Abstract
Understanding the determinants of childhood secondhand smoke (SHS) exposure is important in measuring and preventing exposure to this widespread environmental contaminant. We evaluated the ability of a broad set of factors to explain variability in serum cotinine, reflecting recent exposure, and hair cotinine, reflecting longer-term exposure. We included repeated measures from 223 elementary-school-age asthmatic children residing with a smoker. We used a manual model-building approach and likelihood ratio tests to select a model predicting each biomarker, and also compared the predictive ability of determinants using Akaike Information Criteria. Potential determinants included a comprehensive parent questionnaire, household nicotine, home ventilation characteristics, exposure in vehicles and others’ homes, child demographics, and family social class. Variables in each of these categories remained in the final model for both serum (R2 of 0.61) and hair cotinine (R2 of 0.45). A comprehensive set of factors was required to best predict cotinine. Studies should use biomarkers for the best quantitative assessment of SHS exposure. Hair cotinine may be a problematic measure because it was highly influenced by racial differences that were unexplained by SHS exposure. When biospecimen collection is not possible, a household nicotine measurement is warranted. If only questionnaires are available, multiple questions are required to best characterize exposure, such as number of cigarettes, hours spent in a room with concurrent smoking, maternal smoking, and approximate home size.
Keywords: cotinine, secondhand smoke, questionnaire, nicotine dosimeter
Introduction
Children’s exposure to secondhand smoke (SHS) remains a widespread and severe public health problem. Recent estimates suggest that 40% of US children live with a smoker (Kum-Nji et al., 2006; Wilkinson et al., 2006). It is now well established that SHS contributes to pediatric ear infections, asthma, respiratory infections, and other respiratory disorders (Tutka et al., 2002; DiFranza et al., 2004), with evidence that it impairs neurodevelopment (Mendola et al., 2002; Yolton et al., 2005, 2008; Braun et al., 2008). Accurate quantitative measurement of SHS is therefore important when considered as a primary risk factor and as a confounder of other exposures. In addition, understanding determinants of exposure is useful in designing SHS-reduction policies and interventions.
Biomarkers can objectively and accurately reflect an internal dose integrating all sources of exposure. The nicotine metabolite cotinine is considered the best current measure of SHS exposure. It closely approximates criteria set by the National Research Council for a valid marker and has been positively correlated with risks of SHS-related health problems in children (Benowitz, 1999). Cotinine measured in serum reflects exposure over a few recent days (Jaakkola and Jaakkola, 1997), and that measured in hair reflects exposure over several months (Al-Delaimy, 2002).
Many studies have evaluated predictors of serum, urine, and salivary cotinine. Factors consistently shown to be predictive include quantitative parent report of exposure such as number of cigarettes smoked, home size and ventilation, and child’s race and age. Only a few have evaluated predictors of longer-term exposure as reflected by hair nicotine or hair cotinine (Woodruff et al., 2003; Groner et al., 2005; Wipfli et al., 2008). Many estimations of the association between determinants of exposure and cotinine do not account for other correlated factors, so that reported associations may be spurious or over- or underestimates. Of studies that did use many factors in a multivariable framework, often several variables from different domains independently predict the biomarker of exposure, ranging from parent report of smoking to social class to day of the week (Jarvis et al., 1992; Bakoula et al., 1997; Henschen et al., 1997; Irvine et al., 1997; Mannino et al., 2001; Jurado et al., 2004; Halterman et al., 2008; Kumar et al., 2008). No study has evaluated whether parent report or an environmental measurement better predicts exposure as reflected by a biomarker.
The purpose of this study was to characterize the ability of a broad set of determinants to explain variability in serum and hair cotinine, in part to help researchers decide whether to use questionnaires, biomarkers, or environmental measures of SHS. We used data from the Cincinnati Asthma Prevention Study that included proximal factors such as parent report and an air nicotine dosimeter, measures outside the home environment, and more distal factors that may contribute to cotinine levels, such as home ventilation, child’s race and age, and family social class. We used a multivariable model-building approach to determine which factors were most important in explaining levels of serum cotinine and hair cotinine to quantify the independent contribution of each predictor.
Methods
Study Population
We included participants of a randomized-controlled trial of SHS reduction using air cleaners among elementary-school-age asthmatic children living with a smoker, the Cincinnati Asthma Prevention Study (2000–2003) (Wilson et al., 2005, 2007; Spanier et al., 2006; Yolton et al., 2008). Briefly, of 2240 children with medical records indicating the possibility of asthma, 1678 were screened by telephone for doctor-diagnosed asthma and exposure to five or more cigarettes smoked in and around the home, yielding 341 eligible and 225 enrolled participants. Because we focused on environmental tobacco smoke exposure, we excluded two children with strong evidence of direct smoking: parent report or repeated serum cotinine values >100 ng/ml, yielding a study sample of 223. Data collection took place during a telephone screening interview and during visits to the child’s home just before the intervention (baseline) and at 6 and 12 months. This study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board, and parents provided written informed consent.
Cotinine Measurements
Serum was collected using standard phlebotomy at each study visit and stored at −20°C until analysis. Serum cotinine analyses were performed in the Division of Laboratory Science, National Center for Environmental Health at the Centers for Disease Control and Prevention using high-performance liquid chromatography and atmospheric-pressure ionization tandem mass spectrometry (Bernert et al., 2000, 1997). This method has a limit of detection of 0.05 ng/ml.
Hair was collected by cutting about 20 strands near the root in the occipital region of the head. The root end of the hair was indicated and the hair was stored at room temperature until analysis. Three centimeters of hair proximal to the scalp were cut to roughly capture hair growth in the previous 3 months (Uematsu et al., 1995). Hair cotinine was measured by the Division of Clinical Pharmacology at the University of Toronto using a validated radioimmunoassay procedure after applying intraday and interday reliability assurance standards (Langone et al., 1973; Klein and Koren, 1999; Gareri, 2010). The limit of detection was 0.005 ng/mg.
Environmental Measurements
We measured ambient nicotine in the home using nicotine dosimeter cassettes placed in the room where parents reported that most family activity took place. The sampler was placed at a standardized height of approximately 64 cm away from corners of the room. Cassettes were left in place for 6-month intervals and were collected during the 6 and 12-month home visits. The dosimeters included a filter treated with sodium bisulfate, which binds nicotine after passive diffusion past an air screen. The filter was analyzed using gas chromatography with nitrogen-selective detection with a limit of detection of 0.01 μg per filter (Hammond and Leaderer, 1987). Nicotine concentration was calculated by dividing the number of micrograms of nicotine on the filter by the volume of air samples, which is a function of time due to passive diffusion past the windscreen (Coghlin et al., 1989).
An environmental technician measured the size of each room (length, width, and height), noted housing characteristics that could influence ventilation, and rated the perceived smokiness of the home compared with other study homes.
Parent Questionnaire
Family characteristics and aspects of SHS exposure were collected by interviewer-administered parent questionnaire. We collected details about the number of household members and visitors and their smoking habits around the home. We developed a questionnaire to additionally assess six environments in which elementary-school-aged children spend time: their home, others’ homes, restaurants, other public places, after-school care/activities, and vehicles. We adapted a duration-intensity approach found successful in measuring SHS in adults (Coghlin et al., 1989). For each situation, we asked details about hours spent in this activity on the day before the home visit and the intensity of any cigarette smoke. For example, we would ask if smoking usually occurred in the car, and if the windows were open or closed. Interviewers also administered the Home Observation for Measurement of the Environment (HOME) instrument at 12 months to characterize the nurturing quality of the home environment (Caldwell and Bradley, 1984).
Statistical Analysis
The natural log of cotinine was used in all analyses to normalize model residuals. For each continuous predictor variable, the best of several metrics was selected as indicated by the lowest Akaike Information Criteria (AIC) in models predicting cotinine. The AIC is a measure of the goodness of fit of a model based on the likelihood function with a penalty for a higher number of parameters (Akaike, 1974). Metrics evaluated included the original linear form, a linear plus a quadratic term, a logarithmic transformation, and categorizations created after examining localized regression graphs (LOESS). Twenty hair cotinine values below the limit of detection were replaced with the limit of detection divided by the square root of 2 before log transformation (Hornung and Reed, 1990). Non-home places of SHS exposure were categorized to collapse across small cells and to simplify information without losing predictive ability. For example, because they did not improve predictive ability in bivariate analyses, we combined information about whether smoking occurred “some of the time” or “most of the time” in others’ homes, and whether the windows were open or closed in a vehicle during SHS exposure.
Data from all study visits were used in a repeated measures design correcting for within-subject correlations to maximize precision. The AIC was used to select the optimal correlation structure. We used a linear mixed model with an unstructured correlation matrix for serum cotinine and an exchangeable matrix for hair cotinine. All models were adjusted for study design factors: randomization assignment (intervention, placebo) and study visit (baseline, 6 months, 12 months). The restricted maximum likelihood estimation algorithm was used to obtain β-coefficients and associated standard errors (Harville, 1977). Coefficients were exponentiated in order to give a multiplicative change in cotinine by level of each predictor.
We built multivariable predictive models, separately for serum and hair cotinine. We included factors after review of the extensive literature comparing individual factors and cotinine levels in children. We selected a set of variables that maximized the ability to explain variance in cotinine without including extraneous factors using a manual sequential model-building process, as follows. Determinants were grouped by place and measurement type and entered in the model so that more proximal and important groups (e.g., parent report of smoking at home) were entered before more distal factors (e.g., family social class). Because race was a powerful predictor of hair cotinine (Knight et al., 1996) and also related to many other exposure determinants, race was included in all hair cotinine models. Groups were entered in the following order: (1) parent questionnaire of SHS exposure at home (Bakoula et al., 1997; Peterson et al., 1997); (2) environmental measures such as household nicotine concentration from the nicotine dosimeter (Henderson et al., 1989) and perception of smokiness of the home (Forastiere et al., 1993; Jurado et al., 2004) (3) housing and ventilation characteristics, which may dilute SHS inside the home, including season (Jarvis et al., 1992), ventilation (Bakoula et al., 1997; Johansson et al., 2004), and home size (Henschen et al., 1997; Mannino et al., 2001); (4) SHS exposure outside of the home environment, such as in vehicles and others’ homes (Sexton et al., 2004); (5) child characteristics, such as age (Bakoula et al., 1997; Irvine et al., 1997), child sex (Jarvis et al., 1992; Jurado et al., 2004), and African-American race (Mannino et al., 2001; Sexton et al., 2004; Wilson et al., 2005, 2007); and finally (6) family social class, which may be related to unmeasured more proximal determinants of SHS exposure, such as crowding of the home and the likelihood that the child would encounter other environments with tobacco exposure (Mannino et al., 2001; Jurado et al., 2004).
A group of determinants was retained until the next step if removing all factors in the group resulted in a likelihood ratio P-value <0.10. Alpha=0.10 was chosen because we were interested in building a comprehensive predictive model rather than hypothesis testing. We compared nested models holding the number of observations constant and using the maximum likelihood estimating algorithm in linear mixed models. If the group met these inclusion criteria, then individual factors within the group were considered for removal using likelihood ratio tests, so that the smallest number of important variables was retained. In a few cases, we compared non-nested models using the AIC; for example, comparing the similar variable number of rooms vs home volume and comparing the performance of the nicotine dosimeter to parent report.
To quantify the amount of variability in cotinine explained by increasingly complex models, data from the 12-month visit only was modeled using ordinary least squares linear regression to generate R2 estimates.
To quantify the explanatory ability of individual determinants or groups of determinants such as those from the parent questionnaire, we calculated the reduction in AIC compared to a base model not including that factor.
Results
Participants (Table 1) were about half of African-American race and half white and were of predominantly moderate-to-low social class. Children’s ages ranged from 5 to 12 years at baseline, and 62% were male.
Table 1.
Geometric means and standard deviations of serum cotinine and hair cotinine at baseline stratified by parent and environmental measures of SHS exposure and by housing, child and family characteristics, Cincinnati Asthma Prevention Study (2000–2003)
| N | % | Serum cotinine, ng/ml n=220 |
Hair cotinine, ng/mg n=205 |
|||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| Parent report of SHS at home | ||||||
| Hours at home | ||||||
| 18–24 | 72 | 32 | 1.0 | 3.7 | 0.14 | 3.6 |
| 15<18 | 89 | 40 | 1.4 | 3.3 | 0.14 | 3.7 |
| 0<15 | 62 | 28 | 1.1 | 3.4 | 0.13 | 3.6 |
| Hours in a room with smoking | ||||||
| <2 | 42 | 19 | 2.9 | 2.5 | 0.26 | 2.8 |
| ≤2 | 61 | 27 | 1.6 | 2.4 | 0.16 | 3.6 |
| 0 | 120 | 54 | 0.7 | 3.5 | 0.10 | 3.6 |
| Number of cigarettes/day | ||||||
| 20–104 | 70 | 31 | 2.1 | 2.8 | 0.18 | 4.1 |
| 10<20 | 64 | 29 | 1.1 | 3.4 | 0.12 | 3.0 |
| 0≤10 | 89 | 40 | 0.8 | 3.4 | 0.12 | 3.7 |
| Number of smokers in the home | ||||||
| 3+ | 9 | 4 | 3.2 | 3.5 | 0.22 | 4.3 |
| 2 | 72 | 32 | 1.5 | 2.9 | 0.15 | 3.8 |
| 0 or 1 | 142 | 64 | 1.0 | 3.6 | 0.13 | 3.5 |
| Maternal smoking | ||||||
| Yes | 166 | 74 | 1.5 | 2.8 | 0.16 | 3.5 |
| No | 57 | 26 | 0.6 | 4.5 | 0.08 | 3.5 |
| Paternal smoking | ||||||
| Yes | 77 | 35 | 0.9 | 3.8 | 0.10 | 4.1 |
| No | 146 | 65 | 1.3 | 3.2 | 0.16 | 3.3 |
| Environmental measures of SHS | ||||||
| Household nicotinea (μg/m3) | ||||||
| 1.3–15.8 | 69 | 34 | 2.4 | 2.6 | 0.16 | 3.5 |
| 0.24<1.3 | 64 | 31 | 1.5 | 2.5 | 0.15 | 4.3 |
| 0<0.24 | 72 | 35 | 0.3 | 3.7 | 0.06 | 4.4 |
| Staff perception of smokiness | ||||||
| Smoky | 48 | 22 | 1.8 | 3.0 | 0.18 | 3.2 |
| Not smoky | 163 | 77 | 1.0 | 3.6 | 0.13 | 3.9 |
| Housing and ventilation | ||||||
| Home volume (m3) | ||||||
| 66<168 | 73 | 33 | 1.8 | 3.6 | 0.18 | 3.6 |
| 168<235 | 77 | 35 | 1.4 | 2.7 | 0.18 | 3.1 |
| 235–660 | 73 | 33 | 0.6 | 3.4 | 0.09 | 3.7 |
| Number of rooms | ||||||
| 2–4 | 52 | 23 | 2.1 | 3.1 | 0.18 | 3.5 |
| 5–7 | 120 | 54 | 1.2 | 3.3 | 0.14 | 3.5 |
| 8–13 | 51 | 23 | 0.7 | 3.5 | 0.10 | 3.9 |
| Open floor plan | ||||||
| Traditional | 211 | 95 | 1.2 | 3.4 | 0.14 | 3.6 |
| Open | 11 | 5 | 1.2 | 3.8 | 0.15 | 5.7 |
| Air conditioning | ||||||
| None | 59 | 27 | 1.7 | 3.1 | 0.23 | 3.9 |
| Window unit | 59 | 27 | 1.5 | 2.8 | 0.23 | 2.6 |
| Central air | 102 | 46 | 0.8 | 3.8 | 0.08 | 3.3 |
| Fan running during visit | ||||||
| Yes | 44 | 20 | 1.4 | 3.4 | 0.18 | 3.1 |
| No | 179 | 80 | 1.1 | 3.5 | 0.13 | 3.7 |
| Season | ||||||
| Spring | 59 | 26 | 1.2 | 3.3 | 0.13 | 4.2 |
| Summer | 66 | 30 | 1.0 | 3.7 | 0.20 | 2.7 |
| Winter | 57 | 26 | 1.6 | 3.5 | 0.13 | 3.7 |
| Fall | 41 | 18 | 1.0 | 3.1 | 0.10 | 3.8 |
| SHS outside of home | ||||||
| Vehicles | ||||||
| >1 h | 12 | 6 | 1.9 | 3.6 | 0.20 | 5.1 |
| ≤1 h | 45 | 21 | 1.6 | 3.0 | 0.13 | 3.4 |
| None | 161 | 74 | 1.0 | 3.5 | 0.13 | 3.5 |
| other’s homes | ||||||
| Any | 49 | 22 | 2.0 | 2.4 | 0.16 | 3.7 |
| None | 172 | 77 | 1.0 | 3.7 | 0.13 | 3.6 |
| Child characteristics | ||||||
| African-American race | ||||||
| Yes | 124 | 56 | 1.4 | 3.1 | 0.24 | 3.0 |
| No | 99 | 44 | 1.0 | 3.8 | 0.07 | 3.2 |
| Gender | ||||||
| Female | 85 | 38 | 1.5 | 3.8 | 0.14 | 4.1 |
| Male | 138 | 62 | 1.0 | 3.2 | 0.14 | 3.3 |
| Age (years) | ||||||
| 5<7 | 62 | 28 | 1.2 | 3.9 | 0.12 | 4.0 |
| 7<10 | 103 | 46 | 1.2 | 3.2 | 0.15 | 3.5 |
| 10–12 | 58 | 26 | 1.1 | 3.4 | 0.14 | 3.4 |
| Family social class | ||||||
| Parental education | ||||||
| <HS | 41 | 18 | 2.1 | 3.4 | 0.25 | 3.0 |
| HS Degree | 104 | 47 | 1.1 | 3.4 | 0.11 | 3.6 |
| Some college | 51 | 23 | 1.1 | 3.4 | 0.14 | 4.3 |
| College degree | 27 | 12 | 0.7 | 3.3 | 0.11 | 2.7 |
| Income ($/year) | ||||||
| 0–20 000 | 93 | 42 | 1.8 | 2.7 | 0.20 | 3.1 |
| 20 000–40 000 | 67 | 30 | 1.1 | 3.2 | 0.17 | 3.6 |
| 40 000+ | 54 | 24 | 0.5 | 3.6 | 0.06 | 3.2 |
| Health insurance | ||||||
| Public or none | 125 | 56 | 1.7 | 2.8 | 0.23 | 3.2 |
| Private | 98 | 44 | 0.8 | 3.9 | 0.08 | 3.3 |
| Current marital status | ||||||
| Not married | 138 | 62 | 1.6 | 3.1 | 0.20 | 3.1 |
| Married | 85 | 38 | 0.7 | 3.6 | 0.08 | 3.6 |
| Persons per room | ||||||
| 1–2.25 | 59 | 26 | 1.7 | 3.3 | 0.19 | 3.6 |
| 0.5<1 | 107 | 48 | 1.1 | 3.4 | 0.13 | 3.7 |
| 0.2≤0.5 | 57 | 26 | 0.9 | 3.6 | 0.11 | 3.4 |
| HOME score | ||||||
| 17<45 | 66 | 30 | 1.9 | 3.2 | 0.28 | 3.4 |
| 45≤50 | 62 | 28 | 1.6 | 3.1 | 0.16 | 3.1 |
| 50–59 | 81 | 36 | 0.6 | 3.3 | 0.07 | 3.3 |
SHS, secondhand smoke; M, geometric mean; SD, geometric standard deviation; HS, high school; HOME, Home Observation for Measurement of the Environment (Caldwell and Bradley, 1984).
Nicotine dosimeter reflects levels for the first 6 months of the study.
The children spent on average 17 h at home per day, during which 1.3 h were in a room with someone smoking (range 0–14 h). Thirty-six percent lived with two or more smokers, 74% with a mother and 35% with a father who smoked, with a mean of 16 cigarettes smoked in and around the home per day. Children were exposed to SHS in vehicles and others’ homes (Table 1). Exposure in other places was infrequent: at baseline, two children had exposure during after-school care, five children had any SHS exposure in restaurants, and eight children had exposure in other public places. Consequently, exposure in these places was not considered for multivariable models.
Average values for household nicotine, serum cotinine, and hair cotinine were stable during the study period (Table 2). Spearman’s rank correlations between serum and hair cotinine values overall and by study visit were all 0.5. The intraclass correlation coefficient was 0.78 for serum cotinine and 0.57 for hair cotinine, indicating that cotinine measurements within the same child across time were more similar than measurements between children.
Table 2.
Geometric means and standard deviations of household nicotine, serum cotinine, and hair cotinine by study visit
| Baseline |
6-Month visit |
12-Month visit |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | N | M | SD | |
| Household nicotine (μg/m3) |
0 | 206 | 0.43 | 7.8 | 207 | 0.42 | 8.1 | ||
| Serum cotinine (ng/ml) |
220 | 1.2 | 3.5 | 210 | 1.0 | 4.0 | 213 | 1.1 | 4.5 |
| Hair cotinine (ng/mg) |
205 | 0.14 | 3.6 | 190 | 0.12 | 4.4 | 200 | 0.15 | 3.2 |
N, available sample; M, geometric mean; SD, geometric standard deviation.
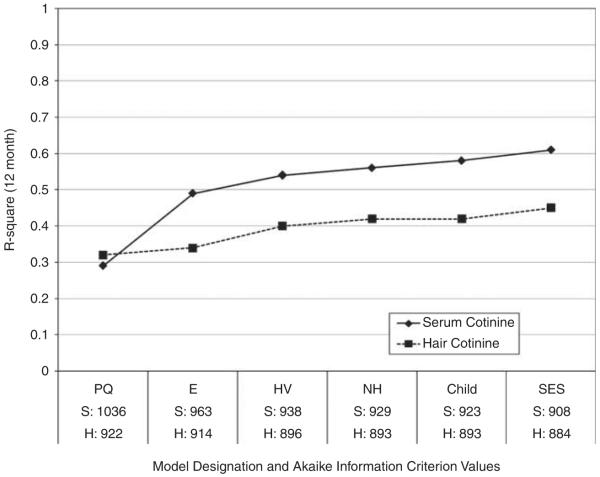
Every group of determinants satisfied our final model inclusion criteria for both serum cotinine and hair cotinine (Figure 1). For both biomarkers, the best predictive model included variables from the parent questionnaire, household nicotine, housing and ventilation observations, and child and social class characteristics. The bulk of variance was explained by the parent questionnaire, household nicotine concentration, and housing and ventilation characteristics. Once these more proximal determinants were included, child characteristics and social class factors met our model inclusion criteria but explained only small amounts of additional variability. The final predictive model for serum cotinine contained 15 factors explaining 61% of the variance and 11 factors for hair cotinine explaining 45% of the variance.
Figure 1.
Predictive ability of increasingly complex models of determinants of serum cotinine and hair cotinine. R2 values were estimated from ordinary least squares linear regression using data from the 12-month visit of the Cincinnati Asthma Prevention Study (2000–2003). Akaike Information Criterion values for models predicting serum cotinine (S) and hair cotinine (H) were estimated from linear mixed models using all available study data. Models included variables that met inclusion criteria as listed in Table 3, from the following additional groups: PQ, parent questionnaire, E, environmental measures, HV, housing and ventilation characteristics, NH, non-home places, Child, child characteristics, SES, family social class characteristics.
Predictors that remained in the final model for both serum and hair cotinine (Table 3) included hours spent in a room with smoking, number of cigarettes smoked, paternal smoking, household nicotine, home size, exposure in vehicles, African-American race, parental education, health insurance, and the HOME scale.
Table 3.
Multiplicative change and 95% confidence intervals for serum and hair cotinine by potential determinants, unadjusted and adjusted, from the Cincinnati Asthma Prevention Study (2000–2003)
| Serum cotinine |
Hair cotinine |
|||
|---|---|---|---|---|
| Unadjusteda n=203/374b |
Adjusteda n=203/374b |
Unadjusteda n=197/348b |
Adjusteda n=197/348b |
|
| Parent report of SHS at home | ||||
| Hours at home | ||||
| Per additional 10 h | 1.1 (0.9, 1.2) | NIM | 1.1 (0.9, 1.3) | NIM |
| Hours in a room with smoking | ||||
| >2 vs 0 | 2.3 (1.6, 3.3) | 1.9 (1.4, 2.6) | 1.5 (1.1, 2.0) | NIM |
| ≤2 vs 0 | 1.8 (1.3, 2.4) | 1.4 (1.1, 1.7) | 1.2 (1.0, 1.6) | NIM |
| Number of cigarettes/day | ||||
| Per 10 additional | 1.3 (1.2, 1.4) | 1.2 (1.1, 1.3) | 1.0 (0.9, 1.2) | 1.1 (1.0, 1.2) |
| Number of smokers in the home | ||||
| 3+ vs 0 or 1 | 1.9 (1.2, 2.8) | NIM | 0.9 (0.5, 1.5) | NIM |
| 2 vs 0 or 1 | 1.3 (1.1, 1.6) | NIM | 1.1 (0.9, 1.4) | NIM |
| Maternal smoking | ||||
| Yes vs no | 2.1 (1.5, 2.9) | 1.5 (1.1, 1.9) | 1.5 (1.1, 2.0) | NIM |
| Paternal smoking | ||||
| Yes vs no | 1.1 (0.8, 1.5) | 1.3 (1.0, 1.7) | 0.7 (0.5, 1.0) | 1.2 (0.9, 1.5) |
| Environmental measures of SHS | ||||
| Household nicotinec (μg/m3) | ||||
| 3 vs 0.5 | 1.9 (1.8, 2.2) | 1.5 (1.4, 1.7) | 1.2 (1.1, 1.4) | 1.1 (1.0, 1.2) |
| Staff perception of smokiness | ||||
| Smoky vs not smoky | 1.5 (1.2, 2.0) | 1.4 (1.1, 1.8) | 1.3 (1.0, 1.6) | NIM |
| Housing and ventilation | ||||
| Home volume (m3) | ||||
| Per 100 m3 smaller | 1.7 (1.4, 2.1) | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.5) | NIM |
| Number of rooms | ||||
| Per 2 fewer | 1.4 (1.3, 1.7) | NIM | 1.3 (1.2, 1.6) | 1.1 (1.0, 1.2) |
| Open floor plan | ||||
| Traditional vs open | 1.2 (0.6, 2.6) | NIM | 1.3 (0.6, 2.5) | NIM |
| Air conditioning | ||||
| None vs central air | 1.8 (1.2, 2.8) | NIM | 3.1 (2.1, 4.5) | 1.5 (1.1, 2.1) |
| Window unit vs central air | 1.8 (1.2, 2.7) | NIM | 2.2 (1.5, 3.2) | 1.1 (0.8, 1.5) |
| Fan running during visit | ||||
| Yes vs no | 0.9 (0.8, 1.8) | NIM | 1.2 (0.9, 1.7) | 1.4 (1.0, 1.9) |
| Season | ||||
| Spring vs fall | 1.4 (1.1, 1.7) | 1.5 (1.2, 1.8) | 1.0 (0.8, 1.3) | NIM |
| Summer vs fall | 1.1 (0.7, 1.7) | 1.3 (1.0, 1.7) | 1.4 (1.0, 2.0) | NIM |
| Winter vs fall | 1.3 (0.8, 1.9) | 1.2 (0.9, 1.6) | 1.1 (0.8, 1.5) | NIM |
| SHSb outside of home | ||||
| Vehicles | ||||
| >1 h vs none | 1.8 (1.2, 2.7) | 1.5 (1.0, 2.3) | 1.3 (0.7, 2.2) | 1.5 (0.9, 2.4) |
| ≤1 h vs none | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.2) | 1.3 (0.9, 1.8) | 1.5 (1.1, 2.0) |
| Others homes | ||||
| Any vs none | 1.2 (0.9, 1.6) | 1.3 (1.0, 1.7) | 1.1 (0.9, 1.4) | NIM |
| Child characteristics | ||||
| African-American race | ||||
| Yes vs no | 1.6 (1.1, 2.3) | 1.2 (0.9, 1.6) | 4.1 (3.1, 5.4) | 3.6 (2.7, 4.9) |
| Gender | ||||
| Female vs male | 1.5 (1.0, 2.2) | 1.3 (1.0, 1.6) | 1.1 (0.8, 1.5) | NIM |
| Age (years) | ||||
| Per 5 years younger | 1.2 (0.7, 1.9) | NIM | 1.0 (0.6, 1.5) | NIM |
| Family social class | ||||
| Parental education | ||||
| <HS vs college degree | 2.4 (1.2, 4.7) | 0.8 (0.5, 1.3) | 2.1 (1.2, 3.9) | 1.2 (0.8, 1.9) |
| HS degree vs college degree | 1.0 (0.5, 1.8) | 0.6 (0.4, 0.9) | 1.3 (0.8, 2.3) | 0.9 (0.6, 1.3) |
| Some college vs college degree | 1.1 (0.5, 2.1) | 0.8 (0.5, 1.2) | 1.2 (0.7, 2.2) | 1.0 (0.7, 1.6) |
| Income ($/year) | ||||
| Per $30,000 reduction | 2.1 (1.7, 2.6) | NIM | 2.0 (1.7, 2.4) | NIM |
| Health insurance | ||||
| Public or none vs private | 2.7 (1.9, 3.9) | 1.6 (1.2, 2.1) | 3.1 (2.3, 4.2) | 1.4 (1.1, 1.9) |
| Currently married | ||||
| No vs yes | 2.2 (1.6, 3.0) | NIM | 2.6 (1.9, 3.4) | NIM |
| Persons per room | ||||
| Additional 1 person per 2 rooms | 1.5 (1.1, 1.9) | NIM | 1.6 (1.3, 2.0) | NIM |
| HOME score | ||||
| Per 10 point reduction | 1.9 (1.5, 2.4) | 1.2 (1.0, 1.5) | 1.8 (1.5, 2.3) | 1.1 (0.9, 1.3) |
SHS, secondhand smoke; NIM, variable not in the final model; HS, high school; HOME Inventory, Home Observation for Measurement of the Environment (Caldwell and Bradley, 1984).
All estimates arise from a linear mixed model predicting log cotinine, adjusted for intervention status (intervention, placebo) and study visit (baseline, 6 months, 12 months). Adjusted estimates additionally include all variables with multiplicative change values given.
Number of observations is listed as children/repeated observations.
To improve model fit we coded nicotine dosimeter values with a log transformation.
African-American race was the strongest predictor of hair cotinine. This variable was associated with an almost fourfold increase both before and after adjusting for other determinants (Table 3).
The multiplicative change value for many variables was similar when included alone and after accounting for all other important factors, indicating little colinearity among these factors (Table 3). For example, parent report that the child was in a room, in which people smoked, for over 2 h compared with 0 h was associated with a 2.3-fold increase (95% confidence interval of 1.6, 3.3) in serum cotinine without accounting for other factors, and a 1.9-fold increase (1.4, 2.6) after accounting for variables in the final model. Similarly, multiplicative change values for the number of cigarettes smoked in the home, staff perception of smokiness, season, exposure in vehicles and others’ homes, and African-American race were all robust to adjustment for other factors.
In contrast, the predictive values of a few variables were attenuated after adjusting for other determinants, suggesting that these variables were serving as proxies of other predictors of cotinine (Table 3). For example, parental education less than high school compared with a college degree was associated with a 2.4-fold (1.2, 4.7) increase in serum cotinine, but after adjustment for other variables was associated a 20% decrease: 0.8 (0.5, 1.3). Other determinants that were less predictive after adjustment included home volume, air conditioning, and health insurance status.
The nicotine dosimeter added predictive ability on top of parent report for both serum and hair cotinine (Figure 1). In side-by-side comparisons (Table 4), household nicotine alone performed markedly better than parent report alone in predicting serum cotinine, as indicated by a reduction in AIC of 91 units for household nicotine but only 37 units for parent report. The predictive ability of the environmental measure of household nicotine did not offer such substantial improvements for hair cotinine (Table 4).
Table 4.
Strength of ability to explain variance in serum cotinine and hair cotinine for questionnaire and environmental measures of environmental tobacco smoke as indicated by reduction in Akaike Information Criteria (AIC), Cincinnati Asthma Prevention Study (2000–2003)
| Reduction in AICa |
||
|---|---|---|
| Serum cotinineb | Hair cotininec | |
| Hours in a room with smoking | 6 | 4 |
| Number of cigarettes/day | 16 | 9 |
| Maternal smoking | 21 | 1 |
| Paternal smoking | −2 | −2 |
| Parent report (4 questions above)d | 37 | 9 |
| Home sizee | 24 | 16 |
| Parent reportd+home sizee | 69 | 25 |
| Household nicotine | 91 | 13 |
| Staff perception of smokiness | 7 | 0 |
AIC values were subtracted from the AIC from a base linear mixed model adjusted for intervention status, study visit, and in the case of hair cotinine, African-American race.
From linear mixed models predicting log serum cotinine with an unstructured correlation matrix, adjusted for intervention status and study visit.
From linear mixed models predicting log hair cotinine with an exchangeable correlation matrix, adjusted for intervention status, study visit, and African-American race.
Parent report includes hours in a room with smoking (three categories as in Table 3), number of cigarettes/day (continuous), maternal smoking (yes/no), and paternal smoking (yes/no).
Home size was measured as home volume in serum cotinine models and number of rooms in hair cotinine models.
Individual parent-report variables associated with the largest reduction in AIC included number of cigarettes/day and maternal smoking (Table 4). Substantially better prediction was achieved by including multiple parent report questions together with an indication of home size.
Discussion
We evaluated potential determinants of childhood SHS exposure assuming that cotinine is the current best measure of internal exposure, including serum cotinine to reflect short-term exposure and hair cotinine to reflect longer-term exposure. Our repeated-measures data set contained a comprehensive parent questionnaire, which included exposure in non-home places where children spend time, an objective environmental measure of exposure in the home, and observations of housing and ventilation characteristics. We used a manual model-building approach, which prioritized more proximal and important predictors over more distal factors, finding that factors in all groups were needed to best predict both serum cotinine and hair cotinine. Our set of determinants explained a substantial proportion of variability in serum (R2 = 0.61, equivalent to a partial correlation of almost 80%) and hair (R2 = 0.45) cotinine. Given differences in individual variability in nicotine and cotinine metabolism, accounting for this proportion of variance may be close to the highest achievable in this context.
If cotinine reflects a biologically important dose of SHS, then questionnaires and environmental measurements of exposure may be insufficient. Misclassification may result from using these simpler measures. Furthermore, future studies may be unable to combine multiple parent-report variables and environmental measures to create one exposure metric. Biomarkers such as cotinine serve as aggregate measures of exposure. Thus, whenever quantitative measurement of SHS exposure is important and wherever practical, biomarkers are indicated. Our study used serum and hair cotinine, but lacked information on other biomarkers of SHS exposure, such as hydroxycotinine or hair nicotine (Benowitz, 1999), which may also prove useful in the accurate quantification of SHS exposure.
In this group of children exposed to SHS in their homes, African-American race was the strongest predictor of hair cotinine, both before and after adjusting for other determinants in our study and in other analyses using these data, associated with an almost fourfold increase (Wilson et al., 2005, 2007). In contrast, African-American race was only associated with a 1.2-fold increase in serum cotinine. The reason for this striking race difference in hair cotinine levels relative to serum cotinine levels is not known. It may be that metabolic differences by race in nicotine metabolism (Perez-Stable et al., 1998; Benowitz et al., 1999) become compounded during accumulation in hair, or that differences in physical properties of hair such as melanin content (Dehn et al., 2001) influence the concentration or measurement of cotinine in hair (Wilson et al., 2007).
Hair cotinine had promise as a biomarker of SHS exposure that reflected long-term exposure with inexpensive, non-invasive collection. However, we believe that the persistence of these unexplained racial differences in hair cotinine levels indicates that hair cotinine is not a reliable biomarker of SHS exposure. At the least, studies that have used hair cotinine must account for African-American race during analyses to assure that the characterization of SHS exposure is not driven by this race difference.
Studies that are unable to use a biomarker should investigate classifying quantitative levels of SHS exposure in children by using nicotine dosimeters, which integrate information on SHS from multiple sources and ventilation factors. This objective measure of household nicotine performed better than a combination of multiple parent-report variables together with home size in predicting serum cotinine. If only questionnaires are available, our results together with those of others (Irvine et al., 1997; Jurado et al., 2004) support that more than one question will improve exposure measurement. Our data indicate that the number of cigarettes smoked at home, the number of hours the child is in a room with concurrent smoking, maternal smoking status, and basic information on home size provide good characterization of SHS exposure. Assessment of number of rooms in a home was almost as good as complete information on home volume.
We chose not to consider interaction terms for our models. Although interactions between factors such as number of cigarettes and home size are reasonable, included variables such as nicotine concentration integrated the amount of smoke and dilution factors to some extent. In addition, our model form forced multiplicative relationships between all factors. Initial exploratory analysis indicated that many interaction terms met inclusion criteria and slightly improved variance explained, but the interpretation of model parameters quickly became unwieldy.
We did not find that children in our study had substantial exposure to SHS in public places or restaurants that may be amenable to policy regulation. In contrast, our results support that smoking in vehicles contributed to a child’s overall SHS exposure, even when measured by questionnaire and after adjustment for smoking habits of the parents. These results support the continued evaluation of regulations to protect children from SHS in private spaces, such as homes and vehicles (Jarvie and Malone, 2008).
Although our study included a wide range of measures of SHS exposure, we were missing some factors that are associated with cotinine in children: smoking bans in vehicles or at home (Bakoula et al., 1997; Johansson et al., 2004) and cotinine levels of the parents (Crawford et al., 1994; Irvine et al., 1997). In addition, some of our variables were imperfectly matched temporally. For example, the nicotine dosimeter integrates exposure over the preceding 6 months, whereas serum cotinine reflects exposure over a recent few days.
We hypothesized that our comprehensive model would account for serum and hair cotinine variability, so that social class characteristics would not add to the model prediction. This hypothesis was not supported. Parental education, health insurance status, and HOME scores added a small amount of predictive ability in explaining both serum and hair cotinine level even after accounting for variables such as nicotine concentration in the home, home size, and child race. That social class factors remained in the predictive models suggests that we are failing to measure all proximal determinants of exposure. These social class variables are likely serving as markers of sources of exposure that were not captured by other variables, perhaps time-activity patterns of the children, type of cigarettes smoked, or societal smoking patterns in the immediate vicinity of the child. The persistence of social class in explaining cotinine, found in our study and others (Mannino et al., 2001; Jurado et al., 2004), also suggests the importance of addressing smoking prevalence at a societal level. The efforts of individual parents may not prove sufficient to shield children from SHS if the children reside in an environment with high levels of cigarette smoking.
Our study sample included elementary-age asthmatic children living with a smoker. The relative importance of the individual determinants found in our sample may differ for children who are not exposed to smoking in their homes. For example, public places or others’ homes are likely to contribute more to total cotinine for children lacking home exposure. The effect of social class may be stronger for children not exposed to SHS at home if social class is acting as a marker for general non-home opportunities for SHS exposure. Generalizability may also be imperfect in regions of the country that have different restrictions on smoking in public places.
We found that a wide range of determinants best explained variance in serum and hair cotinine concentrations among children who lived with a smoker. Hair cotinine may be less useful as a biomarker of SHS exposure because concentrations are dominated by racial differences, even after accounting for exposure differences. Parent report of tobacco smoke exposures, quantitative measurement using nicotine dosimeters, together with information on home size explained the greatest proportion of serum cotinine variance. The explanatory impact of many seemingly redundant variables remained robust to adjustment for many other factors. Our results suggest that questionnaires alone are insufficient to fully quantify pediatric exposure to SHS. We advise future studies examining the effects of tobacco smoke exposure on child health to use biomarkers if possible, or secondly, environmental measures. When only questionnaires can be used, investigators should consider using the number of cigarettes smoked in the home, hours spent with a smoker, maternal smoking status, and a measure of home size.
Acknowledgements
This project was funded by National Heart Lung and Blood Institute R01 H165731 and an environmental epidemiology traineeship, National Institute of Environmental Health Science T32 ES007018. We thank the interviewers, environmental technicians, laboratory personnel, and families who participated in the Cincinnati Asthma Prevention Study; Dr. Julia Klein and her laboratory for hair cotinine analyses; and Dr. Julie Daniels, Dr. Brian Pence, Dr. Caroline Dilworth, and Dr. Mandy Golembesky for their assistance.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- Al-Delaimy WK. Hair as a biomarker for exposure to tobacco smoke. Tob Control. 2002;11(3):176–182. doi: 10.1136/tc.11.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakoula CG, Kafritsa YJ, Kavadias GD, Haley NJ, Matsaniotis NS. Factors modifying exposure to environmental tobacco smoke in children (Athens, Greece) Cancer Causes Control. 1997;8(1):73–76. doi: 10.1023/a:1018487222533. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107(Suppl 2):349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., III Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;291(3):1196–1203. [PubMed] [Google Scholar]
- Bernert JT, Jr, McGuffey JE, Morrison MA, Pirkle JL. Comparison of serum and salivary cotinine measurements by a sensitive high-performance liquid chromatography-tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol. 2000;24(5):333–339. doi: 10.1093/jat/24.5.333. [DOI] [PubMed] [Google Scholar]
- Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, Ann Q, Covey TR, Whitfield WE, Gunter EW, Miller BB, Patterson DG, Jr, Needham LL, Hannon WH, Sampson EJ. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43(12):2281–2291. [PubMed] [Google Scholar]
- Braun JM, Froehlich TE, Daniels JL, Dietrich KN, Hornung R, Auinger P, Lanphear BP. Association of environmental toxicants and conduct disorder in U.S. children: NHANES 2001–2004. Environ Health Perspect. 2008;116(7):956–962. doi: 10.1289/ehp.11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RR. Home Observation for the Measured Environment. University of Arkansas at Little Rock; Little Rock, AR, USA: 1984. [Google Scholar]
- Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol. 1989;130(4):696–704. doi: 10.1093/oxfordjournals.aje.a115391. [DOI] [PubMed] [Google Scholar]
- Crawford FG, Mayer J, Santella RM, Cooper TB, Ottman R, Tsai WY, Simon-Cereijido G, Wang M, Tang D, Perera FP. Biomarkers of environmental tobacco smoke in preschool children and their mothers. J Natl Cancer Inst. 1994;86(18):1398–1402. doi: 10.1093/jnci/86.18.1398. [DOI] [PubMed] [Google Scholar]
- Dehn DL, Claffey DJ, Duncan MW, Ruth JA. Nicotine and cotinine adducts of a melanin intermediate demonstrated by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Chem Res Toxicol. 2001;14(3):275–279. doi: 10.1021/tx000205l. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113(4 Suppl):1007–1015. [PubMed] [Google Scholar]
- Forastiere F, Agabiti N, Dell’Orco V, Pistelli R, Corbo GM, Brancato G, Pacifici R, Zuccaro P, Perucci CA. Questionnaire data as predictors of urinary cotinine levels among nonsmoking adolescents. Arch Environ Health. 1993;48(4):230–234. doi: 10.1080/00039896.1993.9940364. [DOI] [PubMed] [Google Scholar]
- Gareri J, Motherisk Laboratory. Division of Clinical Pharmacology and Toxicology. Hospital for Sick Children . Personal communication regarding hair cotinine reliability testing procedures. Kalkbrenner; Toronto: Jan 14, 2010. Communicated to A. [Google Scholar]
- Groner JA, Hoshaw-Woodard S, Koren G, Klein J, Castile R. Screening for children’s exposure to environmental tobacco smoke in a pediatric primary care setting. Arch Pediatr Adolesc Med. 2005;159(5):450–455. doi: 10.1001/archpedi.159.5.450. [DOI] [PubMed] [Google Scholar]
- Halterman JS, Borrelli B, Tremblay P, Conn KM, Fagnano M, Montes G, Hernandez T. Screening for environmental tobacco smoke exposure among inner-city children with asthma. Pediatrics. 2008;122(6):1277–1283. doi: 10.1542/peds.2008-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond KS, Leaderer BP. A diffusion monitor to measure exposure to passive smoking. Environ Sci Technol. 1987;21(5):494. doi: 10.1021/es00159a012. [DOI] [PubMed] [Google Scholar]
- Harville D. Maximum likelihood approaches to variance component estimation andtorelated problems. J Am Stat Assoc. 1977;72(358):320–338. [Google Scholar]
- Henderson FW, Reid HF, Morris R, Wang OL, Hu PC, Helms RW, Forehand L, Mumford J, Lewtas J, Haley NJ, et al. Home air nicotine levels and urinary cotinine excretion in preschool children. Am Rev Respir Dis. 1989;140(1):197–201. doi: 10.1164/ajrccm/140.1.197. [DOI] [PubMed] [Google Scholar]
- Henschen M, Frischer T, Pracht T, Spiekerkotter E, Karmaus W, Meinert R, Lehnert W, Wehrle E, Kuehr J. The internal dose of passive smoking at home depends on the size of the dwelling. Environ Res. 1997;72(1):65–71. doi: 10.1006/enrs.1996.3688. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- Irvine L, Crombie IK, Clark RA, Slane PW, Goodman KE, Feyerabend C, Cater JI. What determines levels of passive smoking in children with asthma? Thorax. 1997;52(9):766–769. doi: 10.1136/thx.52.9.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola MS, Jaakkola JJ. Assessment of exposure to environmental tobacco smoke. Eur Respir J. 1997;10(10):2384–2397. doi: 10.1183/09031936.97.10102384. [DOI] [PubMed] [Google Scholar]
- Jarvie JA, Malone RE. Children’s secondhand smoke exposure in private homes and cars: an ethical analysis. Am J Public Health. 2008;98(12):2140–2145. doi: 10.2105/AJPH.2007.130856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MJ, Strachan DP, Feyerabend C. Determinants of passive smoking in children in Edinburgh, Scotland. Am J Public Health. 1992;82(9):1225–1229. doi: 10.2105/ajph.82.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Hermansson G, Ludvigsson J. How should parents protect their children from environmental tobacco-smoke exposure in the home? Pediatrics. 2004;113(4):e291–e295. doi: 10.1542/peds.113.4.e291. [DOI] [PubMed] [Google Scholar]
- Jurado D, Munoz C, Luna Jde D, Fernandez-Crehuet M. Environmental tobacco smoke exposure in children: parental perception of smokiness at home and other factors associated with urinary cotinine in preschool children. J Expo Anal Environ Epidemiol. 2004;14(4):330–336. doi: 10.1038/sj.jea.7500329. [DOI] [PubMed] [Google Scholar]
- Kum-Nji P, Meloy L, Herrod HG. Environmental tobacco smoke exposure: prevalence and mechanisms of causation of infections in children. Pediatrics. 2006;117(5):1745–1754. doi: 10.1542/peds.2005-1886. [DOI] [PubMed] [Google Scholar]
- Kumar R, Curtis LM, Khiani S, Moy J, Shalowitz MU, Sharp L, Durazo-Arvizu RA, Shannon JJ, Weiss KB. A community-based study of tobacco smoke exposure among inner-city children with asthma in Chicago. J Allergy Clin Immunol. 2008;122(4):754–759. e1. doi: 10.1016/j.jaci.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Klein J, Koren G. Hair analysis—a biological marker for passive smoking in pregnancy and childhood. Hum Exp Toxicol. 1999;18(4):279–282. doi: 10.1191/096032799678840048. [DOI] [PubMed] [Google Scholar]
- Knight JM, Eliopoulos C, Klein J, Greenwald M, Koren G. Passive smoking in children. Racial differences in systemic exposure to cotinine by hair and urine analysis. Chest. 1996;109(2):446–450. doi: 10.1378/chest.109.2.446. [DOI] [PubMed] [Google Scholar]
- Langone JJ, Gjika HB, Van Vunakis H. Nicotine and its metabolites. Radioimmunoassays for nicotine and cotinine. Biochemistry. 1973;12(24):5025–5030. doi: 10.1021/bi00748a032. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Caraballo R, Benowitz N, Repace J. Predictors of cotinine levels in US children: data from the Third National Health and Nutrition Examination Survey. Chest. 2001;120(3):718–724. doi: 10.1378/chest.120.3.718. [DOI] [PubMed] [Google Scholar]
- Mendola P, Selevan SG, Gutter S, Rice D. Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment Retard Dev Disabil Res Rev. 2002;8(3):188–197. doi: 10.1002/mrdd.10033. [DOI] [PubMed] [Google Scholar]
- Perez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Peterson EL, Johnson CC, Ownby DR. Use of urinary cotinine and questionnaires in the evaluation of infant exposure to tobacco smoke in epidemiologic studies. J Clin Epidemiol. 1997;50(8):917–923. doi: 10.1016/s0895-4356(97)00095-4. [DOI] [PubMed] [Google Scholar]
- Sexton K, Adgate JL, Church TR, Hecht SS, Ramachandran G, Greaves IA, Fredrickson AL, Ryan AD, Carmella SG, Geisser MS. Children’s exposure to environmental tobacco smoke: using diverse exposure metrics to document ethnic/racial differences. Environ Health Perspect. 2004;112(3):392–397. doi: 10.1289/ehp.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier AJ, Hornung R, Lierl M, Lanphear BP. Environmental exposures and exhaled nitric oxide in children with asthma. J Pediatr. 2006;149(2):220–226. doi: 10.1016/j.jpeds.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Tutka P, Wielosz M, Zatonski W. Exposure to environmental tobacco smoke and children health. Int J Occup Med Environ Health. 2002;15(4):325–335. [PubMed] [Google Scholar]
- Uematsu T, Mizuno A, Nagashima S, Oshima A, Nakamura M. The axial distribution of nicotine content along hair shaft as an indicator of changes in smoking behaviour: evaluation in a smoking-cessation programme with or without the aid of nicotine chewing gum. Br J Clin Pharmacol. 1995;39(6):665–669. doi: 10.1111/j.1365-2125.1995.tb05726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JD, Arheart KL, Lee DJ. Accuracy of parental reporting of secondhand smoke exposure: The National Health and Nutrition Examination Survey III. Nicotine Tob Res. 2006;8(4):591–597. doi: 10.1080/14622200600790021. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. Racial differences in exposure to environmental tobacco smoke among children. Environ Health Perspect. 2005;113(3):362–367. doi: 10.1289/ehp.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Kahn RS, Khoury J, Lanphear BP. The role of air nicotine in explaining racial differences in cotinine among tobacco-exposed children. Chest. 2007;131(3):856–862. doi: 10.1378/chest.06-2123. [DOI] [PubMed] [Google Scholar]
- Wipfli H, Avila-Tang E, Navas-Acien A, Kim S, Onicescu G, Yuan J, Breysse P, Samet JM. Secondhand smoke exposure among women and children: evidence from 31 countries. Am J Public Health. 2008;98(4):672–679. doi: 10.2105/AJPH.2007.126631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff SI, Conway TL, Edwards CC, Hovell MF. Acceptability and validity of hair collection from Latino children to assess exposure to environmental tobacco smoke. Nicotine Tob Res. 2003;5(3):375–385. doi: 10.1080/14622200307206. [DOI] [PubMed] [Google Scholar]
- Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2005;113(1):98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Khoury J, Hornung R, Dietrich K, Succop P, Lanphear B. Environmental tobacco smoke exposure and child behaviors. J Dev Behav Pediatr. 2008;29(6):450–457. doi: 10.1097/dbp.0b013e31818d0c21. [DOI] [PMC free article] [PubMed] [Google Scholar]