligation but prior to PVA hydrogel injection and one did not develop MR. The remaining seven animals developed moderate mitral regurgitation. These 7 animals that developed moderate MR or greater underwent acute PVA hydrogel injection. PVA injection resulted in a decrease in MR from moderate to trace (vena contracta: 5.3 ± 0.8 mm vs. 2.3 ± 0.9 mm, chronic MR vs. post PVA; p<0.002). There was a significant decrease in LV ejection fraction following infarction compared to baseline. However, there was no change in LV ejection fraction (chronic MR 42 ± 3 % vs. 40 ± 2 %, post PVA p=ns; Figure 2) and there were no new wall motion abnormalities post PVA injection compared to the chronic MR stage. Figure 3 shows reduction in MR from moderate to trace in an animal following injection of a total of 4 mls of PVA given in 2 aliquots of 2 mls. 3D echocardiography is used to guide injection with visulaization of PVA gel within the myocardium post injection (Figure 4).
Figure 2.
Ejection fraction and volume data at all stages. Ejection fraction decreased significally from baseline to chronic MR stage (p= 0.04), but didn’t change post PVA injection. EDV: end-diastolic volume. ESV: end –systolic volume. EF: ejection fraction.
Figure 3.
Left panel shows moderate ischemic MR that has developed following ligation of left circumflex branches. Right panel: After injection of PVA hydrogel into infarcted myocardium over the papillary muscles, the MR has decreased to trace.
Figure 4.
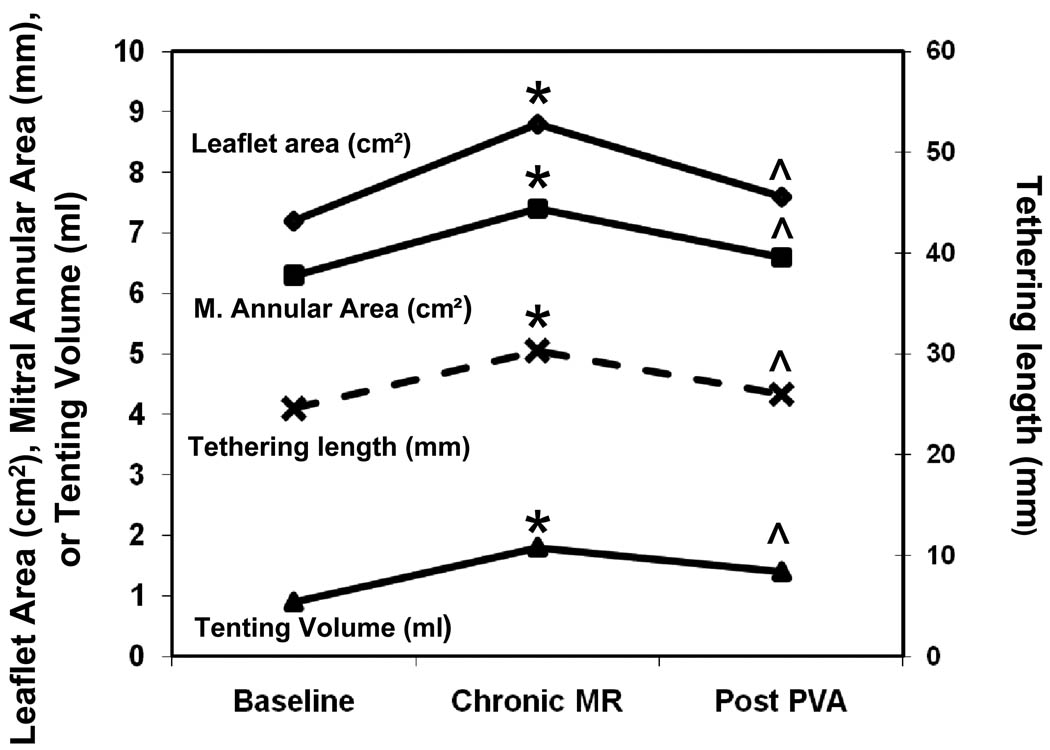
Changes in Mitral Valve Geometry at baseline, post chronic MR and post PVA stages
3D echocardiography examination of mitral valve geometry showed that tethering length (distance from mitral valve to infarcted papillary muscle) increased from 26.2 at baseline to 32.6 mm at chronic MR stage and decreased to 27.6 mm after PVA injection. Similarly, mitral annular area, leaflet closing area and tenting volume increased from baseline to chronic MR stage. These mitral geometric parameters returned to baseline levels with significant differences between chronic MR and post PVA stages (Figure 5).
Figure 5.
Real time 3D echocardiography showing PVA visualized within the myocardium (red circle). PM: papillary muscle. LA: left atrium. Ao: Aortic valve.
Heart rate, left ventricular pressure and maximal dP/dt were unchanged between Chronic MR stages and PVA injection. There were also no significant changes in elastance (Emax), tau (relaxation constant) or the LV diastolic stiffness coefficient after PVA injection (Table 1).These data suggest no detrimental effect on both LV systolic and diastolic function with localized PVA injection into the myocardium.
Table 1.
Hemodynamic and LV Function Data.
| Stage | |||
|---|---|---|---|
| Chronic MR | Post PVA | P* | |
| Heart rate (Beats/min) | 96 ± 11 | 100 ± 9 | ns |
| LV pressure (mmHg) | 76 ± 11 | 75 ± 17 | ns |
| dP/dt (mmHg) | 779 ± 127 | 884 ± 298 | ns |
| Emax, mmHg/mL | 2.6 ± 1 | 3.2 ± 0.8 | ns |
| Tau (ms) | 99 ± 55 | 87 ± 36 | ns |
| Stiffness Coefficient | 0.04 ± 0.03 | 0.05 ± 0.03 | ns |
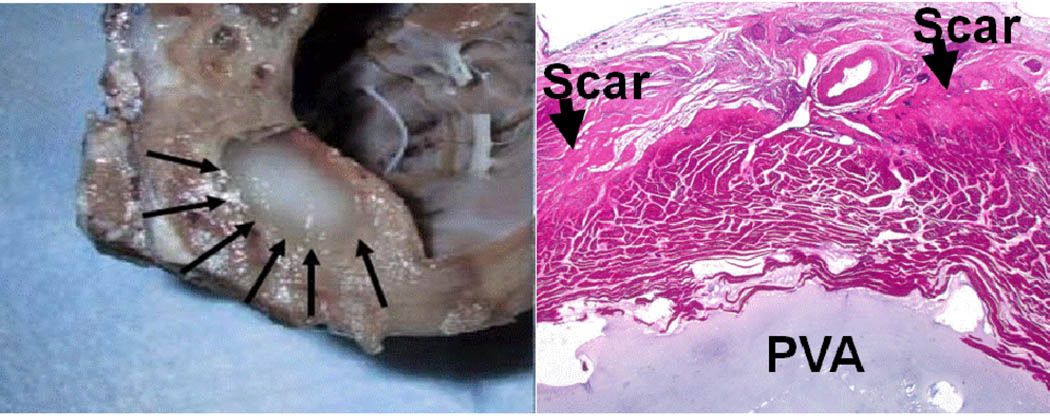
A total of 4.2 +/−0.8 ml of PVA were injected. In 5/7 sheep, between 2–3 injections (each containing 2–3 ml of PVA gel) were performed to achieve MR reduction. Gross anatomical examination showed the PVA hydrogel encapsulated into the myocardium and histology showed that PVA had gelled in a discrete collection adjacent to infarcted myocardium and dense scar (Figure 6).
Figure 6.
A) Gross appearance of PVA hydrogel (arrows) injected into chronic infarction. B) Histology showed PVA (blue) had gelled in a discrete collection adjacent to infarcted myocardium and dense scar (arrows).
Discussion
This study demonstrated efficacy of PVA hydrogel injection in reducing mitral regurgitation in a chronic ischemic model of mitral regurgitation. This appears to occur without detriment to LV systolic or diastolic function.
Polymers have been used in an increasing number of biological applications including plastic and reconstructive, vascular and urological uses 30–33. PVA is a chain of carbons with alternating hydrogen and oxygen. It is made by hydrolysis of polyvinyl acetate, is highly hydrophilic, biologically inert and biocompatible. PVA hydrogel is formed by PVA chains connected by hydroxyl groups, using polyethylene gycol as a gellent to initiate gelling. PVA is currently used as embolization spheres in vascular applications, as cartilage replacement and nerve sheath guides and as rheology modifiers in soft contact lenses for dry eye treatment.17–21 These current PVA applications utilize the solid form of PVA, not a suitable formulation for application in the beating heart. Therefore, an injectable formulation of PVA hydrogel 24 was developed and tested for use as a tissue bulker in infarcted myocardium. PVA can be formulated to be an injectable liquid when heated to 90°C but begins to gel at controlled rates at body temperatures.
PVA injection did not result in a decrease in measures of global LV systolic or diastolic function. Because the PVA injections are focused on a relatively small area of the heart, the reduction in MR likely results from reverse localized remodeling. PVA hydrogel injection may reposition the papillary muscles both by causing tissue displacement and in principle altering the myocardial mechanics so that the underlying myocardium bulges less. The localized nature and relatively small volume of PVA injections would not be expected to have a significant impact if any on LV function or volumes. This was reflected in the lack of change in our measures of LV function following PVA injection compared to the chronic MR stage. However, with extensive PVA injections as might be necessary in large infarction and extensive remodeling, alterations in diastolic LV function may occur and will need to be further assessed.
PVA injections did not result in disruption of the surrounding myocardial fibers. Both MRI studies performed in the acute ischemic MR studies 22 and histological examination showed that PVA injections produced a well-defined cross-linked gel surrounded by necrotic myocardium and fibrous scar. The PVA hydrogel did not disrupt or “ooze” into the surrounding myocardial architecture, rather, the PVA hydrogel forms a stable well encapsulated gel within 5 minutes after injection at body temperatures. The crosslinking of PVA occurs through hydrogen bonding which exceeds the bonding forces likely to be present with adjacent tissue planes.
Initial pilot studies on acute ischemic mitral regurgitation demonstrated proof of concept for PVA injection therapy.22 However, it was critical to demonstrate efficacy in a chronic ischemic model of mitral regurgitation that is the clinically most relevant setting for therapy, reflecting patients presenting with chronic infarction and ischemic MR. Additionally, it was important to establish the ability of PVA hydrogel to form a stable tissue bulking gel not only in acute myocardial infarction but also in chronic infarction where there is an admixture of necrotic myocytes and fibrous scar. The advantage of a polymer based approach is that polymer chemistry has advanced to the point where physical and material characteristics can be modified to adapt to specific clinical situations. For example, injection characteristics may be different in chronic infarction where there is more fibrous tissue than in acute infarction. The formulation of the PVA was modified from the acute studies to decrease viscosity, making it easier to inject into scar yet retain the same gelling profile. Furthermore, the viscosity and rheology of the PVA hydrogel can be further adjusted so that longer but narrower delivery systems used in minimally invasive techniques could potentially be employed.
PVA injection may also function as an “internal stiffener or patch”, acting to limit further remodeling by constraining the myocardium. Injection of collagen into infarcted rat myocardium resulted in a reduction in post myocardial infarction LV remodeling, supporting the concept that localized tissue bulking can limit adverse LV remodeling.34 In addition, finite element modeling of material injection into the myocardium has predicted beneficial effects on ventricular mechanics.35
Clinical Implications
Polymer injection has potential advantages over current techniques of treating ischemic mitral regurgitation. Methods based on solely annular approaches (both surgical and percutaneous) have limitations in that they do not directly modify ischemic ventricular distortion. Previous studies have demonstrated efficacy in experimental models and case studies employing therapies which directly address ventricular distortion or leaflet tethering such as external patching over the infarct, augmentation of the posterior leaflet by insertion of a pericardial patch, or surgical relocation of the papillary muscle.12–16 Yet, the proposed ventricular or leaflet approaches remain relatively invasive. PVA hydrogel injection has the potential to be less invasive, and hence may be more widely applied. PVA hydrogel technique can also be tailored to the individual patient, adjusting for the variable degree of tethering among patients, with potential benefit to involve both papillary muscles and annular and subannular levels.
Limitations
This study examined acute PVA injections into chronic ischemic MR and the efficacy of chronic PVA injections in maintaining MR reduction remains to be determined in future studies.
PVA hydrogel injections may be limited in globally dilated and dysfunctional left ventricles as this may require more extensive reverse remodeling than possible with localized tissue bulking therapy. However, it could be possible to make serial sequential injections over both papillary muscles to achieve symmetric reverse remodeling of PM displacement.
Conclusion
PVA hydrogel injections improve coaptation and reduce remodeling chronic MR without impairing LV systolic or diastolic function. This new approach offers a potential alternative for relieving ischemic mitral regurgitation by correcting papillary muscle position, thus relieving tethering that causes ischemic mitral regurgitation.
Funding Sources and Acknowledgements
This work was supported in part by NIH/National Institute for Biomedical Imaging and Bioengineering (NIBIB) R21 EB005294, NIH/(National Heart Lung Blood Institute R01 HL092101 (JH), NIH/ (National Heart Lung Blood Institute) NHLBI R01 038176 and K24 HL67434, grant 07CVD04 of Fondation Leducq, Paris, France (RAL) and Echo-Investigator Award from the American Society of echocardiography (JH), and an American Society of Echocardiography Career Development Award and Spanish Society of Cardiology (post-residency grant) (JS).
References
- 1.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997;96:827–833. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 3.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation. 1997;96:1826–1834. doi: 10.1161/01.cir.96.6.1826. [DOI] [PubMed] [Google Scholar]
- 4.Komeda M, Glasson JR, Bolger AF, Daughters GT, 2nd, Ingels NB, Jr, Miller DC. Papillary muscle-left ventricular wall "complex". J Thorac Cardiovasc Surg. 1997;113:292–300. doi: 10.1016/s0022-5223(97)70326-x. [DOI] [PubMed] [Google Scholar]
- 5.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 6.Gazoni LM, Kern JA, Swenson BR, Dent JM, Smith PW, Mulloy DP, Reece TB, Fedoruk LM, Lisle TC, Peeler BB, Kron IL. A change in perspective: results for ischemic mitral valve repair are similar to mitral valve repair for degenerative disease. Ann Thorac Surg. 2007;84:750–757. doi: 10.1016/j.athoracsur.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 7.McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, Shiota T, Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Mihaljevic T, Lam BK, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM, Blackstone EH, Lytle BW. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 9.Tatha SAOJ, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Disease. 2001;11:11–18. [PubMed] [Google Scholar]
- 10.Guy TSt, Moainie SL, Gorman JH, 3rd, Jackson BM, Plappert T, Enomoto Y, St John-Sutton MG, Edmunds LH, Jr, Gorman RC. Prevention of ischemic mitral regurgitation does not influence the outcome of remodeling after posterolateral myocardial infarction. J Am Coll Cardiol. 2004;43:377–383. doi: 10.1016/j.jacc.2003.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;110 Suppl 1:II85–II90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 12.Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse ventricular remodeling reduces ischemic mitral regurgitation: echo-guided device application in the beating heart. Circulation. 2002;106:2594–2600. doi: 10.1161/01.cir.0000038363.83133.6d. [DOI] [PubMed] [Google Scholar]
- 13.Mishra YK, Mittal J, Jaguri P, Trehan N. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation:1-year results. Ann. Thorac. Surg. 2006;81:42–46. doi: 10.1016/j.athoracsur.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002;74:600–601. doi: 10.1016/s0003-4975(02)03749-9. [DOI] [PubMed] [Google Scholar]
- 15.Langer F, Rodriguez F, Cheng A, Ortiz S, Nguyen TC, Zasio MK, Liang D, Daughters GT, Ingels NB, Miller DC. Posterior mitral leaflet extension: an adjunctive repair option for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2006;131:868–877. doi: 10.1016/j.jtcvs.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski LG, Hunziker PR, Tanabe H, Scherrer-Crosbie M, Sullivan S, Levine RA. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation. 2000;101:2756–2763. doi: 10.1161/01.cir.101.23.2756. [DOI] [PubMed] [Google Scholar]
- 17.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000 Jul;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 18.Ku DB. Reinforced Uncrosslinked Poly (vinyl alcohol) Cryogel. Atlanta, GA: Georgia Tech Research Corporation; 1999. [Google Scholar]
- 19. http://salumedica.com/history.htm.
- 20.Hassan CM. Structure and Applications of Poly(Vynyl Alcohol) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. Advances in Polymer Science. 2000;153:37–65. [Google Scholar]
- 21.Hassan CM, Stewart JE, Peppas NA. Diffusional characteristics of freeze/thawed poly(vinyl alcohol) hydrogels: applications to protein controlled release from multilaminate devices. Eur J Pharm Biopharm. 2000;49:161–165. doi: 10.1016/s0939-6411(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 22.Hung J, Solis J, Guerrero JL, Braithwaite GJ, Muratoglu OK, Chaput M, Fernandez-Friera L, Handschumacher MD, Wedeen VJ, Houser S, Vlahakes GJ, Levine RA. A novel approach for reducing ischemic mitral regurgitation by injection of a polymer to reverse remodel and reposition displaced papillary muscles. Circulation. 2008;118:S263–S269. doi: 10.1161/CIRCULATIONAHA.107.756502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, Edmunds LH., Jr Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57:432–439. doi: 10.1016/0003-4975(94)91012-x. [DOI] [PubMed] [Google Scholar]
- 24.Ruberti JW, Braithwaite GJC. “Systems and methods for controlling and forming polymer gels”. 7,485,670. US Patent. 2008
- 25.Handschumacher MD, Lethor JP, Siu SC, Mele D, Rivera JM, Picard MH, Weyman AE, Levine RA. A new integrated system for three-dimensional echocardiographic reconstruction: development and validation for ventricular volume with application in human subjects. J Am Coll Cardiol. 1993:743–753. doi: 10.1016/0735-1097(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 26.Mele D, Vandervoort P, Palacios I, Rivera JM, Dinsmore RE, Schwammenthal E, Marshall JE, Weyman AE, Levine RA. Proximal jet size by Doppler color flow mapping predicts severity of mitral regurgitation. Circulation. 1995;91:746–754. doi: 10.1161/01.cir.91.3.746. [DOI] [PubMed] [Google Scholar]
- 27.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 28.Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, Levine RA. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res. 1973;32:314–322. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- 30.Pagel PS, Kampine JP, Schmeling WT, Warltier DC. Alteration of left ventricular diastolic function by desflurane, isoflurane, and halothane in the chronically instrumented dog with autonomic nervous system blockade. Anesthesiology. 1991;74:1103–1114. doi: 10.1097/00000542-199106000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Kershen RT, Atala A. New advances in injectable therapies for the treatment of incontinence and vesicoureteral reflux. Urol Clin North Am. 1999;26:81–94. doi: 10.1016/s0094-0143(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 32.Nimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997;40:675–682. doi: 10.1097/00006123-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Radovan C. Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74:482–492. doi: 10.1097/00006534-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 35.Wall ST. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]