Abstract
Aims
Thrombospondin-1 (TSP1), via its necessary receptor CD47, inhibits nitric oxide (NO)-stimulated soluble guanylate cyclase activation in vascular smooth muscle cells, and TSP1-null mice have increased shear-dependent blood flow compared with wild-type mice. Yet, the endothelial basement membrane should in theory function as a barrier to diffusion of soluble TSP1 into the arterial smooth muscle cell layer. These findings suggested that endothelial-dependent differences in blood flow in TSP1-null mice may be the result of direct modulation of endothelial NO synthase (eNOS) activation by circulating TSP1. Here we tested the hypothesis that TSP1 inhibits eNOS activation and endothelial-dependent arterial relaxation.
Methods and results
Acetylcholine (ACh)-stimulated activation of eNOS and agonist-driven calcium transients in endothelial cells were inhibited by TSP1. TSP1 also inhibited eNOS phosphorylation at serine1177. TSP1 treatment of the endothelium of wild-type and TSP1-null but not CD47-null arteries inhibited ACh-stimulated relaxation. TSP1-null vessels demonstrated greater endothelial-dependent vasorelaxation compared with the wild type. Conversely, TSP1-null arteries demonstrated less vasoconstriction to phenylephrine compared with the wild type, which was corrected upon inhibition of eNOS. In TSP1-null mice, intravenous TSP1 blocked ACh-stimulated decreases in blood pressure, and both intravenous TSP1 and a CD47 agonist antibody acutely elevated blood pressure in mice.
Conclusion
TSP1, via CD47, inhibits eNOS activation and endothelial-dependent arterial relaxation and limits ACh-driven decreases in blood pressure. Conversely, intravenous TSP1 and a CD47 antibody increase blood pressure. These findings suggest that circulating TSP1, by limiting endogenous NO production, functions as a pressor agent supporting blood pressure.
Keywords: Thrombospondin-1, eNOS, CD47, Vasorelaxation, Blood pressure
1. Introduction
The vascular endothelium is a critical regulator of blood vessel tone through its production of the bioactive gas nitric oxide (NO). NO is generated in the endothelium by the conversion of l-arginine to l-citrulline catalysed by the NADPH-dependent enzyme endothelial NO synthase (eNOS), which requires Ca2+/calmodulin, flavin adenine dinucleotide, flavin mononucleotide, and tetrahydrobiopterin (BH4) as co-factors.1,2 Constitutive production of NO by endothelial cells promotes blood flow by inhibiting vascular smooth muscle cell (VSMC) contracture and platelet aggregation.3 NO-stimulated cGMP synthesis causes VSMC relaxation and decreases arterial vascular resistance by stimulating the dephosphorylation of myosin light chain-2.4 As such, endothelial NO is a central regulator of vascular health and blood pressure.
Recently, we reported that the matricellular protein thrombospondin-1 (TSP1), via its necessary receptor CD47, limits NO signalling in vascular cells through inhibiting NO-stimulated cGMP production by soluble guanylate cyclase (sGC).5–7 TSP1-null, and CD47-null mice both show increased shear-dependent changes in tissue blood flow and greater decreases in blood pressure to an NO challenge compared with wild-type animals.8,9 However, in the absence of disease or injury, TSP1 is primarily found as a soluble circulating blood protein and in platelet alpha granules. With a mass of 450 kDa, TSP1 is theoretically too large to cross the endothelial basement membrane and thus, under normal conditions, should not directly engage the VSMC layer of resistance arteries. Thus it was not clear how circulating TSP1 limited tissue and blood flow responses unless it also limited primary eNOS activation and endogenous NO production. Our present work tested the hypothesis that TSP1 regulates eNOS activation and endothelial-dependent vasorelaxation.
2. Methods
Detailed Materials and Methods are available online in the Supplementary material.
2.1. Animals
Wild-type, TSP1-null, and CD47-null mice were housed under pathogen-free conditions and had ad libitum access to filtered water and standard rat chow. Experiments and handling and care of animals conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
2.2. Reagents
TSP1 was isolated from fresh human platelets as previously described10 or purchased from Athens Research & Technology (Athens, GA, USA). The recombinant signature domain of TSP1 (E123CaG1) was kindly provided by Dr Deane Mosher (University of Wisconsin at Madison). All other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA).
2.3. Cell cultures
Bovine aortic endothelial cells (BAEC) and human umbilical vein endothelial cells (HUVEC) were obtained from Lonza Group Ltd (Switzerland) and maintained in manufacturer provided growth medium. Murine endothelial cells were harvested as previously described.5
2.4. Intracellular cyclic nucleotide measurement
Intracellular cGMP levels were determined using an enzyme immunoassay (Amersham, GE Healthcare, UK) as previously described.5 Data were normalized to microgram of protein with a BCA Assay kit (Pierce Biotechnology, Rockford, IL, USA).
2.5. eNOS activation assay
Endothelial cells were serum starved in endothelial basal medium plus 0.1% BSA. Treatment agents were added for 15 min prior to cell stimulation using acetylcholine (ACh) (10 µmol/L). Cells then received [3H]-l-arginine (Perkins Elmer). The assay was terminated and cell lysate added to pre-equilibrated Dowex-50-H+ columns. Equal volumes of eluate were quantified using a 1900CA Liquid Scintillation Analyzer (Packard). All conditions were assessed in triplicate, and all experiments were repeated at least three times.
2.6. Endothelial cell calcium flux
Agonist-induced Ca2+ release was monitored in endothelial cells in the presence or absence of 2.2 nmol/L TSP1 or other treatment agents using live cell imaging. For single-cell imaging, cells were serum starved 24 h prior to experiments and loaded with 5 µmol/L Fluo-4AM ester for 20 min prior to experiment. Images were acquired every 3 s on a Zeiss LSM 5 confocal microscope (Carl Zeiss, Thornwood, NY, USA) at ×20, 512 × 512. For en face calcium measurements in fresh aortas from wild-type mice, vessel segments were loaded for 1 h with Fluo-4AM. Images were acquired every 3 s at 10× magnification and a resolution of 512 × 512.
2.7. Immunoprecipitation and western blotting
Cells and tissue (arterial, skeletal muscle, and lung) were homogenized in lysis buffer and western blots or immunoprecipitation for the indicated target proteins were performed.
2.8. Analysis of tissue eNOS expression
Cells and tissue (arterial, skeletal muscle and lung) obtained from age- and sex-matched wild-type, TSP1-null, and CD47-null mice were snap frozen in liquid nitrogen and pulverized. Chilled lysis buffer was added to pulverized tissue and incubated on ice for 10 min. Each lysate was passed through a 23-gauge needle 10 times, briefly sonicated, and then centrifuged for 10 min at 10 000 g. The supernatants were snap frozen and stored at −80°C for later analysis by western.
2.9. Analysis of tissue sGC expression
Murine lung tissue (wild type, TSP1-null and CD47-null) was harvested and processed with a Dounce homogenizer with ice cold RIPA lysis buffer. Protein content was determined, cell lysates subjected to SDS–PAGE on 4–12% gels, and the separated proteins transferred to Immobilon-P transfer membranes. sGCα1 was detected with a polyclonal anti-sGCα1 antibody, sGCβ1 was detected with a polyclonal anti-sGCβ1 antibody, and actin was detected with a monoclonal anti-α-actin antibody.
2.10. Ex vivo arterial dilation studies
Arterial segments (3 m in length) were mounted in a dual wire myograph system (Multi Myograph Model 610M), allowed to equilibrate, and tone normalized to 9.98 mN. Concentration-response curves to phenylephrine (PE) were carried out and a dose that produced 80% maximum contraction (EC80) was chosen for establishing vascular tone.
2.11. Internal blood pressure measurement via telemetry
Blood pressure was measured by implanted telemetric transducers as previously described.9 For a discussion of dosage determination, see the detailed Supplementary material online, Materials and Methods.
2.12. Statistics
All studies were performed a minimum of three times. Statistical significance was calculated using two-way ANOVA as appropriate with a P-value <0.05 taken as significant using standard software (Origin 7, Origin Labs). Comparison of dose-response curves was carried out using a two-way ANOVA on non-linear regression fitted curves, followed by a Bonferroni post hoc test.
3. Results
3.1. TSP1 limits basal eNOS activity
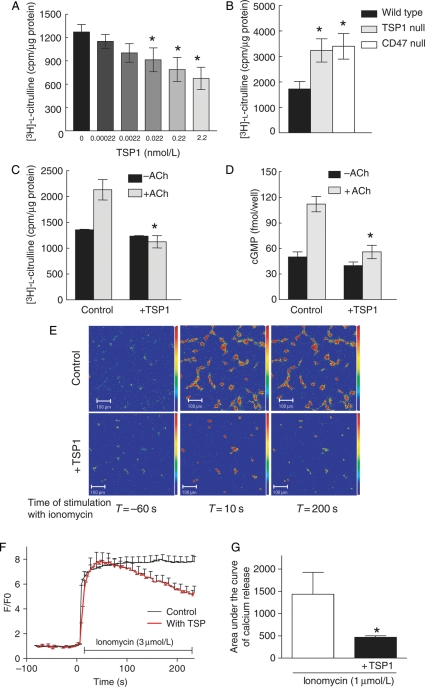
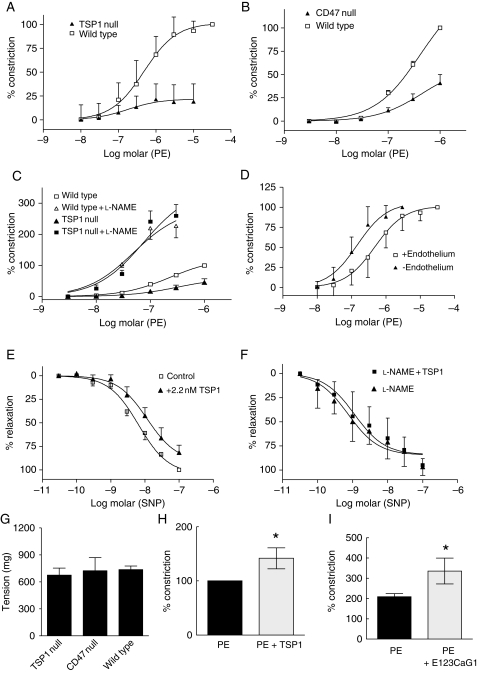
TSP1 inhibition of NO-stimulated cGMP accumulation and functional responses in cultured vascular cells can be explained by CD47-dependent inhibition of the downstream target sGC,5–7 but upstream inhibition of eNOS activation could also contribute to these results. To examine this, endothelial cells were treated with TSP1 for 15 min, and eNOS activity was assessed by conversion of [3H]-l-arginine to [3H]-l-citrulline (Figure 1A). TSP1 inhibited basal eNOS activity at concentrations ≥0.022 mol/L. Consistent with a role for the TSP1 receptor CD47 in limiting eNOS activity both TSP1- and CD47-null endothelial cells demonstrated increased basal enzyme activity (Figure 1B). Due to protein adsorption onto surfaces and the known sequestering of TSP1 by tissue matrix and proteoglycans, subsequent studies were performed with 2.2 nmol/L of TSP1 which is more than the minimal active concentration determined under serum-free conditions (and a concentration 10-fold higher than the mean plasma level of healthy persons11,12) but still within the range reported in certain disease situations.
Figure 1.
TSP1 inhibits basal- and agonist-stimulated eNOS activity and modulates calcium transients. BAEC or wild-type, TSP1-null, and CD47-null endothelial cells (5 × 105 cells/well) were serum starved for 24 h and incubated in minimal medium with the indicated amounts of TSP1 (A), without any treatments (B) or with TSP1 (2.2 nmol/L) followed by ACh stimulation (10 µmol/L) (C) in l-arginine free minimal medium prior to adding [3H]-l-arginine. [3H]-l-citrulline synthesis was determined as described and is presented normalized to total protein. Treatment wells were done in triplicate and all experiments were repeated three times. *P< 0.05 compared with untreated (A), wild-type (B), or ACh alone (C). HUVEC (5 × 105 cells/well) were serum starved for 24 h and incubated in serum/additive free medium + 0.1% BSA and stimulated with ACh (10 µmol/L) ± TSP1 (2.2 nmol/L). Intracellular cGMP levels were determined using an enzyme immunoassay (D). HUVEC were serum starved in basal medium overnight, loaded with fluo-4-AM for 20 min, and then pre-treated with/without TSP1 (2.2 nmol/L) followed by ionomycin (3 µmol/L). Images were acquired every 3 s (×20, 259 × 259). Data analysis was performed using Pascal 3.2 software (LSM5 Pascal Zeiss) (E). Colour images are representative of quantification in (E) (fluorescence intensity bar on right). Analysed relative changes in fluorescence intensity over time (F/F0) as the result of three independent experiments (untreated cells n = 113, TSP1-treated cells n = 64, +SD) (F). Measurement of the [Ca2+]i flux in situ in fresh aortic segments from wild-type mice was performed using fluo-4-AM following treatment with/without TSP1 (2.2 nmol/L 15 min) followed by ionomycin (3 µmol/L) (G). Results expressed as the change in area under the curve. Results are the mean ± SD of six vessels treated with ionomycin and four vessels treated with ionomycin + TSP1. *P< 0.05 compared with ionomycin alone.
3.2. TSP1 blocks eNOS activation by ACh
ACh is a physiological activator of eNOS.13,14 Endothelial cells treated with ACh demonstrated increased l-citrulline production, and this was blocked by pre-treatment with 2.2 nmol/L TSP1 (Figure 1C). TSP1 also blocked ACh-stimulated increases in cGMP levels (Figure 1D).
3.3. TSP1-mediated inhibition of eNOS activation does not occur through substrate limitation
Post-translational regulation of eNOS occurs through multiple mechanisms including control of its substrates and co-factors.2,15 We excluded one mechanism for indirect inhibition of NO synthesis by verifying that TSP1 does not inhibit carrier-mediated uptake of l-arginine into endothelial cells under conditions in which citrulline synthesis was inhibited (data not shown).
3.4. TSP1 modulates agonist-stimulated calcium transients in endothelial cells and murine aortas
ACh and ionomycin both activate eNOS in a calcium-dependent manner.16–18 HUVEC showed a sustained rise in intracellular calcium in response to ionomycin. However, the sustained phase of Ca2+ stimulated by ionomycin was reduced in cells treated with TSP1 compared with control cells (Figure 1D and E). En face confocal imaging of the endothelium in wild-type arteries further demonstrated that TSP1 applied to the endothelial layer significantly suppressed the agonist-driven calcium wave (Figure 1F) (quantified as the area under the curve).
3.5. TSP1 inhibits agonist-stimulated phosphorylation of eNOS
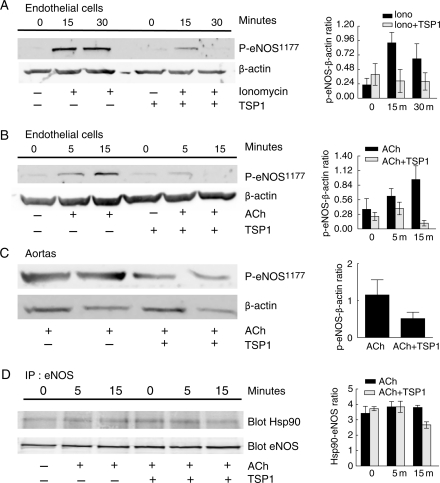
eNOS activation by ACh and ionomycin is associated with increased phosphorylation at residue serine1177 in human eNOS.19 Treatment of HUVEC with TSP1 blocked both ionomycin- and ACh-stimulated eNOS phosphorylation at serine1177 (Figure 2A and B). Aortic segments from wild-type mice treated with ACh showed enhanced eNOS phosphorylation at serine1177 which was blocked by endothelial cell treatment with TSP1 (Figure 2C).
Figure 2.
TSP1 inhibits agonist-stimulated phosphorylation of eNOS. HUVEC were serum starved and pre-treated with TSP1 (2.2 nmol/L 15 min) followed by addition of ionomycin (3 µmol/L) (A) or ACh (10 µmol/L) for the times indicated (B). Lysates were prepared and western blot analysis of eNOS phosphorylation at serine1177 determined. A representative blot of three experiments for each treatment is shown. Arterial segments from wild-type mice were pre-treated with TSP1 (2.2 nmol/L 15 min) followed by ACh (10 µmol/L) ± and lysates blotted against eNOS phosphorylation at serine1177 (C). A representative blot of three separate experiments is presented. HUVEC were serum starved and pre-treated with TSP1 (2.2 nmol/L 15 min) followed by ACh (10 µmol/L) for the indicated times. eNOS was immunoprecipitated and then western blotted against Hsp90 (D). A representative blot of three separate experiments is presented. For western blots, expression was quantified by densitometry calculated by measuring intensity of bands using Image J and normalized to either β-actin or eNOS expression.
3.6. TSP1 inhibits agonist-stimulated interactions between eNOS and Hsp90
Hsp90 is a chaperone protein that associates with eNOS and promotes correct folding and activation of the enzyme.20 Consistent with its ability to limit eNOS activation, we found on co-immunoprecipitation that TSP1 inhibited ACh-stimulated association of eNOS with Hsp90 (Figure 2D).
3.7. Soluble TSP1 inhibits eNOS-dependent arterial relaxation
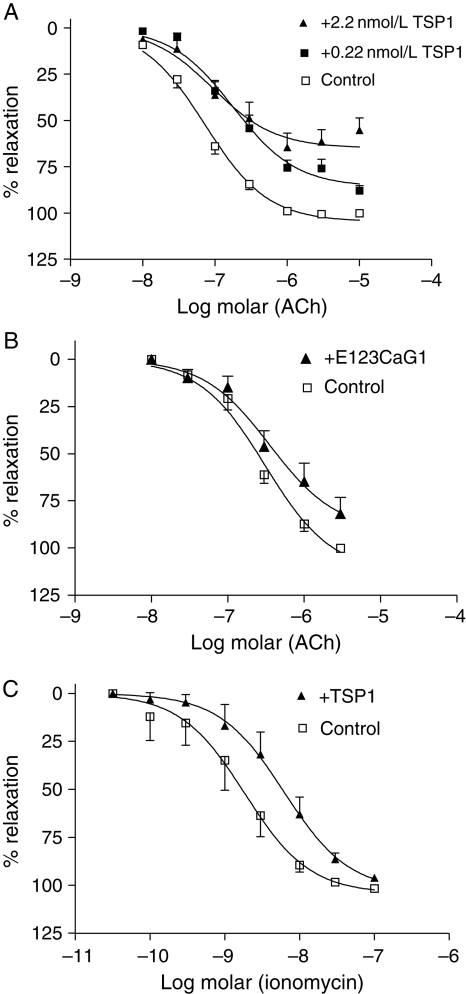
Effects of TSP1 on endothelium-dependent relaxation of murine thoracic aortas were studied with myography. Arterial relaxation to ACh in wild-type vessels was inhibited following endothelial treatment with physiologic levels of TSP1 (0.22 nmol/L) (Figure 3A), while higher concentrations (2.2 nmol/L) further shifted the response curve to ACh and decreased the maximum vasorelaxation response. The signature domain of TSP1, E123CaG1, that specifically binds CD47 also inhibited ACh-stimulated vasorelaxation (Figure 3B and Table 1). Ionomycin, through activating eNOS, can stimulate arterial relaxation21 and TSP1 also inhibited ionomycin-stimulated vasorelaxation (Figure 3C).
Figure 3.
TSP1 limits agonist-stimulated vasorelaxation. The luminal endothelium of wild-type vessels was pre-treated with exogenous TSP1 [0.22 and 2.2 nmol/L, (A)] or a recombinant domain of TSP1 [E123CaG1, 2.2 nmol/L, (B)] for 15 min and vasorelaxation to ACh determined. Results are the mean ± SD of 16 control vessels, 10 TSP1 (2.2 nmol/L)-treated vessels, six TSP1 (0.22 nmol/L)-treated vessels, and five E123CaG1 domain (2.2 nmol/L)-treated vessels. P< 0.0001 for both doses of TSP1 and P< 0.0063 for recombinant domain compared with untreated. Vasorelaxation of pre-contracted aortic segments from wild-type mice was determined in the presence of ionomycin (3 µmol/L) ± TSP1 (2.2 nmol/L) (C). Results are the mean ± SD of five vessels per treatment group, P< 0.0008 compared with untreated. Maximal relaxation in control vessels was set to 100% relaxation and treated vessels normalized to untreated. All fitted curves were analysed by two-way ANOVA followed by the Bonferroni post-test.
Table 1.
Myograph vasodilation response to ACh of murine thoracic aortas
| Figure | Treatment | EC50 | Rmax (% changed) |
|---|---|---|---|
| 3A | 2.2 nM TSP1 | 9.288E−08 | 64.90 |
| 0.22 nM TSP1 | 1.802E−07 | 77.18 | |
| Control | 7.321E−08 | 100.00 | |
| 3B | 2.2 nM E123CaG1 | 3.549E−07 | 82.11 |
| Control | 3.109E−07 | 100.00 | |
| 3C | 2.2 nM TSP1 | 6.225E−09 | 96.04 |
| Control | 1.854E−09 | 100.00 | |
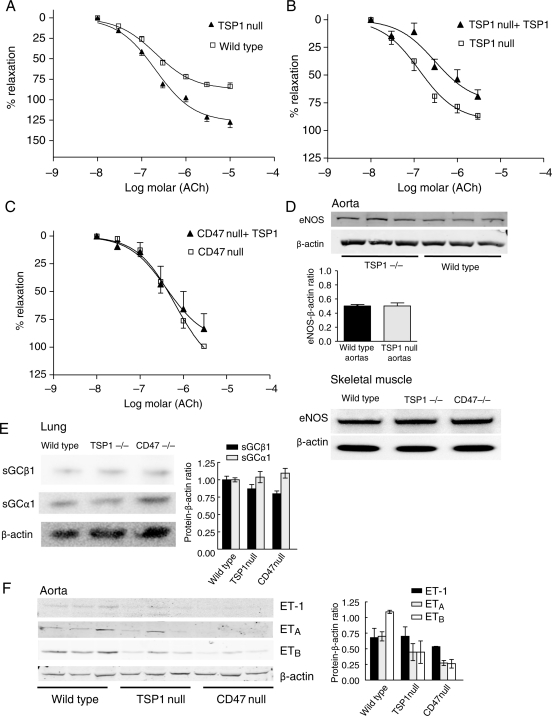
| 4A | WT | 2.104E−07 | 87.24 |
| TSP1 null | 2.249E−07 | 126.14 | |
| 4B | TSP1 null | 1.532E−07 | 100.00 |
| TSP1 null +2.2 nM TSP1 | 2.499E−07 | 82.86 | |
| 4C | CD47 | 5.542E−07 | 100.00 |
| CD47 +2.2 nM TSP1 | 4.453E−07 | 82.01 |
3.8. CD47 is necessary for TSP1 inhibition of endothelial-dependent vasorelaxation
eNOS-dependent arterial relaxation is enhanced in TSP1-null vessels compared with the wild-type (Figure 4A and Table 1). CD47-null arteries also demonstrated enhanced ACh-stimulated vasodilation compared with the wild-type (data not shown). Conversely, treatment of the endothelial lining of TSP1-null vessels (Figure 4B), but not CD47-null vessels (Figure 4C), with TSP1 inhibited ACh-stimulated vasorelaxation (Figure 4B).
Figure 4.
CD47 is necessary for TSP1 inhibition of eNOS-dependent arterial relaxation. Vasorelaxation dose-response curves of wild-type and TSP1-null vessels were determined to ACh (A). Data represent the mean ± SD of six TSP1-null and four control vessels. P< 0.001 TSP1-null compared with wild-type on two-way ANOVA and Bonferroni post-test. Vasorelaxation to ACh was determined in TSP1- (B) and CD47-null (C) aortic segments before and after the treatment of the luminal endothelium with TSP1 (2.2 nmol/L). Data represent the mean ± SD of four TSP1-null vessels and five CD47-null vessels. Two-way ANOVA analysis and Bonferroni post-test of the significance of TSP1 treatment of TSP1-null arteries on ACh-stimulated vasorelaxation showed P< 0.0028 compared with untreated TSP1-null arteries. Lysates from thoracic aortic segments (n = 3 vessels per strain) and vastus medialis muscle biopsies from age- and sex-matched wild-type and null mice were probed by western analysis for total eNOS (D). For each tissue type, representative blots of three experiments are presented. Lung tissue from age- and sex-matched wild-type, TSP1- and CD47-null mice was processed for western analysis of sGC subunits α1 and β1 (E). A representative blot of three experiments is presented. Lysates from thoracic aortic segments from age-matched male wild-type, TSP1- and CD47-null mice (n = 3 animals per strain) were probed via western analysis for ETA, ETB, and ET-1 (F). For western blots, expression was quantified by densitometry calculated by measuring intensity of bands using Image J and normalized to β-actin expression.
Altered responses in the null vessels might result from differences in eNOS or sGC protein levels. However, eNOS protein levels did not differ in arterial segments or skeletal muscle samples from wild-type and null mice (Figure 4D). Likewise, sGC protein levels were comparable in wild-type and TSP1-null mice. However, a change in the ratio between sGCβ1 and sGCα1 protein levels was noted in CD47-null samples (Figure 4E). Enhanced vasorelaxation of null arteries could also result from downregulation of vasoconstrictor pathways. Endothelin-1 (ET-1) is a key endogenous vasoconstrictor.22 Analysis of ET-1 and its receptors ETA and ETB in arteries from wild-type and null mice demonstrated no significant differences in protein levels between wild-type and TSP1-null vessels, but ETA and ETB levels were significantly decreased in CD47-null vessels (Figure 4F).
3.9. TSP1 potentiates arterial vasoconstriction
Arterial tone represents a balance between vasorelaxation and vasoconstriction.23 Consistent with their enhanced endothelial-dependent vasorelaxation, TSP1- and CD47-null arteries demonstrated decreased vasoconstriction to PE compared to wild-type vessels (Figure 5A and B). Inhibition of eNOS with l-nitro-arginine methyl ester (l-NAME) corrected resistance to PE-stimulated vasoconstriction in TSP1-null (Figure 5C) and CD47-null arteries (see Supplementary material online, Figure S1A), as did manual removal of the endothelium (data not shown), suggesting that elevated eNOS activity, in the absence of TSP1 and CD47, accounts in part for decreased responses to PE in TSP1- and CD47-null vessels. This is also supported by our findings that wild-type vessels (similar to null vessels) demonstrate increased vasoconstriction to PE on mechanical removal of the endothelium (Figure 5D). Additionally, TSP1 treatment of endothelium-intact wild-type vessels blunts vasorelaxation to the combination of exogenous NO from sodium nitroprusside (SNP) and endogenous NO from eNOS (Figure 5E). However, when the eNOS component of relaxation is removed by pre-treating the vessels with l-NAME, TSP1 is incapable of inhibiting SNP-stimulated vasodilation (Figure 5F). Similarly, vasodilation in endothelial-intact wild-type arteries to the pure NO donor diethylenetriamine NO (DETA/NO) is inhibited by soluble TSP1 and l-NAME pre-treatment blocks this effect (see Supplementary material online, Figure S1B and C). In an endothelial-denuded vessel, TSP1 could gain access to the smooth muscle cells to inhibit NO-stimulated relaxation, and an intact endothelial layer should represent a relative barrier to diffusion of soluble TSP1 into the VSMC layer of arterial segments. However, in light of reports that l-NAME inhibition of NOS increases arterial sensitivity to sydnonimine and SNP,24,25 responses of l-NAME treated wild-type arterial segments (Figure 5F and see Supplementary material online, Figure S1C) suggest that exogenous TSP1 may also alter sensitivity of the VSMC compartment to exogenous NO. Differences between wild-type and null arteries due to altered vasoconstrictor signalling in the null vessels are unlikely because responses to KCl were similar in wild-type, TSP1-null, and CD47-null vessels (Figure 5G). Finally, in keeping with the ability of TSP1 to limit eNOS-dependent vasorelaxation, treatment of the endothelium of wild-type arteries with TSP1 (Figure 5H) or a CD47-binding domain of TSP1, E123CaG1 (Figure 5I), significantly potentiates PE-stimulated vasoconstriction.
Figure 5.
TSP1 potentiates PE-stimulated vasoconstriction. Vasoconstriction to PE was determined in wild-type and TSP1-null arteries (A) or wild-type and CD47-null arteries (B). Data represent the mean ± SD of eight vessels from each strain (A) and 12 vessels from each strain (B). Two-way ANOVA analysis and Bonferroni post-test of significance of PE-stimulated vasoconstriction in wild-type vs. TSP1- and CD47-null arteries showed P< 0.0001. Vasoconstriction to PE±l-NAME (100 µmol/L) was determined in wild-type and TSP1-null arteries (C). Data represent the mean ± SD of five vessels of each strain. Two-way ANOVA analysis and Bonferroni post-test of significance of PE-stimulated vasoconstriction in wild-type vs. TSP1-null vessels showed P< 0.0001 compared with no difference in significance in vasoconstriction in wild-type and null vessels treated with PE + l-NAME. Changes in arterial tone to PE were determined in wild-type vessels with and without endothelium (D), in wild-type vessels with intact endothelium to SNP ± TSP1 (2.2 nmol/L) (E) and in l-NAME (100 µmol/L) treated wild-type vessels to SNP ± TSP1 (2.2 nmol/L) (F). Data represent the mean ± SD of 26 vessels with intact endothelium, 11 denuded vessels, four vessels treated with SNP, and four vessels treated with SNP + TSP1. Two-way ANOVA analysis and Bonferroni post-test of significance between endothelial intact vs. denuded vessels showed P< 0.0001 and between vessels treated with SNP vs. SNP + TSP1 showed P< 0.0003. Vasoconstriction in wild-type, TSP1-null, and CD47-null vessels was determined to KCl [100 mmol/L, (G)]. Data represent the mean ± SD of four vessels in each treatment group. P =0.510 and 0.916 wild type compared with TSP1 and CD47 null, respectively. Vasoconstriction of wild-type arteries was determined in response to an EC80 dose of PE (1 µmol/L) ± TSP1 (2.2 nmol/L) (H) or a recombinant fragment of the signature domain of TSP1 (E123CaG1, 2.2 nmol/L) (I). Data represent the mean ± SD of four vessels in each treatment group. P< 0.05 compared with PE alone (H and I).
3.10. Circulating TSP1 limits ACh-stimulated changes in mean arterial pressure
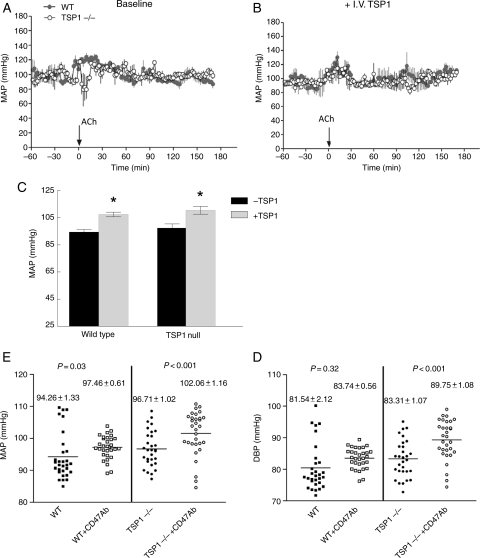
To assess the in vivo role of circulating TSP1 in regulating eNOS-dependent vasorelaxation and blood pressure, age-matched male wild-type and TSP1-null mice bearing telemetric pressure transducers were treated with ACh (0.08 μg/gram weight i.v.; see the Supplementary material online, Materials and Methods for details on dosage determination) and mean arterial pressure (MAP) measured (Figure 6A). TSP1-null mice demonstrated a significant decrease in MAP following ACh treatment. A similar dose of ACh in wild-type animals did not result in a change in MAP, suggesting that circulating levels of TSP1 in the blood are sufficient to limit eNOS activation by this dose of ACh. The administered dose of ACh minimally altered heart rate in either strain of animals (data not shown), suggesting that the hypotensive effects in TSP1-null animals were due to endothelial-dependent vasorelaxation and not secondary to ACh-stimulated bradycardia. Importantly, pre-treating TSP1-null mice with intravenous TSP1 (14.7 pmol/gram weight), which was previously shown to significantly elevate circulating plasma TSP1 levels for over 6 h,26 blocked the ACh-stimulated decrease in blood pressure in null mice (Figure 6B).
Figure 6.
Circulating TSP1 limits endothelial-dependent changes in blood pressure and is a hypertensive on acute administration. Age-matched male wild-type and TSP1-null mice bearing telemetric pressure transducers were treated with i.v. ACh (0.08 μg/gram weight) and MAP determined (A). TSP1-null mice were pre-treated with intravenous TSP1 (14.7 pmol/gram body weight) and followed 3 h later by i.v. ACh and MAP determined (B). Age-matched male wild-type and TSP1-null mice were treated with intravenous TSP1 (22 pmol/gram body weight) and MAP determined via telemetry transducer (C). Results represent the mean ± SD over a 2 h time interval post-administration of four animals of each strain. P< 0.05 compared with untreated. Age-matched wild-type and TSP1-null male mice received a CD47 antibody (clone 301, 4 μg/gram weight) or vehicle (normal saline) and the change in MAP and diastolic blood pressure determined via telemetric transducer. Results represent the mean ± SD over a 2 h time interval post-administration of eight animals of each strain.
3.11. Systemic administration of TSP1 increases blood pressure in mice
Circulating levels of TSP1 have been found to be elevated in several conditions associated with decreased tissue blood flow and elevated blood pressure including type I diabetes,27 dermatomyositis,28 systemic sclerosis,29 and sickle cell disease.30 Treating transducer bearing wild-type and TSP1-null mice with intravenous TSP1 (22 pmol/gram body weight) acutely elevated MAP in wild-type and TSP1-null mice (P< 0.05, Figure 6C).
3.12. A CD47 antibody alters blood pressure in wild-type and TSP1-null mice
To further explore the role of TSP1–CD47 signalling on blood pressure, mice were treated with CD47 antibody and blood pressure measured via telemetric transducer. Mimicking intravenous TSP1, antibody engagement of CD47 increased mean and diastolic blood pressure significantly in TSP1-null mice and MAP in wild-type mice (Figure 6D and E). An isotype-matched control antibody did not change blood pressure (data not shown).
4. Discussion
TSP1, via its receptor CD47, limits NO signalling in isolated endothelial cells, VSMC, and platelets by inhibiting NO-stimulated cGMP production by sGC.5–7 On the basis of its mass of 450 kDa, TSP1 is theoretically too large to cross the subendothelial basement membrane in normal physiologic states. Therefore, circulating TSP1 should not directly influence VSMC NO/cGMP signalling in intact vessels and yet TSP1- and CD47-null mice demonstrate enhanced shear-dependent changes in blood flow,8 and vascular endothelial cells from null animals demonstrate increased basal cGMP levels.31 We demonstrate herein that circulating TSP1 in vivo blocks endothelial-dependent decreases in blood pressure through limiting both basal- and agonist-stimulated eNOS activation. Equally significant, intravenous TSP1 elevates blood pressure. This can be explained by our finding that TSP1–CD47 signalling inhibits eNOS activation in endothelial cells and that null endothelial cells have inherently greater eNOS activity. Thus, TSP1 limits production of the diffusible vasodilator NO. This mechanism is consistent with our observations that addition of TSP1 to the luminal compartment in wild-type and TSP1-null, but not in CD47-null arteries, decreases ACh-stimulated vasodilation. Further, treatment of the endothelium of wild-type arterial segments with TSP1, and a recombinant domain of TSP1 that binds to CD47, by limiting eNOS activity, potentiates PE-stimulated vasoconstriction.
ACh and ionomycin stimulation of endothelial cells are associated with the activation of the PLC-IP3 pathway, leading to Ca2+-dependent eNOS activation and NO production.32–34 TSP1 modulates Ca2+ transients in endothelial cells, blocks phosphorylation of eNOS at serine1177, and limits association with Hsp90. Thus, TSP1 modifies eNOS activity through altering cellular calcium signalling, protein phosphorylation, and protein–protein association. ACh interacts with M1 and M3 receptors on endothelial cells and through the heterotrimeric protein Gαq elevates IP3 and intracellular Ca2+.35 CD47 in complex with several integrins is believed to function as an atypical G-coupled protein receptor.36 In endothelial cells, a CD47 antibody that blocked neutrophil activation also inhibited fibronectin-stimulated changes in cellular calcium.37 Expanding upon these findings, our results support a novel role for TSP1 in regulating calcium in endothelial cells.
Interestingly, TSP1 inhibited Ca2+ transients in endothelial cells stimulated by ionomycin, but ionomycin does not act as a simple ionophore at the plasma membrane.38 Rather, ionomycin also increases Ca2+ release from internal stores. Furthermore, thrombin has been shown to inhibit ionomycin-stimulated Ca2+ flux in HUVEC,39 indicating that physiological inhibition of this flux is possible.
Blood pressure is determined by several factors including arterial tone.40,41 Blockade of NO production using the NOS inhibitor l-NAME leads to hypertension in animals and people.42,43 Hypertension in eNOS-null mice has been associated with increased TSP1 expression.44 Conversely, a recent clinical series noted an association between patients with hypotension and abnormally low levels of circulating TSP1.45 Consistent with the ability of circulating TSP1 to inhibit eNOS activation and endothelial-dependent vasorelaxation, TSP1-null animals treated with ACh demonstrated a significant decrease in MAP compared with wild-type mice. Intravenous replacement of TSP1 in null mice blocked the ACh-stimulated drop in blood pressure, and in wild-type and null mice both intravenous TSP1 and a CD47 antibody (that mimics TSP1) elevated blood pressure. Also we now show that in the absence of vasoactive challenge, CD47-null mice show lower resting MAP, SBP, and DBP compared with the wild type (Table 2). Taken together, the results presented herein suggest that circulating TSP1 provides a rheostat to limit eNOS activation and NO production and in doing so exerts a pressor activity upon blood pressure.
Table 2.
In vivo resting blood pressure differences (Δ)—wild-type and CD47-null mice
| Variable | Δ | P-value |
|---|---|---|
| MAP | 4.72 ± 0.5 | 0.001 |
| SBP | 6.85 ± 0.48 | 0.006 |
| DBP | 4.48 ± 2.01 | 0.0176 |
| Pulse pressure | 0.81 ± 0.97 | 0.527 |
| Activity | 0.19 ± 0.03 | 0.817 |
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: J.S.I. is Chair of the Scientific Advisory Board of Vasculox, Inc. (St Louis, MO, USA) and Founder of Radiation Control Technologies, Inc. (Pittsburgh, PA, USA).
Funding
NIH grant K22 CA128616 (J.S.I.) and Intramural Research Program of the NIH, NCI, Center for Cancer Research (D.D.R.), and NIDDK (J.S.).
Supplementary Material
References
- 1.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. doi:10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. doi:10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 3.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 4.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. doi:10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 5.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. doi:10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71:785–793. doi: 10.1016/j.cardiores.2006.05.024. doi:10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, et al. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. doi:10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. doi:10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, et al. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts DD, Cashel J, Guo N. Purification of thrombospondin from human platelets. J Tissue Cult Methods. 1994;16:217–222. doi:10.1007/BF01540654. [Google Scholar]
- 11.Kao KJ, Klein PA. A monoclonal antibody-based enzyme-linked immunosorbent assay for quantitation of plasma thrombospondin. Am J Clin Pathol. 1986;86:317–323. doi: 10.1093/ajcp/86.3.317. [DOI] [PubMed] [Google Scholar]
- 12.Tan BK, Syed F, Lewandowski KC, O'Hare JP, Randeva HS. Circadian oscillation of circulating prothrombotic thrombospondin-1: ex vivo and in vivo regulation by insulin. J Thromb Haemost. 2008;6:1827–1830. doi: 10.1111/j.1538-7836.2008.03123.x. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa XF, Gonzalez DR, Martinez AD, Duran WN, Boric MP. ACh-induced endothelial NO synthase translocation, NO release and vasodilatation in the hamster microcirculation in vivo. J Physiol. 2002;544:883–896. doi: 10.1113/jphysiol.2002.021972. doi:10.1113/jphysiol.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton D, Ruan L, Sood SG, Li C, Zhang Q, Venema RC. Agonist-stimulated endothelial nitric oxide synthase activation and vascular relaxation. Role of eNOS phosphorylation at Tyr83. Circ Res. 2008;102:497–504. doi: 10.1161/CIRCRESAHA.107.162933. doi:10.1161/CIRCRESAHA.107.162933. [DOI] [PubMed] [Google Scholar]
- 15.Fleming I, Busse R. Signal transduction of eNOS activation. Cardiovasc Res. 1999;43:532–541. doi: 10.1016/s0008-6363(99)00094-2. doi:10.1016/S0008-6363(99)00094-2. [DOI] [PubMed] [Google Scholar]
- 16.Suh SH, Vennekens R, Manolopoulos VG, Freichel M, Schweig U, Prenen J, et al. Characterisation of explanted endothelial cells from mouse aorta: electrophysiology and Ca2+ signalling. Pflugers Arch. 1999;438:612–620. doi: 10.1007/s004249900085. doi:10.1007/s004240051084. [DOI] [PubMed] [Google Scholar]
- 17.Danthuluri NR, Cybulsky MI, Brock TA. ACh-induced calcium transients in primary cultures of rabbit aortic endothelial cells. Am J Physiol. 1988;255:H1549–H1553. doi: 10.1152/ajpheart.1988.255.6.H1549. [DOI] [PubMed] [Google Scholar]
- 18.Ghigo D, Alessio P, Foco A, Bussolino F, Costamagna C, Heller R, et al. Nitric oxide synthesis is impaired in glutathione-depleted human umbilical vein endothelial cells. Am J Physiol. 1993;265:C728–C732. doi: 10.1152/ajpcell.1993.265.3.C728. [DOI] [PubMed] [Google Scholar]
- 19.Gentile MT, Vecchione C, Maffei A, Aretini A, Marino G, Poulet R, et al. Mechanisms of soluble beta-amyloid impairment of endothelial function. J Biol Chem. 2004;279:48135–48142. doi: 10.1074/jbc.M407358200. doi:10.1074/jbc.M407358200. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49:134–140. doi: 10.1016/j.vph.2008.06.008. doi:10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miike T, Shirahase H, Kanda M, Kunishiro K, Kurahashi K. Regional heterogeneity of substance P-induced endothelium-dependent contraction, relaxation, and -independent contraction in rabbit pulmonary arteries. Life Sci. 2008;83:810–814. doi: 10.1016/j.lfs.2008.09.025. doi:10.1016/j.lfs.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Masaki T, Yanagisawa M, Takuwa Y, Kasuya Y, Kimura S, Goto K. Cellular mechanism of vasoconstriction induced by endothelin. Adv Second Messenger Phosphoprotein Res. 1990;24:425–428. [PubMed] [Google Scholar]
- 23.Marin J, Sanchez-Ferrer CF. Role of endothelium-formed nitric oxide on vascular responses. Gen Pharmacol. 1990;21:575–587. doi: 10.1016/0306-3623(90)91002-9. [DOI] [PubMed] [Google Scholar]
- 24.Busse R, Pohl U, Mulsch A, Bassenge E. Modulation of the vasodilator action of SIN-1 by the endothelium. J Cardiovasc Pharmacol. 1989;14(Suppl. 11)):S81–S85. doi: 10.1097/00005344-198906152-00015. [DOI] [PubMed] [Google Scholar]
- 25.Ralevic V, Mathie RT, Alexander B, Burnstock G. NG-nitro-L-arginine methyl ester attenuates vasodilator responses to acetylcholine but enhances those to sodium nitroprusside. J Pharm Pharmacol. 1991;43:871–874. doi: 10.1111/j.2042-7158.1991.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 26.Volpert OV, Lawler J, Bouck NP. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastases via thrombospondin-1. Proc Natl Acad Sci USA. 1998;95:6343–6348. doi: 10.1073/pnas.95.11.6343. doi:10.1073/pnas.95.11.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayraktar M, Dundar S, Kirazli S, Teletar F. Platelet factor 4, beta-thromboglobulin and thrombospondin levels in type I diabetes mellitus patients. J Int Med Res. 1994;22:90–94. doi: 10.1177/030006059402200204. [DOI] [PubMed] [Google Scholar]
- 28.Lutz J, Huwiler KG, Fedczyna T, Lechman TS, Crawford S, Kinsella TR, et al. Increased plasma thrombospondin-1 (TSP-1) levels are associated with the TNF alpha-308A allele in children with juvenile dermatomyositis. Clin Immunol. 2002;103:260–263. doi: 10.1006/clim.2001.5212. doi:10.1006/clim.2001.5212. [DOI] [PubMed] [Google Scholar]
- 29.Macko RF, Gelber AC, Young BA, Lowitt MH, White B, Wigley FM, et al. Increased circulating concentrations of the counteradhesive proteins SPARC and thrombospondin-1 in systemic sclerosis (scleroderma). Relationship to platelet and endothelial cell activation . J Rheumatol. 2002;29:2565–2570. [PubMed] [Google Scholar]
- 30.Browne P, Shalev O, Hebbel RP. The molecular pathobiology of cell membrane iron: the sickle red cell as a model. Free Radic Biol Med. 1998;24:1040–1048. doi: 10.1016/s0891-5849(97)00391-2. doi:10.1016/S0891-5849(97)00391-2. [DOI] [PubMed] [Google Scholar]
- 31.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. doi:10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 32.Cheung DW, Chen G. Calcium activation of hyperpolarization response to acetylcholine in coronary endothelial cells. J Cardiovasc Pharmacol. 1992;20(Suppl. 12)):S120–S123. doi: 10.1097/00005344-199204002-00034. [DOI] [PubMed] [Google Scholar]
- 33.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Sources of Ca2+ in relation to generation of acetylcholine-induced endothelium-dependent hyperpolarization in rat mesenteric artery. Br J Pharmacol. 1997;120:1328–1334. doi: 10.1038/sj.bjp.0701027. doi:10.1038/sj.bjp.0701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moncada S, Higgs EA. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- 35.Landry Y, Niederhoffer N, Sick E, Gies JP. Heptahelical and other G-protein-coupled receptors (GPCRs) signaling. Curr Med Chem. 2006;13:51–63. doi:10.2174/092986706775197953. [PubMed] [Google Scholar]
- 36.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. doi:10.1016/S0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MA, Brown EJ, Fazeli B. A 50-kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J Biol Chem. 1993;268:19931–19934. [PubMed] [Google Scholar]
- 38.Morgan AJ, Jacob R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J. 1994;300(Pt 3):665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neylon CB, Irvine RF. Thrombin attenuates the stimulatory effect of histamine on Ca2+ entry in confluent human umbilical vein endothelial cell cultures. J Biol Chem. 1991;266:4251–4256. [PubMed] [Google Scholar]
- 40.Crews DE, Williams SR. Molecular aspects of blood pressure regulation. Hum Biol. 1999;71:475–503. [PubMed] [Google Scholar]
- 41.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93:715–724. doi: 10.1113/expphysiol.2007.039545. doi:10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai H, Hara H, Tsai AG, Tsuchida E, Intaglietta M. Constriction of resistance arteries determines L-NAME-induced hypertension in a conscious hamster model. Microvasc Res. 2000;60:21–27. doi: 10.1006/mvre.2000.2240. doi:10.1006/mvre.2000.2240. [DOI] [PubMed] [Google Scholar]
- 43.Swislocki A, Eason T, Kaysen GA. Oral administration of the nitric oxide biosynthesis inhibitor, N-nitro-L-arginine methyl ester (L-NAME), causes hypertension, but not glucose intolerance or insulin resistance, in rats. Am J Hypertens. 1995;8:1009–1014. doi: 10.1016/0895-7061(95)00161-1. doi:10.1016/0895-7061(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 44.Kosugi T, Heinig M, Nakayama T, Connor T, Yuzawa Y, Li Q, et al. Lowering blood pressure blocks mesangiolysis and mesangial nodules, but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am J Pathol. 2009;174:1221–1229. doi: 10.2353/ajpath.2009.080605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Zhao Q, Han Q, Gao M, Zhang N. Serum thrombospondin-1 is altered in patients with hemorrhagic fever with renal syndrome. J Med Virol. 2008;80:1799–1803. doi: 10.1002/jmv.21270. doi:10.1002/jmv.21270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.