Abstract
Nine patients who became diabetic after upper-abdominal exenteration and liver transplantation were given pancreatic islet-cell grafts obtained from the liver donor (eight cases), a third-party donor (one), or both (four). Two patients were diabetic when they died of infections after 48 and 109 days, as was a third patient who died of tumour recurrence after 178 days. The other 6 are alive 101–186 days postoperatively, and five are insulin-free or on insulin only during night-time parenteral alimentation. C-peptide increased 1·7 to 3·3 fold in response to intravenous glucose in these five patients who have had glycosylated haemoglobin in the high normal range. However, the kinetics of the C-peptide responses to intravenous glucose in all eight patients tested revealed an absent first-phase release and a delayed peak response consistent with transplantation and/or engraftment of a suboptimal islet cell mass. The longest survivor, who requires neither parenteral alimentation nor insulin, is the first unequivocal example of successful clinical islet-cell transplantation.
Introduction
Long-term reversal of hyperglycaemia following islet transplantation has been reported in animal models of diabetes,1 but not in patients,2–5 though Scharp’s group in St Louis5 has lately reported one encouraging case to an American Diabetes Association meeting (see ]AMA 1990; 264: 427). Clinical failure has been attributed to poor harvesting and organ preservation techniques, inadequate procedures for isolating and purifying islets, and failure to control islet-directed immune destruction. However, improvements in techniquef6,7 have lately increased the yield and purity of functionally competent islet grafts,2,5,8–10 and the potent new immunosuppressive agent FK 506 has made control of rejection easier.11,12 Using these advances, we have given islet allografts to patients rendered apancreatic by upper-abdominal exenteration.13,14
Patients and methods
Patients
Nine patients aged 8–58 years underwent upper-abdominal exenteration for tumours too extensive to be removed with less drastic procedures. Liver, pancreas, spleen, stomach, duodenum, proximal jejunum, and terminal ileum and, in three cases, the ascending and transverse colon were removed.13,14 A cadaveric liver orthotopic allograft was done14 and the graft portal vein was anastomosed to the recipient superior mesenteric vein. Arterialisation was from the recipient aorta or coeliac axis (fig 1). A 14G catheter with a heparin lock was placed in a superior mesenteric venous tributary. Bowel continuity was re-established and biliary drainage was via a choledochojejunostomy (fig 1).
Fig 1.
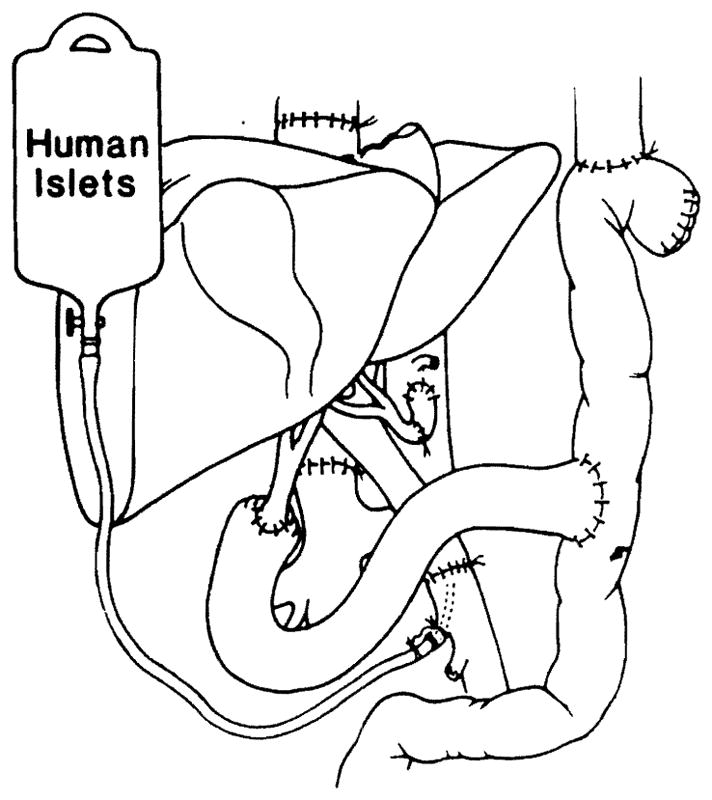
Liver and pancreatic islet transplantation after upper abdominal exenteration.
Organ procurement
The cadaveric donors were the same ABO type as the recipient except for the second pancreas donor (type O) for patient 8, who was type B. HLA matching was random, and the antigens matched were zero or one of a possible 4 in the 4 cases in which complete information was available. There were two positive cytotoxic cross-matches (patients 2 and 4).
The livers and pancreases were obtained from multi-organ donors.7,13 In situ perfusion of the abdominal aorta was with 1500–2000 ml of University of Wisconsin solution (UWS). An additional 500–1000 ml UWS were infused directly into the liver via the portal vein which was encircled below the catheter tip to prevent retrograde leakage. Venous hypertension of the pancreas was avoided by venting the portal and/or splenic vein. The specimens were immersed in UWS and packed in ice.
The pancreas of the liver donor was the source of the primary islet graft for eight patients. Four recipients were given islets from a second donor 1–2 days after the first infusion. Patient 7 was given his single islet infusion from a third-party donor 1 day after the principal operation.
Islet preparation and administration
Cold ischaemia time of the thirteen pancreases averaged 8 h (range 5–14h). The islets were isolated.10 The pancreatic duct was cannulated and 250–300 ml Hank’s solution containing 1–2 mg/ml collagenase (Boehringer-Mannheim) was injected. The separated islets were purified with a discontinuous gradient of ‘Ficoll’ powder (Sigma) in Euro-Collins solution,5 at specific densities of 1·108, 1·096, and 1·037. A COBE 2991 cell separator (COBE Laboratories, Lakewood, Colorado) was used for purification.15,16 Islet preparation took 4 h on average.
The islet cells were suspended in 250 ml Hank’s solution; 100 μl samples were stained with dithizone17 to assess total islet yield,16,18,19 which was standardised. Islet particles varied in size from 50 to over 250 μm. The heterogenously sized islets were converted to “150 μm equivalents”,20 and the contribution of the different size groups to the total islet volume was then expressed in μl.
The preparation was pelleted and suspended in 100 ml Hank’s solution containing 10% human albumin and infused into the portal vein catheter over 20–30 min. Portal venous pressure was measured and portal flow was assessed by colour doppler ultrasonography. When not in use, the portal vein catheter was flushed every 6 h with 2 ml saline containing heparin 100 units/ml. The catheter was extracted after completion of the last infusion(s).
Postoperative management
All patients were maintained on continuous parenteral hyperalimentation for about 2 weeks after surgery, with solutions containing 20–25% dextrose, 10–20% lipids, 3·5–5· 0% crystalline aminoacids, electrolytes, trace metals, and vitamins including folic acid. Subsequently, the patients ate as desired. 15 to 40 non-enteric coated tablets per day of pancreatic extract (pancrelipase USP) were administered with meals. The daytime diet was supplemented with 1000–1500 kcal parenteral alimentation overnight until the body weight was stable without intravenous supplementation. Regular insulin (‘Humulin’; Eli Lilly) was always added to the intravenous solutions to maintain the blood glucose at 5–7 mmol/l.
Immunosuppression with FK 506 began with intravenous doses 0·075 mg/kg every 12 h followed by 0·15 mg/kg orally every 12 h. The dose was adjusted on clinical grounds and by monitoring plasma FK 506 levels. Rejection was treated with a single intravenous injection of 1g hydrocortisone or methylprednisolone; that failed in two patients, who were given OKT3 10mg daily for 5 days (‘Orthoclone’; Ortho Pharmaceutical). Only patient 3 was given maintenance steroid therapy.
Assessment of islet function
Basal and stimulated (6 min after 1 mg glucagon intravenously) C-peptide levels were measured after the exenteration and liver transplantation but before infusion of islets. There were no C-peptide responses. After islet transplantation, blood glucose and C-peptide were monitored. An intravenous glucose tolerance test (IVGTT), selected as a provocative test of C-peptide secretion, was done every 2–4 weeks. Glycosylated haemoglobin (HbA1c) was measured before and every 6 weeks after transplantation.
Results
Survival
Patient 2 died of recurrent cancer after 178 days. Patients 3 and 4 died with rejection, sepsis, and multiple organ failure (table I). All three were insulin dependent. No islets were found post mortem in the liver of patient 3; several well-granulated islets were identified in patient 4 by indirect immunoperoxidase stain. Necropsy was not done in patient 2. Three patients recovered from the procedures despite liver rejection (patient 2), cytomegalovirus and candida infections (patient 6), and systemic enterobacter sepsis (patient 8). All six survivors have normal liver function and no evidence of tumour recurrence after 3–6 months of follow-up.
TABLE 1.
RECIPIENTS AND OUTCOME*
| No | Perioperative complications | Insulin requirements† | Outcome | Details of islet grafts |
||
|---|---|---|---|---|---|---|
| Donor age(s) | Islets (× 103) | Endocrine vol (μl) | ||||
| 1 (15, F) | None | NIR-S | Full activity | 18 | 474 | 839 |
| 2 (8, M) | Moderate rejection (liver) day 19, treated with FK506 | IR | Died 178 days | 7 | 562 | 993 |
| 3 (55, M) | Severe rejection (liver) day 18, treated with OKT3 | IR | Died 48 days post-transplant | 36 | 205 | 363 |
| 4 (36, F) | Clinical rejection day 5, treated with OKT3; intractable cytomegalovirus infection and respiratory failure | IR | Died 109 days post-transplant | 19 | 289 | 510 |
| 5 (58, M) | None | NIR-S | Full activity | 26, 19 | 369, 209 | 653, 369 |
| 6 (52, F) | Invasive cytomegalovirus infection, candidaemia | IR | Recuperating at home | 18, 40 | 105, 153 | 186, 270 |
| 7 (46, M) | None | NIR-S | Full activity | 18 | 285 | 504 |
| 8 (44, F) | Sepsis with transient renal failure | NIR-U | Full activity | 17, 35 | 459, 267 | 811, 472 |
| 9 (33, F) | None | NIR-S | Full activity | 31, 50 | 205, 127 | 363, 224 |
Follow-up to 15 July 1990
NIR-S = non-insulin requiring, stable, preprandial blood glucose < 7·8 mmol/l HbA1c < 6·2% (3·9–5·9 normal) ketosis resistant; NIR-U = non-insulin requiring, unstable preprandial blood glucose 7·8–11·2 mmol/l HbA1c < 7%. ketosis resistant IR = Insulin requiring
Pancreatic islet yield
The final preparations constituted a packed volume of less than 3 ml. The number of 150 μm islet equivalents varied from 105 000 to 562 000, corresponding to a volume of endocrine tissue of 186–993 μl (table I). The intraportal injection(s) did not affect portal pressure and haemodynamics or cause any adverse effects. In patient 6, a liver biopsy sample taken 17 days post-transplantation contained a morphologically normal islet in one portal triad of the hepatic graft.
Islet function
No patient acquired ketonuria. The three who died were not able to eat regularly and were given insulin continuously during total parenteral alimentation except at the time of IVGTT.
Patient 1 is insulin-free after more than 6 months and has not required parenteral nutrition support since the fourth postoperative month. She lives a normal life and has gained 3 kg over the past 2 months. Patients 5, 7, 8, and 9 are not on exogenous insulin, except during the night-time parenteral hyperalimentation which has been required periodically to prevent weight loss. Patient 6 is on insulin 0·1 units/kg daily and is a treatment failure.
The fasting blood sugar levels in the 5 patients who were insulin independent and who were tested after withdrawal of night-time parenteral alimentation for several days averaged less than 7·8 mmol/l, and random non-fasting levels during the day time were below 8·4 mmol/l in four. HbA1c levels were high normal in four patients and slightly abnormal in the other (table II).
TABLE II.
FASTING AND NON-FASTING BLOOD GLUCOSES AND GLYCOSYLATED HAEMOGLOBIN IN SIX SURVIVING PATIENTS
| Case | Blood glucose (fasting) (mmol/l) | Blood glucose (non-fasting) (mmol/l) | HbA1c (%) |
|---|---|---|---|
| 1 | 5·4 (0·5) | 5·8 (0·5) | 6·0 |
| 5 | 5·9 (0·2) | 6·1 (0·2) | 5·8 |
| 6 | 8·7 (0·5) | 10·1 (0·3) | 5·8 |
| 7 | 5·7 (0·5) | 7·5 (0·5) | 5·1 |
| 8 | 7·2 (0·7) | 10·8 (0·5) | 6·6 |
| 9 | 7·6 (0·3) | 8·3 (0·3) | 5·6 |
As mean (and SEM)
C-peptide was detectable in plasma of all patients for as long as they were studied. C-peptide responses to intravenous glucose were demonstrable in all patients, the latest results being summarised in table III. C-peptide kinetics are shown in fig 2 for four patients not on daytime exogenous insulin (patient 8 was excluded from the computations because of a high basal C-peptide level, attributed to renal failure). The peak C-peptide response was delayed at 60 min, a 5–10 min peak being normal, and blood glucose did not return to basal levels until 150–180 mm.
TABLE III.
C-PEPTIDE RESPONSE TO 0·5 g/kg INTRAVENOUS GLUCOSE
| Case | Follow-up (days)* | C-peptide (pmol/ml) |
Week post-transplant of last test | |
|---|---|---|---|---|
| Basal | Peak | |||
| 1 | 185 | 0·96 | 2·8 | 16 |
| 2 | 178 (dead) | 0·66 | 1·14 | 9·6 |
| 3 | 48 (dead) | 0·78 | 0·90 | 1·5 |
| 4 | 109 (dead) | 2·22 | 2·52 | 5 |
| 5 | 153 | 1·68 | 4·08 | 12 |
| 6 | 121 | 1·68 | 3·6 | 2·6 |
| 7 | 118 | 1·0 | 3·3 | 7 |
| 8 | 102 | 5·0† | 8·4 | 6 |
| 9 | 101 | 1·4 | 3·24 | 2·4 |
To July 15, 1990
Serum creatinine 354 μmol/l at time of test
Fig 2.
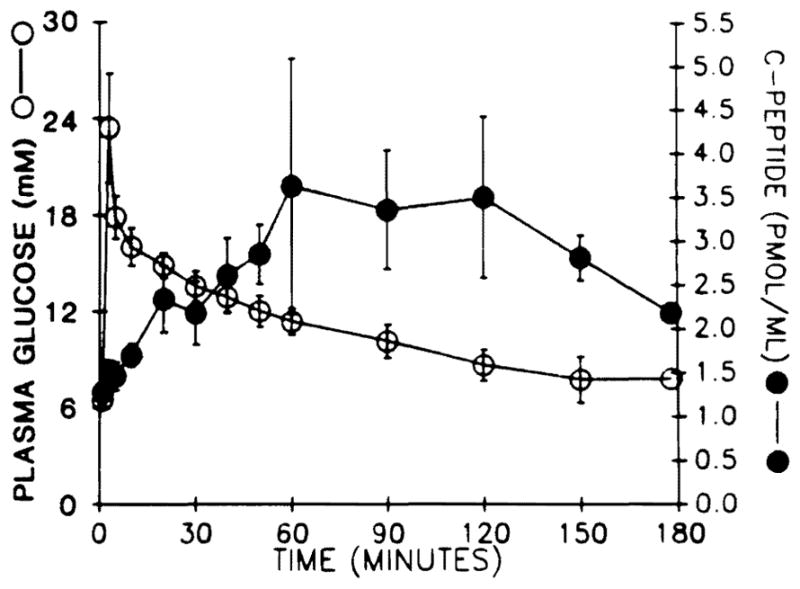
Glucose disappearance and C-peptide responses after administration of intravenous glucose (0·5 g/kg) in patients 1, 5, 7, 9.
Discussion
The conditions of these procedures have both advantages and disadvantages for the evaluation of islet allotransplantation. The foremost advantage is the assurance that any islet function was from the graft, all native pancreas having been removed. In all nine patients there was no C-peptide response to glucagon in the brief interval between surgery and the islet infusion. A hypothetical advantage, for which there is experimental support,21 is that the immune barrier to islet acceptance might be lowered by the presence of a liver from the same donor, as in eight of the cases. However, the patient (case 7) whose pancreas was procured from a third-party donor achieved one of the best results; in this case the pancreas donor and the recipient had a complete HLA mismatch except for one DR antigen. There are also disadvantages. All the recipients had severe weight loss and required long periods of parenteral alimentation with solutions that contained insulin. Even with the resumption of diet, parenteral infusions overnight were frequently needed to prevent malnutrition. These were stopped for several days or weeks when metabolic tests were done so that the patients were free of exogenous insulin. Postoperative infections and other complications had a potential confounding effect on the results in half the cases, and patient 2 had early tumour recurrences which precluded metabolic stability or long survival. Patients 2 and 4 had positive cytotoxic cross-matches with their liver-islet donors. Although their livers functioned throughout the survival period, they ultimately became diabetic.
Despite the difficulties, early islet allograft function was demonstrable in every recipient. This function has been sustained in five patients. However, no patient has normal insulin secretory kinetics: even where fasting and random non-fasting blood sugar was normal first-phase release in the C-peptide responses to intravenous glucose was absent and the peak response was delayed. These findings are characteristic in animal models of reduced islet-cell mass22–24 and in the prolonged prodrome of spontaneous type I diabetes mellitus.25 They also resemble the results in pancreatectomised dogs given inadequate numbers of islet allografts.26 The dog studies had suggested that correction might be more complete in patients given the islets from two donors but this did not happen, and 2 of the best clinical results (cases 1 and 7) were in patients given islets from single pancreases. Silent rejection of islets without commensurate injury to the liver graft is one possibility, islets being highly immunogenic.2–5 FK 506 can be diabetogenic,27 and an even greater vulnerability to hyperglycaemia is caused by steroid therapy. However, only one of our patients was on maintenance prednisone.
This series suggests that islet transplantation in man is feasible but it is too early for widespread clinical trials to be justified. The metabolic correction is incomplete and finding multiple islet donors–should this in fact prove to be necessary–will make islet transplantation impracticable on a large scale, unless procedures for long-term culture or cryopreservation of islets are developed.
Acknowledgments
Supported by research grants from the Veterans Administration, project grant DL 29961 and DK 25802 from the National Institutes of Health, and the Diabetes Research Institute Foundation, Miami, Florida.
References
- 1.Gray DWR, Morris PJ. Developments in isolated pancreatic islet transplantation. Transplantation. 1987;43:321–31. doi: 10.1097/00007890-198703000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Alejandro R, Mintz DH, Noel J, Latif Z, Koh N, Russel E, Miller J. Islet cell transplantation in type I diabetes mellitus. Transplant Proc. 1987;19:2359–61. [PubMed] [Google Scholar]
- 3.Sharp DW, Lacy PE, Ricordi C, et al. Human islet transplantation in patients with type I diabetes. Transplant Proc. 1989;21:2744–45. [PubMed] [Google Scholar]
- 4.Warnock GL, Kneteman NM, Ryan EA, Halloran PF, Rabinovitch A, Rajotte RV, et al. Continued function of pancreatic islets after transplantation in type I diabetes. Lancet. 1989;ii:570–72. doi: 10.1016/s0140-6736(89)90701-0. [DOI] [PubMed] [Google Scholar]
- 5.Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990;39:515–18. doi: 10.2337/diab.39.4.515. [DOI] [PubMed] [Google Scholar]
- 6.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–76. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343–48. [PMC free article] [PubMed] [Google Scholar]
- 8.Gray DWR, McShane P, Grant A, Morris PJ. A method for the isolation of islet of Langerhans from the human pancreas. Diabetes. 1984;33:1055–61. doi: 10.2337/diab.33.11.1055. [DOI] [PubMed] [Google Scholar]
- 9.Warnock GL, Ellis D, Rajotte RV, Davidson I, Baekkeskov S, Egebjerg J. Studies of the islolation and viability of human islets of Langerhans. Transplantation. 1988;45:957–63. doi: 10.1097/00007890-198805000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;ii:1000–04. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK 506. JAMA. 1990;264:63–67. [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374–386. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzakis A, Todo S, Starzl TE. Upper abdominal exenteration with liver replacement: a modification of the cluster procedure. Transplant Proc. 1990;22:273–74. [PMC free article] [PubMed] [Google Scholar]
- 15.Lake SP, Basset D, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes. 1989;38 (suppl 1):143–45. doi: 10.2337/diab.38.1.s143. [DOI] [PubMed] [Google Scholar]
- 16.Alejandro A, Strasser S, Zucker PF, Mintz DH. Isolation of pancreatic islets from dogs. Transplantation. doi: 10.1097/00007890-199008000-00007. (in press) [DOI] [PubMed] [Google Scholar]
- 17.Latif Z, Noel J, Alejandro R. A simple method for staining of fresh and cultured islets. Transplantation. 1988;45:827–30. [PubMed] [Google Scholar]
- 18.Alderson D, Kneteman NM, Olack BJ, Scharp DW. Isolation and quantification of canine islet tissue for transplantation. Transplantation. 1987;43:579–81. doi: 10.1097/00007890-198704000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Ricordi C, Socci C, Davalli A, Staudacher C, Baro P, Vertova A, Sassi I, Gavazzi F, Pozza G, Di Carlo V. Isolation of the elusive pig islet. Surgery. 1990;107:688–94. [PubMed] [Google Scholar]
- 20.Ricordi C, Gray DWR, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Latina. doi: 10.1007/BF02581331. (in press) [DOI] [PubMed] [Google Scholar]
- 21.Morris PJ. Combined liver and pancreatic islet transplantation in the rat. Transplantation. 1983;36:230–231. [PubMed] [Google Scholar]
- 22.Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983;71:1544–53. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kano Y, Kanatsuna T, Nakamura N, et al. Defect of the first phase insulin secretion to glucose stimulation in the perfused pancreas of the nonobese diabetic (NOD) mouse. Diabetes. 1986;35:486–90. doi: 10.2337/diab.35.4.486. [DOI] [PubMed] [Google Scholar]
- 24.Reddy S, Bibby NJ, Fisher SL, Elliot RB. Longitudinal study of first phase insulin release in the BB rat. Diabetologia. 1986;29:802–07. doi: 10.1007/BF00873220. [DOI] [PubMed] [Google Scholar]
- 25.Srikanta S, Ganda OP, Eisenbarth GS, Soeldner JS. Islet-cell antibodies and beta-cell function in monozygotic triplets and twins initially discordant for Type I diabetes mellitus. N Engl J Med. 1983;308:322–25. doi: 10.1056/NEJM198302103080607. [DOI] [PubMed] [Google Scholar]
- 26.Alejandro R, Latif Z, Polonsky KS, Shienvold FL, Civantos F, Mintz DH. Natural history of multiple intrahepatic canine islet allografts during and following administration of cyclosporine. Transplantation. 1988;45:1036–41. doi: 10.1097/00007890-198806000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. doi: 10.1097/00000658-199009000-00008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


