Abstract
The gene transcripts encoding both the AF8 and AF2 neuropeptides of the nematode Ascaris suum have been identified, cloned, and sequenced. The AF8 transcript (afp-3) encodes five identical copies of AF8; each peptide-encoding region is flanked by the appropriate dibasic or monobasic cleavage processing sites. The AF2 transcript (afp-4) encodes three identical copies of AF2 along with the appropriate cleavage sites. In contrast, the afp-1 transcript (Edison et al. [1997] Peptides 18:929 –935) encodes six different AF peptides (AF3, 4, 10, 13, 14, 20) which all share a –PGVLRFamide C-terminus but have different N-terminal sequences. By using in situ hybridization, gene transcript expression patterns of afp-1, afp-3, and afp-4 (As-flp-18, As-flp-6, and As-flp-14, respectively, in the naming convention proposed by Blaxter et al. [1997] Parasitol Today 13:416 – 417) were determined in the adult A. suum anterior nervous system. Each gene transcript can be localized to a different subset of neurons. These subsets of neurons are different from the subsets of Caenorhabditis elegans neurons that were shown to express identical or similar peptides by the use of promoter GFP constructs
INDEXING TERMS: neuropeptides, FMRFa-like peptides, AF2, AF3, AF8, Caenorhabditis elegans
The FMRFamide-like peptide (FLP) family is large and distributed across many taxa. It is the most thoroughly studied neuropeptide family in nematodes (Davis and Stretton, 1996; Geary et al., 1999; Li et al., 1999a,b; McVeigh et al., 2005, 2006), and has been found in expressed sequence tag (EST) libraries of all nematode species studied (McVeigh et al., 2006). In the parasitic nematode Ascaris suum, our laboratory has identified 24 AF peptides (Ascaris FMRFamide-like peptides) through direct isolation or mass spectrometry (Cowden et al., 1989; Cowden and Stretton, 1993, 1995; Davis and Stretton, 1996, 2001; Yew et al., 2003, 2005, 2007) and five more putative AF peptides through gene cloning (Nanda and Stretton, unpubl.). Members of the AF family have short sequences of less than 15 amino acids; they typically share a common RFamide C-terminal sequence and are distinguished by their differing N-terminal extensions. FMRFamide-like immunoreactivity is found in roughly 60% of A. suum neurons (Cowden et al., 1993), and the AF peptides have been shown to produce a variety of potent effects on locomotory behavior and on the neuromuscular system (Cowden et al., 1989; Cowden and Stretton, 1993; Pang et al., 1995; Geary et al., 1999; Davis and Stretton, 2001; Trailovic et al., 2005; Verma et al., 2007), as well as the pharynx (Brownlee et al., 1999). For some peptides, correlated changes in levels of cyclic AMP have been found (Reinitz et al., 2000; Thompson et al., 2003).
A fundamental question about the AF peptides is how individual peptides, despite the similarities in their C-terminal sequences, are able to exert a diversity of physiological effects. Differences in N-terminal sequence among these peptides probably confer differential properties through interactions with different specific receptors in target neurons. Electrophysiological experiments show that different AF peptides can exert dramatically different effects on individual identified neurons (Davis and Stretton, 2001). For example, among the 18 AF peptides tested, AF2 produces the strongest depolarization of dorsal excitor type 2 (DE2) motorneurons, while AF8 leads to hyper-polarization in these neurons. Furthermore, the same AF peptide can have different effects on different neurons; for example, AF15 increases the input resistance of DE2 and decreases the input resistance of the dorsal inhibitory motorneuron (DI). In muscle cells, the same AF peptide can produce different effects on contraction in different locations: AF8 contracts ventral muscle and relaxes dorsal muscle (Maule et al., 1995). Last, but not least, the spatial organization of sites of release of AF peptides, and of the receptors that respond to them, must also make a large contribution to the overall functioning of the system.
Together these data suggest that the cellular organization of the intercellular signaling systems mediated by AF peptides is likely to have an important role in modulating A. suum behavior. Previously we used immunocytochemistry and mass spectroscopy of dissected ganglia to determine the cellular expression patterns of processed peptides (Sithigorngul et al., 1990, 1996; Sithigorngul and Stretton, 1991; Cowden et al., 1993; Yew et al., 2005). Each of these methods has its limitations. Cellular localization of individual peptides with antibodies depends on the specificity of the antibody; tests for crossreactivity with other endogenous peptides are limited to known peptides from A. suum, and these are only a fraction of those that are present—many more peptides remain to be discovered and sequenced. Dissected ganglia contain multiple neurons (2–41 neurons), so mass spectrometry of ganglia can confirm the presence of the peptide among the population of neurons, but not identify which specific neuron(s) contains the peptide.
In this article we describe an in situ hybridization (ISH) protocol that we developed for A. suum as an independent technique for detecting the cellular expression patterns of three AF peptide-encoding genes, encoding eight different peptides. We first identified and sequenced the afp-3 and afp-4 gene transcripts (afp: Ascaris FMRFamide-like precursor protein) that encode AF8 (KSAYMRFamide) and AF2 (KHEYLRFamide), respectively. We then used ISH with riboprobes to localize the afp-1 transcript, which encodes six peptides sharing C-terminal –PGVLRFamide (AF3, 4, 10, 13, 14, and 20), and the afp-3 and afp-4 gene transcripts within neurons. The results we obtained are congruent with the results from immunocytochemistry and mass spectrometry, but they make the description of the cellular expression patterns of these peptides much more robust.
MATERIALS AND METHODS
Animals
Live Ascaris suum were collected from pig small intestines at a regional slaughterhouse, and maintained at 37°C in phosphate-buffered saline (140 mM sodium chloride, 10 mM sodium phosphate, pH 7.0–7.5). They were used on the day of collection.
RNA isolation and cDNA preparation
Twenty flash-frozen A. suum heads were ground in liquid nitrogen, and, following cell lysis and DNA digestion, total RNA was isolated with a Clontech (Palo Alto, CA) RNA II Nucleospin Kit. First-strand cDNA was generated from the isolated RNA by reverse transcription polymerase chain reactions (RT-PCR) using Superscript II RNase H-reverse transcriptase and an oligo(dT) primer (Superscript First Strand Synthesis System for RT-PCR; Life Technologies, Bethesda, MD) in a Perkin Elmer (Norwalk, CT) Gene Amp PCR System 2400 thermal cycler. Head polyA RNA, isolated from head total RNA with an Oligotex mRNA kit (Qiagen, Chatsworth, CA), was used in Rapid Amplification of cDNA Ends (RACE) reactions. Subsequent PCR and 5′-RACE reactions used either the Perkin Elmer instrument or an Eppendorf (Hamburg, Germany) Mastercycler Gradient.
Degenerate primer design
AF8 primer design
To obtain the 5′ end of the AF8 gene transcript, four degenerate reverse primers (AF8-REV1: CCRAAICGCATRTAIGCIGA, AF8-REV2: CCRAAYCTCATRTAIGCIGA, AF8-REV3: CCRAAICGCATRTAIGCRCT, and AF8-REV4: CCRAAYCTCATRTAIGCRCT; R = A/G; Y = C/T; I = inosine) were designed against the amino acid sequence KSAYMRFG (Cowden and Stretton, 1995). Glycine was presumed to provide the necessary C-terminus amidation precursor (Kreil, 1984; Bradbury and Smyth, 1987). These reverse primers were each used with the trans-spliced leader SL1-specific forward primer (SL1: GGTTTAATTACCCAAGTTTGAG) (Nilsen et al., 1989). The 3′ end of the AF8 transcript was found in EST sequences in the NCBI database (GenBank Access. nos. BM283911, BM282749, BM281685, BM281997, BM281921, BM281944, BM28062, BM282409). The complete sequence was confirmed with control PCRs that used CAATCGAAGTACGAAATTAT as forward primer and either CAGGGATACAATTTAGACGTGAG or AACGACGAGAAGTTTATAG as reverse primers.
AF2 primer design
The 3′ end of the AF2 transcript (218 bp) was found in an EST on the BlaxterLab Nematode BLAST Server (Gen-Bank Access. no. BI593869). To confirm this sequence (region 11–218 bp), a PCR was run with the forward primer (AF2F-TEST CCGATTCGGAAAAAAGGAAG) and reverse primer (AF2R-TEST CACAAGTTGTACGTGTTTATATG). To obtain the 5′ end of the transcript, 5′-RACE primers were used in a BD SMART RACE cDNA Amplification kit (ClonTech) (AF2-5RACE1: ACGCACGGAAGACGTTGTTGTGCAATTA, AF2-5RACE2: GACCAAATCTGAGATACTCGTGCTTCCT, and AF2-5RACE3: ATCGGAGGTATTCGTGCTTCCTTTTTTC). 5′-RACE reactions were run according to manufacturer’s instructions using head polyA RNA. Two reverse primers (AF2-REV1: CACAAGTTGTACGTGTTTATAT and AF2-REV2: ATATGATGCATATTTAGCGATA) were also designed for use with the SL1 forward primer to determine whether the AF2 transcript contains this 5′ spliced leader sequence. PCR, cloning, and sequencing steps were carried out as described below. Primers were designed with the use of Primer3 (Rozen and Skaletsky, 2000) and NetPrimer software (Premier Biosoft International, Palo Alto, CA) and were synthesized by Operon Technologies (Alameda, CA) or Integrated DNA Technologies (Skokie, IL).
Cloning and sequencing
Hot-lid, time-release PCR reactions were carried out with A. suum cDNA and the AmpliTaq Gold PCR kit (Applied Biosystems, Foster City, IL). Both positive and negative controls were run concurrently. Positive controls used primers designed to amplify a specific region of the A. suum collagen transcript (GenBank Access. no. AF035410). Negative controls were run without cDNA template (i.e., using the product from an RT-PCR reaction run without reverse transcriptase). All PCR products were separated by agarose gel electrophoresis and bands were excised and purified (Qiaquick Gel Purification, Qiagen). The purified DNA of each novel band was ligated into an activated pCR II TOPO dual promoter T-vector plasmid, and then used to transform chemically competent Escherichia coli cells (TOPO TA Cloning, Invitrogen, La Jolla, CA). Recombinant colonies were selected using alpha-complementation and grown individually. Plasmids from each sample were isolated and purified (QIAprep Spin Miniprep kit, Qiagen). Sequencing reactions were run using Big Dye fluorescent dye terminator regents (Applied Biosystems) and then purified using the CleanSEQ magnetic bead system (Agencourt, Beverly, MA). Sequences were determined by the UW-Madison Biotechnology DNA Sequencing Center; sequence electrophorograms were viewed on Chromas (v. 2.2) software, and were screened for possible vector contamination using VecScan (National Center for Biotechnology Information, NCBI, NIH, Bethesda, MD). Sequences were analyzed using Jellyfish software (LabVelocity) and the ExPASy proteomics server (Swiss Institute of Bioinformatics; Gasteiger et al., 2003). Signal peptides were predicted from SignalP 2.0 and TargetP 1.01 software (Center for Biological Sequence Analysis; Technical University of Denmark).
Riboprobe synthesis
The 290 –524 bp region of the afp-1 gene transcript (GenBank Access. no. U15279; Edison et al., 1997), the 191– 411 bp region of the afp-4 transcript (GenBank Access. no. AY386834), and the 354 –575 bp region of the afp-3 transcript (GenBank Access. no. AY386833) were each amplified using standard PCR techniques and inserted into a pCRII TOPO dual promoter vector (Invitrogen). The transcript target regions of each riboprobe are shown in Figure 1. Each insert was sequenced to confirm the fidelity of the sequence and to determine its orientation within the vector. The construct was linearized (EcoRV and SpeI from New England Biolabs, Beverly, MA) and purified (Qiaquick PCR Purification kit, Qiagen), then used as a template for the synthesis of antisense and sense riboprobes from each insert (Maxiscript SP6/T7kit, Ambion, Austin, TX, and digoxigenin-11-dUTP, Roche Applied Science, Indianapolis, IN). Excess nucleotides were removed (NucAway Spin Columns, Ambion). Probe integrity and concentration were determined by gel electrophoresis with Sybr Gold staining (Molecular Probes, Eugene, OR) and nylon membrane dot blots (Roche Applied Science protocol).
Figure 1.
The nucleotide sequence and the deduced amino acid sequence of the afp-3, afp-4, and afp-1 cDNAs. The riboprobe’s targeted regions are indicated by the bold nucleotides. The putative start sites are in parentheses. The peptide-encoding amino acid sequences are bold and underlined. The peptide-forming cleavage sites are in italics. The bold forward slash indicates the predicted signal peptide cleavage site. An SL1 spliced leader nucleotide sequence is present and underlined in afp-4 and afp-1. A dash signifies a stop codon. Numbering of sequences is bold for amino acids, and unbold for nucleotides. A: (top panel) AF8-encoding cDNA (GenBank Access. no. AY386833). B: (middle panel) AF2-encoding cDNA (GenBank Access. no. AY386834). C: (bottom panel) afp-1 cDNA (Edison et al., 1997; GenBank Access. no. U15279).
In situ hybridization
ISH experiments were performed to localize the afp-1, afp-3, and afp-4 gene transcripts. An additional experiment using both afp-3 and afp-4 probes together was performed to distinguish afp-3 and afp-4 labeling in the dorsal ganglion.
The A. suum wholemount preparations were made by injecting collagenase into female worms ligatured with string, as described previously (Johnson and Stretton, 1985). This procedure dissociates muscle cells from the body wall, exposing the nervous system embedded in the hypodermis. The fully collagenased head (the anterior 2 cm of the worm) was removed and dissected in a Sylgard dish containing 100 mM phosphate buffer (PB, pH 7.4), by cutting longitudinally between the dorsal nerve cord and a lateral line and then cutting the lips to allow removal of the pharynx. The tissue was pinned out flat, and any remaining muscle gently removed with forceps or by agitating the dish. After several rinses with PB, the preparations were fixed at 4°C for 5 hours with 4% paraformaldehyde (PFA) in 100 mM PB, pH 7.4, with gentle rocking. The preparations were washed and dehydrated: 150 mM NaCl (5 min), 50% ethanol (3 min), 70% ethanol (3 min), 95% ethanol (3 min), 100% ethanol (2 × 5 min). These and all other washes in the ISH procedure that are not specified otherwise were performed at room temperature on a rocker. The preparations were stored in 100% ethanol overnight at −20°C.
The preparations were rehydrated with 150 mM NaCl (3 min), and then 100 mM PB (3 min). The heads were briefly refixed with 4% PFA in PB (15 min), and then rinsed with 100 mM PB (2 × 5 min). The preparations were deproteinized as follows: Tris-HCl/CaCl2 buffer (20 mM Tris; 2 mM CaCl2, pH 7.6; 5 min), proteinase K (2 μg/mL in Tris-HCl/CaCl2 buffer; 15 min at 37°C on a rocker), Tris-HCl/CaCl2 buffer (5 min), and 100 mM PB (5 min). The preparations were refixed in 4% PFA in 100 mM PB (5 min) and washed with 100 mM PB (4 × 5 min). Each head was put into a separate well of a chambered cell culture slide and incubated in 100 μL of hybridization buffer (sodium chloride/sodium citrate SSC 4× [Sambrook et al., 2001], 50% formamide, Denhardt’s solution 5× [Sambrook et al., 2001], tRNA 250 μg/mL) for 1 hour at room temperature, without rocking. The hybridization buffer was replaced with 100 μL digoxigenin-labeled antisense riboprobe diluted in hybridization buffer (5–50 pg/μL riboprobe in hybridization buffer), and the chambers were coverslipped. In all experiments, two types of controls, with digoxigenin-labeled sense riboprobe and with riboprobe absent, were run concurrently. As noted in the Results (see below), no staining of neurons was observed in control preparations. Preparations were incubated overnight in a sealed humid box at 50°C without rocking, and then washed and treated with RNAse A to digest unbound single stranded riboprobe: SSC 1× (15 min), SSC 0.25× (2 × 10 min at 50°C, rocking), RNAse A (10 μg/mL, in SSC 2×, 1 hour at 37°C with rocking), SSC 2× (2 × 5 min), and Tris-HCl/NaCl buffer (50 mM Tris-HCl; 300 mM NaCl, pH 7.6; 5 min). Nonspecific sites were saturated using blocking buffer (50 mM Tris-HCl, 300 mM NaCl pH 7.6, 1% Blocking Powder [Roche], 30 min). Bound digoxigenin-labeled probe was detected by incubation with anti-digoxigenin Fab fragments conjugated to alkaline phosphatase (Roche Applied Science) diluted in blocking buffer (1:500) overnight. This commercial antibody reagent was raised in sheep against a digoxigenin hapten and purified by ion exchange chromatography and by immunoadsorption to isolate IgG, which was digested with papain to produce Fab fragments and then conjugated with alkaline phosphatase. After washing with Tris-HCl/NaCl buffer (3 × 10 min), endogenous alkaline phosphatases were inhibited in levamisole buffer (1μM levamisole in 50 mM Tris-HCl buffer; 300 mM NaCl; 50 mM MgCl2; pH 9.5; 2 × 10 min). The heads were visualized with an NBT/BCIP staining buffer (45 μL of 75 mg/mL nitroblue tetrazolium salt in dimethylformamide, 35 μL of 50 mg/mL 5-bromo-4-chloro-3-indolyl phosphate toluidinium salt in dimethylformamide in 10 mL levamisole buffer) in the dark with frequent monitoring with a dissecting microscope. Staining times varied depending on the intensity of staining of the neurons and the background staining of hypodermal tissue. The staining reaction was stopped with distilled water. Head preparations were then mounted and coverslipped with an aqueous based mounting medium (Crystal Mount, Biomedia, Foster City, CA) and photomicrographs taken with a Kodak HIII camera on a Zeiss Universal microscope. Images were imported into Adobe Photoshop (San Jose, CA) as black and white images, and in some cases, especially for neurons in the lateral lines where the hypodermis contributed some background, the contrast and/or evenness of illumination was manipulated to improve the visualization of the stained neuron(s). The stained neurons differed in color from the background, and could be recognized easily in the microscope. Neurons were named using the Caenorhabditis elegans nomenclature (White et al., 1986); this usage is based on the striking morphological similarity of individual neurons in A. suum and C. elegans (e.g., Stretton et al., 1978; Angstadt et al., 1989; Sithigorngul et al., 1996).
RESULTS
AF gene transcripts
afp-3 (AF8) gene transcript
Degenerate PCR experiments using the AF8-REV4 and SL1 primers produced the 5′ end of the AF8 transcript and included two KSAYMRFG translated sequences. A BLAST search revealed several cDNA sequences that matched the 3′ end (277–362 bp) of our sequence (GenBank Access. nos. BM283911, BM282749, BM281685, BM281997, BM281921, BM281944, BM282409). A nucleotide alignment of these sequences displayed a clear consensus sequence with at most one sequence diverging for 1–2 nucleotides at any given position in the first 500 bp. To confirm the validity of the AF8 gene transcript sequence, additional PCR reactions were performed using specific primers generated from the original sequences to amplify the following regions (23–729 bp, 23–752 bp). These PCRs resulted in the expected sequences. The complete AF8 transcript sequence (Fig. 1A) is 795 bases long and contains an open reading frame of 156 amino acids. This protein encodes five predicted copies of the AF8 peptide each flanked at both ends by either a dibasic (KR) or monobasic (R) cleavage site (Veenstra, 2000). Each AF8-encoding region also ends in a C-terminal glycine, which is a known precursor for amidation. This transcript also includes the 5′ trans-spliced-leader SL1.
afp-4 (AF2) gene transcript
We obtained a full-length afp-4 transcript from 5′-RACE experiments using the AF2-5RACE3 primer. This sequence extended the original sequence found on the Blaxter BLAST database (GenBank Access. no. B1593869) by 424 bp, showing that the full transcript includes 641 bp (Fig. 1B). The new sequence differed from the Blaxter sequence by having one less adenine in a run of adenines beginning at 444 bp. This results in the restoration of the first AF2 coding region which was out of phase in the Blaxter sequence. The corrected sequence has four potential start codons; the first potential start site is the most likely since it has the strongest prediction of a signal peptide. The afp-4 sequence encodes three predicted copies of AF2 spaced apart only by dibasic (KR) cleavage sites. Each AF2-encoding region also possesses the C-terminal glycine necessary for later amidation. PCR reactions, designed to determine the presence or absence of the SL1 5′ trans-spliced leader sequence on the afp-4 transcript, produced no detectable amplified product, and we conclude that this transcript does not contain SL1.
Signal peptides
Both the AF8 and AF2 precursor protein sequences included predicted N-terminal signal peptides generally required for entrance into the secretory pathway (Fig. 1A,B). The most likely signal peptide cleavage site for the AF8 precursor protein sequence was between position 17 and 18 (VCA-LR), and for the AF2 precursor protein it was between position 21 and 22 (VSA-DA). The presence of a predicted signal peptide for the afp-1-encoded precursor protein has already been reported (Edison et al., 1997).
Localization of afp-1, afp-3, and afp-4 transcripts
The afp-1, afp-3, and afp-4 ISH experiments show that each gene transcript has a distinct expression pattern (Figs. 2– 6). Gene expression in the head wholemount preparation was restricted to neurons and each gene-specific riboprobe labeled a characteristic subset of neurons in the cephalic nervous system. The definitive identification of neurons stained after ISH is more difficult than after immunocytochemistry (ICC). In ICC, anti-peptide staining throughout the cytoplasm usually allows visualization of both the cell body and the processes; in ISH, on the other hand, only the cell bodies of neurons are stained, and important diagnostic features that come from the morphology of the processes of the neurons are not available. The criteria for identification of individual neurons stained by ISH are therefore restricted to the shape, position, and size of the cell body. In some cases these features are sufficient to definitively identify neurons, and in others the neuron can only be assigned to a class.
Figure 2.
Diagrams of the head ganglia of A. suum, modified from Goldschmidt (1908). The preparation has been split near the dorsal axis and opened flat. Neuronal cell bodies and commissural processes are shown. The nerve ring (NR), ventral ganglion (VG), dorsal ganglion (DG), lateral ganglia, and retrovesicular ganglion (RVG) are indicated. LLL: left lateral line; RLL: right lateral line; VC ventral nerve cord; DC: dorsal nerve cord; DeC: deirid commissures; AC: amphidial commissures. The central diagram is an overall view of the head ganglia. Surrounding boxes are magnifications of individual ganglia showing the location of identified neurons. The DG contains ALA and RID (RMED is in the nerve ring). The VG contains the identified neurons AVK, RIS, AIY and AIM (not yet distinguished from each other), RIR, AVL, and RIH (RMEV is in the nerve ring). The RVG contains two AVFs, two RIFs, RIG, and interneuron B (INTB). The lateral ganglia contain the “large” neuron and ADE, the anterior deirid.
Figure 6.

ISH with both afp-3 and afp-4 (AF2 and AF8) antisense probes. The RID and the ALA neurons are both heavily stained in the dorsal ganglion (DG). Scale bar = 100 μm.
In all trials, concurrently run controls using either digoxigenin-labeled sense riboprobes or no-riboprobe controls showed no neuronal staining and minimal background. Examples are shown in Figures 3E,F and 4D. The sequence target regions of each ISH probe are shown in Figure 1.
Figure 3.
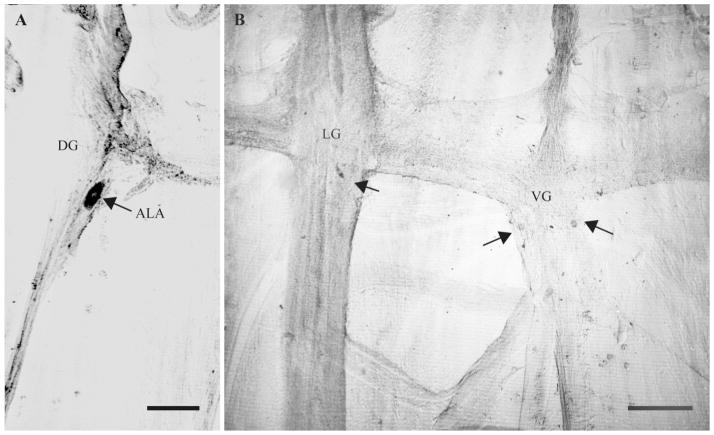
ISH with afp-1 antisense probe. A: A single neuron, RID, is stained in the dorsal ganglion (DG). In the left lateral ganglia (LG) the “large” neuron (long arrow) is heavily stained and a medium neuron near the amphidial commissure (medium arrow) is lightly stained. In this preparation a spherical neuron near the nerve ring is lightly stained (arrowhead). B: The same neurons are stained in the right lateral ganglion, as are five neurons in the ventral ganglion (VG). A and B are from the same preparation. C: The RIG neuron at the posterior end of the RVG is heavily stained and four other neurons are more lightly stained. D: The second RIG neuron, located posterior to the RVG in the ventral cord, is also heavily stained. E,F: Control ISH with afp-1 sense strand probe. VG and LG: E; RVG: F. No neurons are stained. Scale bars = 100 μm.
afp-1 (AF3, 4, 10, 13, 14, 20); 17 preparations
Dorsal ganglion (DG)
The dorsal ganglion contains two neurons, the ALA and RID neurons (Fig. 2). The shapes of their cell bodies are usually easily recognized: ALA has a characteristic anterior elongation that gives rise to two neurites that extend into each side of the nerve ring, and RID sends a process around the nerve ring, then along the dorsal nerve cord. In addition, the ALA neuron is usually posterior to the RID neuron. In all preparations there was staining in one cell, and in all but two cases this neuron could be identified as the RID neuron (Fig. 3A) from its characteristic position and shape. The staining intensity was highly variable, and was strong in only three preparations.
Ventral ganglion (VG)
There were between two and five neurons stained with variable intensity, none of it strong. These neurons included an unpaired neuron in the middle of the ganglion (either RIR or AVL) and two pairs of medium-sized cells in the posterior half (Fig. 3B).
Lateral ganglia (LG)
In all preparations, two pairs of neurons were stained, usually strongly. The first pair comprised the so-called “large” neurons (Sithigorngul et al., 1996), located in the LG roughly midway between the amphidial commissure and the deirid commissure (Fig. 3A,B) on each side; the second pair were medium-sized neurons near the amphidial commissure (Fig. 3B). Only one preparation showed medium intensity staining of a medium-sized cell with a spherical cell body (unusual in A. suum—most neuronal cell bodies are fusiform), whereas in most preparations this cell, located near the nerve ring, was unstained or very lightly stained (Fig. 3A,B).
Retrovesicular ganglion (RVG)
The most prominent staining was in the RIG neuron, a small monopolar cell near or at the posterior end of the ganglion (Fig. 3C). This cell is a member of a bilateral pair, each of which projects a process anteriorly to enter one or other side of the nerve ring (Angstadt et al., 1989). One RIG neuron is in the posterior RVG; its partner is located in the ventral nerve cord posterior to the RVG, and this cell was also very strongly stained (Fig. 3D). There was also weak to medium intensity staining in three or four medium to large neurons in the RVG. In three preparations there was very weak staining of the RIF neurons (described below).
Control preparations with sense riboprobes are shown in Figure 3E,F, and show no specific staining.
afp-4 (AF2); 10 preparations
Dorsal ganglion
There was strong staining of the RID neuron in all preparations (Fig. 4A). One preparation showed weak staining of the second neuron in the DG, the ALA neuron.
Figure 4.
ISH with afp-4 (AF2) antisense probe. A: The RID neuron in the dorsal ganglion (DG) is heavily stained. The ventral ganglion (VG) has two lightly stained neurons. The lateral ganglia (LG) have staining in the “large” neuron (short arrow) and in a small neuron near the amphidial commissure (long arrow). B: In a different preparation, the VG and one LG, containing the same stained neurons as in A, but with additional lightly stained neurons in the VG and LG. C: Retrovesicular ganglion (RVG), showing intense staining of the pair of RIF neurons and light staining of four other neurons. D: Control ISH with AF2 sense probe. No neuronal staining is seen. Scale bars = 100 μm.
Ventral ganglion
The staining of the neurons in the ventral ganglion was more variable, and never strong. Often the staining seemed to be concentrated in the nucleus of neuronal cell bodies (Fig. 4A,B). In one preparation no neurons were stained, and in others between one and five neurons were stained with moderate or weak intensity. These neurons were medium to large cells toward the posterior end of the ganglion.
Lateral ganglia
In each lateral ganglion there was strong staining of the “large” lateral neuron, located near the deirid commissure, and of a small neuron in the amphidial ganglion (Fig. 4A,B). Some preparations also showed weaker staining of one or two additional medium to small neurons just anterior to the large cell.
Retrovesicular ganglion
The two RIF neurons (Angstadt et al., 1989) were intensely stained in seven preparations (Fig. 4C), a single intensely stained RIF was found in one preparation, and two preparations lacked stained RIF neurons. The RIF neurons are a bilateral pair of very small monopolar cells that are located at or very near the anterior end of the RVG. Within the ventral cord they are near the medial (with respect to the intact worm) surface of the cord, which has the least surrounding hypodermal tissue. These neurons are delicate and may be lost, probably due to mechanical agitation. One preparation showed weak or medium intensity staining of six other neurons in the RVG.
A control preparation exposed to sense riboprobe is shown in Figure 4D. No specific staining is seen.
afp-3 (AF8); 14 preparations
Dorsal ganglion
In all preparations a single neuron was stained, in most cases intensely (Fig. 5A). Preparations were also viewed by phase microscopy to detect unstained neurons, and in nine preparations it was clear that the stained neuron was the more posterior of the two DG neurons, so it could be positively identified as the ALA neuron. In the other five preparations there was more overlap between the two neurons, so the second neuron could not be distinguished with sufficient clarity to identify it by its shape; it was therefore hard to identify the two neurons with certainty in these five preparations.
Figure 5.
ISH with afp-3 (AF8) antisense probe. A: The ALA neuron is heavily stained in the dorsal ganglion (DG). B: A pair of small cells in the anterior part of the ventral ganglion (VG) is lightly stained (arrows), as was a pair of neurons in the lateral ganglion (LG) near the nerve ring (arrow; only one LG is shown). Scale bars = 100 μm.
Ventral ganglion
One pair of small anterior neurons stained with medium intensity in eight preparations (Fig. 5B). In six preparations the staining intensity was low overall, and no stained neurons were seen in the VG.
Lateral ganglia
The lateral ganglia showed strong to medium intensity staining of a pair of small neurons near the nerve ring in 12 preparations (Fig. 5B). Even though the remaining two preparations were very weakly stained overall, these same neurons were seen. In seven preparations a second pair of small neurons near the nerve ring was stained with weak to medium intensity (not shown).
Retrovesicular ganglion
None of the preparations had stained neurons in the RVG. Control preparations showed no staining (data not shown).
Dual afp-3 and afp-4 ISH
Afp-3 and afp-4 gene expression patterns are for the most part strikingly different. Each transcript was each detected in only one large cell body in the DG. To confirm that the two probes labeled two different cells, the afp-3 and afp-4 riboprobes were applied simultaneously. The resulting preparations clearly show the presence of two labeled cells which can be recognized as the ALA and RID neurons (Fig. 6). Based on the shape and relative position of the stained cell bodies, we identified RID as the AF2-expressing neuron, and ALA as the AF8-expressing neuron.
DISCUSSION
AF2 and AF8 gene transcripts
The presence of a predicted signal peptide sequence in both afp-3 and afp-4 transcripts suggests that the peptides they encode are secreted products. The afp-4 transcript encodes three copies of AF2 (KHEYLRFamide), while the afp-3 transcript encodes five copies of AF8 (KSAYMRF-amide). Multiple copies of the same peptide encoded by the same transcript may be a means of signal amplification. This could be one of the mechanisms by which neurons that express peptides encoded by different genes control the relative ratio of the peptides that are secreted.
For the identical amino acid sequence repeats of both AF2 and AF8, the codon usage is variable, implying positive selection pressure for the amino acid sequence. Strong selective pressure on the amino acid sequence is also suggested by comparative data from other nematode species, thanks to the EST libraries assembled by the Blaxter laboratory and their collaborators (Parkinson et al., 2004). AF2 is conserved in 20 parasitic nematode species, as well as in C. elegans and C. briggsae; AF8 is conserved in 14 parasitic nematode species and in C. elegans and C. briggsae (McVeigh et al., 2005). In most cases the predicted precursor protein also contains multiple copies of the peptide, as in A. suum.
Also notable is that, unlike other AF transcripts (afp-1, afp-3, afp-6; Edison et al., 1997; Yew et al., 2007), the afp-4 transcript is not preceded by the trans-spliced SL1 sequence. Addition of the SL1 leader sequence is an essential step in the pre-mRNA maturation of many A. suum and C. elegans gene transcripts. C. elegans has a number of different spliced leaders (SL1–5) (Ross et al., 1995). SL1 and SL2 have been found in several other nematode species (Blumenthal, 2005). In C. elegans SL2 is used to trans-splice downstream transcripts in operons; SL2 has not been described in A. suum. Experiments show that SL1 can enhance translation in vitro (Maroney et al., 1995) but, so far, this has not been verified in vivo. While the precise role of SL1 itself is not yet clear, its importance in the nematode system appears critical as the deletion of the SL1 gene causes C. elegans embryonic death (Ferguson et al., 1996).
Gene expression
Each riboprobe specific to a given transcript produces an overall staining pattern that is fairly consistent and shows a consensus pattern. Each of the AF gene expression consensus patterns is distinct and includes a different subset of neurons. Indeed, differences between the staining patterns of individual AF gene transcripts far outweigh variability among preparations using a single riboprobe. This variability includes differences in the intensity of individual neuronal staining and also in the numbers of neurons stained in a given ganglion, and may be caused either by actual differences in the gene expression patterns of individual A. suum, and/or by experimental artifact. Variability in gene expression among individual A. suum would not be surprising. A. suum is, after all, a parasite whose genetic variability is unknown, and at present cannot be raised to adulthood in the laboratory. Although all our experiments were performed on large female worms, the specimens were collected from the small intestines of infected host swine at a large facility where we have no knowledge of the history of the host animal or its physiological state. Gene expression in A. suum could also be affected by the inevitable environmental differences due to gender, breed type, or drug treatment histories of host animals, over which we have no control.
Variability caused by experimental factors should also be considered. Several steps of the ISH process are potential sources of variation among preparations. Tissue dissociation by collagenase and subsequent dissection are possible sources of experimental artifact. There is variable efficacy of collagenase-induced dissociation of muscle from the body wall. In less completely digested preparations, undissociated muscle is removed mechanically, which may in turn damage underlying neuronal structures. Differences in dissection may also differentially expose neuronal structures during permeabilization, fixation, and hybridization steps, which may also contribute to staining differences.
Despite these caveats, the results of our A. suum ISH preparations compare very favorably with ISH results obtainable in other model systems. A. suum is one of the few experimental systems available in which many individual cells can be reliably identified strictly by anatomical differences. This advantage cannot, however, be entirely exploited in ISH. Since staining is restricted to the soma, definitive identification is possible only with neurons having particularly distinctive cell body size, shape, or position.
Our finding that the afp-1, afp-3, and afp-4 gene transcripts all show different cellular expression patterns suggests that at least part of the differences between the intercellular signaling systems mediated by different AF peptides are controlled at the transcriptional level. Widespread gene expression patterns suggest that AF2, AF8, and the afp-1 peptides play a complex role in neuromodulation. Since the cells present in nematode ganglia do not appear to be grouped together by function (White et al., 1986), it is difficult to make inferences on the significance of unidentified stained neurons on the basis of ganglion location alone.
The difference between afp-3 and afp-4 staining in the dorsal ganglion raises some interesting speculations. Both the ALA and RID neurons have processes that extend along the length of the worm, which would allow the possibility of their directly affecting locomotory behavior. The ALA neuron, which expresses afp-3, has two bilaterally symmetrical long processes that travel along each of the lateral lines, ending in varicose elongations in the lateral tail ganglia. In contrast, the RID motorneuron, which expresses afp-4, has a single extended process that innervates dorsal body muscles (Donmoyer and Stretton, unpubl.). This raises the intriguing hypothesis that differential AF gene expression may be a means of controlling the activity of the different somatic muscle fields (cf. Maule et al., 1995).
Comparison with C. elegans
The identification of individual neurons in ISH preparations of A. suum is often difficult, since usually only cell bodies are stained. The most important clues for definitive cellular identification in antibody-stained preparations, namely, the course of the process(es) of each neuron, are missing. Nevertheless, there are some neurons for which cell body shape, size, and position are sufficiently distinctive to allow rigorous identification (Fig. 2). These include the two neurons, ALA and RID, in the dorsal ganglion, two neurons (RMED and RMEV) in the nerve ring, six of the 13 neurons in the RVG (two AVFs, AVG, RIG, and two RIFs), the second RIG located in the ventral nerve cord posterior to the RVG, 10 of the 33 neurons in the ventral ganglion (two AVKs, RIS, AVL, RIR, RIH, two AIY+AIM), two ADEs in the lateral ganglia, and all 69 motor neurons in the ventral nerve cord (11 DE1, 7 DE2, 9 DE3, 6 DI, 13 VI, 12 VE1, and 11 VE2). In total, these 92 neurons account for 30% of the neurons in the adult female A. suum. We can make a reasonable guess of the identity of several other neurons in our ISH preparations. For the purpose of comparison with C. elegans, strict cellular identification is crucial, so we prefer to be conservative and confine our comparisons to those neurons for which we have no doubt about identity.
In the dorsal ganglion of A. suum, ALA expresses the afp-3 transcript, encoding AF8. RID expresses the afp-1 transcript, encoding the six related peptides AF3, AF4, AF10, AF13, AF14, and AF20, together with the afp-4 transcript, which encodes AF2. The presence of the processed peptides in these cells has been examined by mass spectrometry of single dissected dorsal ganglia (Yew et al., 2005). AF2, AF8, and all six members of the afp-1 peptide family were found, adding to the robustness of the description of the peptide expression of these cells. Specific antibodies against AF2 and AF8 have not yet been generated, but an antibody that recognizes all six of the afp-1 peptides stains RID in the A. suum DG (Sithigorngul and Stretton, unpubl.). Mass spectrometry on single dissected neurons will be even more informative, and these experiments are in progress (Jarecki J, Andersen K, Vestling M, and Stretton A, unpubl.).
In C. elegans, gene expression patterns have been explored through the use of constructs that include ≈2–3.5 kbp of upstream sequences of each flp (FMRFamide-like peptide) gene ligated to a green fluorescent protein (GFP) reporter gene (Kim and Li, 2004). In some cases, there is agreement on the cellular expression pattern in A. suum and C. elegans. For example, the A. suum RIG neurons strongly express the afp-1 transcript, and the antibody that recognizes the six peptides encoded by afp-1 strongly stains the RIG neurons (Sithigorngul and Stretton, unpubl.); in C. elegans, the flp-18 GFP construct (flp-18 encodes peptides closely related to the afp-1-encoded AF peptides; Husson et al., 2005) labels the RIG neurons. However, by using GFP constructs there is no expression of flp-6 (encodes AF8; identical sequence in C. elegans) in ALA, which contains AF8 in A. suum, or in RID (Kim and Li, 2004). Similarly, in RID, which expresses AF2 and the afp-1 peptides in A. suum, in C. elegans there is no expression of flp-18 (the expression of flp-14, which encodes AF2, has not been reported in C. elegans; Kim and Li, 2004). One of the most interesting interpretations of these results is that A. suum and C. elegans can express identical (or very closely related) peptides in different neurons. These two nematodes are estimated to have diverged evolutionarily about 550 million years ago (Vanfleteren et al., 1994), yet they have nervous systems with neuronal morphologies that are so strikingly similar that they are virtually scale models of each other. In addition, the sequences of neuropeptides are highly conserved, not just between these nematodes, but across many of the 30 parasitic nematodes for which EST libraries have been examined (Parkinson et al., 2004; McVeigh et al., 2005). It is very tempting to conclude that differences in behavior controlled by these structurally similar nervous systems are generated, at least in part, by differences in the cellular expression patterns of these powerful neuromodulators. However, gene expression patterns determined by the use of GFP constructs must be interpreted with caution until they are confirmed by independent methods. Already, some of the reported GFP expression patterns in C. elegans have been challenged by the use of SAGE (serial analysis of gene expression): of 49 genes showing mRNA expression in isolated ASE neurons only 28 were detected by GFP expression (Etchberger et al., 2007). These results included five neuropeptide-encoding genes (ins-22, flp-25, flp-13, flp-1, and nlp-21), of which only three (ins-22, flp-25, and flp-13) gave GFP expression. Similarly, the expression of two flp genes (flp-5 and flp-21) with positive GFP expression in ASE neurons (Kim and Li, 2004; Li and Kim, 2008) was not detected in the mRNA sampling. There are several possible reasons for these discrepancies (both apparent false positives and false negatives), the most likely of which involves our incomplete understanding of the details of the regulatory mechanisms that control the expression of these particular genes. In making a GFP construct, it is hard to know whether the included control sequences are complete (Kim and Li, 2004). Cis-regulatory modules may in some cases be located at considerable distances 5′ to the transcription initiation site (Dean, 2006). Such modules have also been found in intronic or 3′-untranslated or untranscribed regions (Wenick and Hobert, 2004), which were not included in the GFP constructs used for determining flp gene expression. The role of microRNAs in control of expression by posttranscriptional interactions with 3′-untranslated regions (Ambros, 2004) is an additional reason for inclusion of these regions in reporter constructs. The use of constructs might also affect normal control by changing the stoichiometry of the DNA and transcription factors (TFs), leading, for example, to the lack of sufficient TFs to cover all the relevant DNA binding sites in the transgenic cell (Hobert and Loria, 2006). Since the world of TFs and other regulatory proteins is often intricate and interactive, and may involve multiple molecular interactions, responses to concentration changes in any of the components may well be nonlinear, and result in qualitatively or quantitatively abnormal expression. There is a need for methods that determine the endogenous expression of native peptides, and/or the transcripts that encode them, in wildtype C. elegans. Unfortunately, ISH has not yet been successful in C. elegans neurons, and the use of peptide-specific antibodies in C. elegans has only been reported for AF1 (Kim and Li, 2004).
Other nematodes
ISH results in the plant parasitic nematode Globodera pallida (Kimber et al., 2002), with probes against the G. pallida transcripts for AF2, AF8, and the afp-1-related peptides, showed neuronal staining. The identification of individual neurons in this nematode is difficult, but the tentative identification of the stained neurons in no case agreed with the localization by GFP constructs in C. elegans, nor with the localization reported in A. suum in the present article. This reinforces the suggestion that there may be widespread differences in the cellular distribution of neuropeptides in the neurons of different nematodes, and that this may be one of the factors leading to species-specific differences in behavior in different nematode species with nervous systems that are very similar in their morphology.
Acknowledgments
We thank Steven Nanda, Gary Lyons, Joanne Yew, David Hsu, and Molly Nyholm for advice and discussion, and the following colleagues who critically reviewed the article: Philippa Claude, Jessica Jarecki, India Viola, Christopher Konop, Catharine Reinitz, Katherine Andersen, Alison Crane, Andrew Miller, Megan Ramaker, and Jennifer Knickelbine. We thank Bill Feeny for help with the figures.
Grant sponsor: National Institutes of Health (NIH); Grant number: RO1AI15429.
LITERATURE CITED
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Angstadt JD, Donmoyer JE, Stretton AOW. Retrovesicular ganglion of the nematode Ascaris. J Comp Neurol. 1989;284:374–388. doi: 10.1002/cne.902840305. [DOI] [PubMed] [Google Scholar]
- Blaxter ML, Guiliano DB, Scott AL, Williams SA. A unified nomenclature for filarial genes. Parasitol Today. 1997;13:416–417. doi: 10.1016/s0169-4758(97)01140-x. [DOI] [PubMed] [Google Scholar]
- Blumenthal T. The C. elegans Research Community. Wormbook. 2005. Trans-splicing and operons. [DOI] [PubMed] [Google Scholar]
- Bradbury AF, Smyth DG. Biosynthesis of the C-terminal amide in peptide hormones. Biosci Rep. 1987;7:907–916. doi: 10.1007/BF01122123. [DOI] [PubMed] [Google Scholar]
- Brownlee DJ, Walker RJ. Actions of nematode FMRFamide-related peptides on the pharyngeal muscle of the parasitic nematode, Ascaris suum. Ann N Y Acad Sci. 1999;897:228–238. doi: 10.1111/j.1749-6632.1999.tb07894.x. [DOI] [PubMed] [Google Scholar]
- Cowden C, Stretton AOW. AF2, an Ascaris neuropeptide: isolation, sequence, and bioactivity. Peptides. 1993;14:423–230. doi: 10.1016/0196-9781(93)90127-3. [DOI] [PubMed] [Google Scholar]
- Cowden C, Stretton AOW. Eight novel FMRFamide-like neuropeptides isolated from the nematode Ascaris suum. Peptides. 1995;16:491–500. doi: 10.1016/0196-9781(94)00211-n. [DOI] [PubMed] [Google Scholar]
- Cowden C, Stretton AOW, Davis RE. AF1, a sequenced bioactive neuropeptide isolated from the nematode Ascaris suum. Neuron. 1989;2:1465–1473. doi: 10.1016/0896-6273(89)90192-x. [DOI] [PubMed] [Google Scholar]
- Cowden C, Sithigorngul P, Brackley P, Guastella J, Stretton AOW. Localization and differential expression of FMRFamide immunoreactivity in the nematode Ascaris suum. J Comp Neurol. 1993;333:455–468. doi: 10.1002/cne.903330311. [DOI] [PubMed] [Google Scholar]
- Davis RE, Stretton AOW. The motornervous system of Ascaris: electrophysiology and anatomy of the neurons and their control by neuromodulators. Parasitology. 1996;113:S97–117. doi: 10.1017/s0031182000077921. [DOI] [PubMed] [Google Scholar]
- Davis RE, Stretton AOW. Structure-activity relationships of 18 endogenous neuropeptides on the motor nervous system of the nematode Ascaris suum. Peptides. 2001;22:7–23. doi: 10.1016/s0196-9781(00)00351-x. [DOI] [PubMed] [Google Scholar]
- Dean A. On a chromosome far, far away: LCRs and gene expression. Trends Genet. 2006;22:38–45. doi: 10.1016/j.tig.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Edison AS, Messinger LA, Stretton AOW. afp-1: a gene encoding multiple transcripts of a new class of FMRFamide-like neuropeptides in the nematode Ascaris suum. Peptides. 1997;18:929–935. doi: 10.1016/s0196-9781(97)00047-8. [DOI] [PubMed] [Google Scholar]
- Etchberger JF, Lorch A, Sleumer MC, Zapf R, Jones SJ, Marra MA, Holt RA, Moerman DG, Hobert O. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev. 2007;21:1653–1674. doi: 10.1101/gad.1560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KC, Heid PJ, Rothman JB. The SL1 trans-spliced leader RNA performs an essential embryonic function in Caenorhabditis elegans that can also be supplied by SL2 RNA. Genes Dev. 1996;10:1543–1556. doi: 10.1101/gad.10.12.1543. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPasy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary TG, Marks NJ, Maule AG, Bowman JW, Alexander-Bowman SJ, Day TA, Larsen MJ, Kubliak TM, Davis JP, Thompson DP. Pharmacology of FMRFamide-related peptides in helminths. Ann N Y Acad Sci. 1999;897:212–227. doi: 10.1111/j.1749-6632.1999.tb07893.x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. Das Nervensystem von Ascaris lumbricoides und megalocephala. Z Wiss Zool. 1908;9:73–136. [Google Scholar]
- Hobert O, Loria P. Uses of GFP in Caenorhabditis elegans. Methods Biochem Anal. 2006;47:203–226. doi: 10.1002/0471739499.ch10. [DOI] [PubMed] [Google Scholar]
- Husson SJ, Clynen E, Baggerman G, De Loof A, Schoofs L. Discovering neuropeptides in Caenorhabditis elegans by two dimensional liquid chromatography and mass spectrometry. Biochem Biophys Res Commun. 2005;335:76–86. doi: 10.1016/j.bbrc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Stretton AOW. Localization of choline acetyl-transferase within identified motoneurons of the nematode Ascaris. J Neurosci. 1985;5:1984–1992. doi: 10.1523/JNEUROSCI.05-08-01984.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Li C. Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J Comp Neurol. 2004;475:540–550. doi: 10.1002/cne.20189. [DOI] [PubMed] [Google Scholar]
- Kimber MJ, Fleming CC, Prior A, Jones JT, Halton DW, Maule AG. Localization of Globodera pallida FMRFamide-related peptide encoding genes using in situ hybridization. Int J Parasitol. 2002;32:1095–1105. doi: 10.1016/s0020-7519(02)00084-x. [DOI] [PubMed] [Google Scholar]
- Kreil G. Occurrence, detection, and biosynthesis of carboxy-terminal amides. Methods Enzymol. 1984;106:218–223. doi: 10.1016/0076-6879(84)06023-7. [DOI] [PubMed] [Google Scholar]
- Li C, Kim K. The C. elegans Research Community. Wormbook. 2008. Neuropeptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Kim K, Nelson LS. FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. Brain Res. 1999a;848:26–34. doi: 10.1016/s0006-8993(99)01972-1. [DOI] [PubMed] [Google Scholar]
- Li C, Nelson LS, Kim K, Nathoo A, Hart AC. Neuropeptide gene families in the nematode Caenorhabditis elegans. Ann N Y Acad Sci. 1999b;897:239–252. doi: 10.1111/j.1749-6632.1999.tb07895.x. [DOI] [PubMed] [Google Scholar]
- Maroney PA, Denker JA, Darzynkiewicz E, Laneve R, Nilsen TW. Most mRNAs in the nematode Ascaris lumbricoides are trans-spliced: a role for spliced leader addition in translational efficiency. RNA. 1995;1:714–723. [PMC free article] [PubMed] [Google Scholar]
- Maule AG, Geary TG, Bowman JW, Marks NJ, Blair KL, Halton DW, Shaw C, Thompson DP. Inhibitory effects of nematode FMRFamide-related peptides (FaRPs) on muscle strips from Ascaris suum. Invert Neurosci. 1995;1:255–265. doi: 10.1007/BF02211027. [DOI] [PubMed] [Google Scholar]
- McVeigh P, Leech S, Mair GR, Marks NJ, Geary TG, Maule AG. Analysis of FMRFamide-like peptide (FLP) diversity in phylum Nematoda. Int J Parasitol. 2005;35:1043–1060. doi: 10.1016/j.ijpara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- McVeigh P, Geary TG, Marks NJ, Maule AG. The FLP-side of nematodes. Trends Parasitol. 2006;22:385–396. doi: 10.1016/j.pt.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Nilsen TW, Shambaugh J, Denker J, Chubb G, Faser C, Putnam L, Bennett K. Characterization and expression of a spliced leader RNA in the parasitic nematode Ascaris lumbricoides var. suum. Mol Cell Biol. 1989;9:3543–3547. doi: 10.1128/mcb.9.8.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang FY, Mason J, Holden-Dye L, Franks CJ, Williams RG, Walker RJ. The effects of the nematode peptide KHEYLRF-amide (AF2) on the somatic musculature of the parasitic nematode Ascaris suum. Parasitology. 1995;110:353–362. doi: 10.1017/s003118200008094x. [DOI] [PubMed] [Google Scholar]
- Parkinson J, Mitreva M, Whitton C, Thompson M, Daub J, Martin J, Schmid R, Hall N, Barrell B, Waterston RH, McCarter JP, Blaxter ML. A transcriptome analysis of the phylum Nematoda. Nat Genet. 2004;36:1259–1267. doi: 10.1038/ng1472. [DOI] [PubMed] [Google Scholar]
- Reinitz CA, Herfel HG, Messinger LA, Stretton AOW. Changes in locomotory behavior and cAMP produced in Ascaris suum by peptides from Ascaris suum or Caenorhabditis elegans. Mol Biochem Parasitol. 2000;111:185–197. doi: 10.1016/s0166-6851(00)00317-0. [DOI] [PubMed] [Google Scholar]
- Ross LH, Freedman JH, Rubin CS. Structure and expression of novel spliced leader RNA genes in Caenorhabditis elegans. J Biol Chem. 1995;270:22066–22075. doi: 10.1074/jbc.270.37.22066. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Misener S, Krawetz SA, editors. Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell D, Sambrook J. Molecular cloning, a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2001. [Google Scholar]
- Sithigorngul P, Stretton AOW. Differential distribution of AF1, a FMRFamide-like neuropeptide in Ascaris nervous system revealed by specific monoclonal antibodies. Abstr Soc Neurosci. 1991;17:279. [Google Scholar]
- Sithigorngul P, Stretton AOW, Cowden C. Neuropeptide diversity in Ascaris: an immunological study. J Comp Neurol. 1990;294:362–376. doi: 10.1002/cne.902940306. [DOI] [PubMed] [Google Scholar]
- Sithigorngul P, Cowden C, Stretton AOW. Heterogeneity of Cholecystokinin/gastrin-like immunoreactivity in the nervous system of the nematode Ascaris suum. J Comp Neurol. 1996;370:427–442. doi: 10.1002/(SICI)1096-9861(19960708)370:4<427::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Stretton AOW, Fishpool RM, Southgate E, Donmoyer JE, Walrond JP, Moses JER, Kass IS. Structure and physiological activity of the motor neurons of the nematode Ascaris. Proc Nat Acad Sci U S A. 1978;75:3493–3497. doi: 10.1073/pnas.75.7.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DP, Davis JP, Larsen MJ, Coscarelli EM, Zinser EW, Bowman JW, Alexander-Bowman SJ, Marks NJ, Geary TG. Effects of KHEYLRFamide and KNEFIRFamide on cyclic adenosine monophosphate levels in Ascaris suum somatic muscle. Int J Parasitol. 2003;33:199–208. doi: 10.1016/s0020-7519(02)00259-x. [DOI] [PubMed] [Google Scholar]
- Trailovic SM, Clark CL, Robertson AP, Martin RJ. Brief application of AF2 produces long lasting potentiation of nAChR responses in Ascaris suum. Mol Biochem Parasitol. 2005;139:51–64. doi: 10.1016/j.molbiopara.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Vanfleteren JR, dePeer YV, Blaxter ML, Tweedie SAR, Trotman C, Lu L, Van Hauwaert M-L, Moens L. Molecular genealogy of some nematode taxa as based on cytochrome C and globin amino acid sequences. Mol Phylog Evol. 1994;3:92–101. doi: 10.1006/mpev.1994.1012. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch Insect Biochem Physiol. 2000;43:49–65. doi: 10.1002/(SICI)1520-6327(200002)43:2<49::AID-ARCH1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Verma S, Robertson AP, Martin RJ. The nematode neuropeptide, AF2 (KHEYLRF-NH2), increases voltage-activated calcium currents in Ascaris suum muscle. Br J Pharm. 2007;151:888–899. doi: 10.1038/sj.bjp.0707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenick AS, Hobert O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell. 2004;6:757–770. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Yew JY, Dikler S, Stretton AO. De novo sequencing of novel neuropeptides directly from Ascaris suum tissue using matrix-assisted laser desorption/ionization time-of-flight/time-of-flight. Rapid Commun Mass Spectrom. 2003;17:2693–2698. doi: 10.1002/rcm.1240. [DOI] [PubMed] [Google Scholar]
- Yew JY, Kutz KK, Dikler S, Messinger L, Li L, Stretton AO. Mass spectrometric map of neuropeptide expression in Ascaris suum. J Comp Neurol. 2005;488:396–413. doi: 10.1002/cne.20587. [DOI] [PubMed] [Google Scholar]
- Yew JY, Davis R, Dikler S, Nanda J, Reinders B, Stretton AO. Peptide products of the afp-6 gene of Ascaris suum have different biological actions. J Comp Neurol. 2007;502:872–882. doi: 10.1002/cne.21357. [DOI] [PubMed] [Google Scholar]