Abstract
Reducing the heart's temperature by 2–5°C is a potent cardioprotective treatment in animal models of coronary artery occlusion. The anti-infarct benefit depends upon the target temperature and the time at which cooling is instituted. Protection primarily results from cooling during the ischaemic period, whereas cooling during reperfusion or beyond offers little protection. In animal studies, protection is proportional to both the depth and duration of cooling. An optimal cooling protocol must appreciably shorten the normothermic ischaemic time to effectively salvage myocardium. Patients presenting with acute myocardial infarction could be candidates for mild hypothermia since the current door-to-balloon time is typically 90 min. But they would have to be cooled quickly shortly after their arrival. Several strategies have been proposed for ultra-fast cooling, but most like liquid ventilation and pericardial perfusion are too invasive. More feasible strategies might include cutaneous cooling, peritoneal lavage with cold solutions, and endovascular cooling with intravenous thermodes. This last option has been investigated clinically, but the results have been disappointing possibly because the devices lacked capacity to cool the patient quickly or cooling was not implemented soon enough. The mechanism of hypothermia's protection has been assumed to be energy conservation. However, whereas deep hypothermia clearly preserves ATP, mild hypothermia has only a modest effect on ATP depletion during ischaemia. Some evidence suggests that intracellular signalling pathways might be responsible for the protection. It is unknown how cooling could trigger these pathways, but, if true, then it might be possible to duplicate cooling's protection pharmacologically.
Keywords: Cardioprotection, Cooling, Hypothermia, Infarction, Ischaemia
1. Introduction
Therapeutic whole-body hypothermia has been considered for centuries.1 For example, the Russian approach to resuscitation of a patient in cardiac arrest in 1803 consisted of covering him with snow and then hoping for the resumption of a spontaneous circulation.1 A century and a half later, the beneficial effect of therapeutic hypothermia during cardiac surgery was proved in canine models.2,3 Outside of the operating room, hypothermia has been demonstrated to protect the brain following cardiac arrest in both animal models and humans.4,5 The American Heart Association and the European Resuscitation Council both recommend the use of hypothermia in comatose patients resuscitated from cardiac arrest to improve the subsequent neurological recovery.6,7
Besides hypothermia's beneficial effect during cardiac surgery or following cardiac arrest, it has also been clearly demonstrated that even very mild hypothermia can greatly increase the heart's tolerance to myocardial ischaemia, resulting in decreases of infarct size in animal models of coronary artery occlusion. Mild hypothermia (body temperature down to 32°C) has the advantage that the heart continues to pump normally and no extracorporeal support is needed. However, despite the encouraging results in animals, clinical trials of mild hypothermia in patients being revascularized for acute myocardial infarction have yielded surprisingly disappointing effects on infarct size.8–10 One goal of the present review is to consider why past trials have failed and propose what might be done to make cooling-induced cardioprotection more effective. We will review the literature to see whether an optimal target temperature and cooling method can be recommended. Finally, the mechanism of mild cooling's protection will be revisited. Whereas deep hypothermia stops the heart and clearly preserves ATP during ischaemia, cooling to 34°C has only a modest effect on ATP depletion during ischaemia11 but it is as protective to the rabbit heart as is ischaemic preconditioning.12 That has caused some to suspect that the mechanism of the cardioprotective effect of mild hypothermia might be more complex than energy preservation alone.13
2. Physiological effects of mild hypothermia
Hypothermia has distinct physiological effects. Hypothermia can be classified as mild (32–35°C), moderate (28–32°C), severe (20–28°C), or profound (<20°C). Mild hypothermia is the only one to be used for a whole-body therapeutic purpose, as lower temperatures are increasingly life-threatening. Below 28°C, the heart spontaneously fibrillates in most mammalian species. Conversely, mild whole-body hypothermia is remarkably well tolerated by both animals and humans. The physiological effects of mild hypothermia include a decrease in the heart rate and a subsequent fall in cardiac output (−7% for every 1°C),3,14 whereas stroke volume and mean arterial pressure remain unchanged. Cooling of the skin provokes an increase in systemic vascular resistance. With respect to regional haemodynamics, cerebral blood flow and intracranial pressure decrease,3,14 whereas renal blood flow and subsequent diuresis are increased.15 Mild hypothermia also attenuates the cerebral metabolic rate (−6 to −7% for every 1°C).16 Intestinal motility is also depressed by mild hypothermia. Blood pH changes by +0.016 unit for every 1°C decrease, and serum potassium decreases as a result of its enhanced cellular uptake.17 Hypothermia-induced hypokalaemia should be corrected with caution during hypothermia since raising serum K+ usually leads to hyperkalaemia during subsequent rewarming. Other adverse effects include increased infection risk, a mild coagulopathy,17 and hyperglycaemia caused by decreased plasma insulin levels.18
3. The infarct-limiting property of cooling: experimental in vivo investigations
3.1. Proof of concept of the myocardial infarct-limiting property of hypothermia
Heart temperature is known to be a major determinant of infarct size in animal models of acute myocardial ischaemia.19–21 As an example, infarct size resulting from 30 min of regional myocardial ischaemia in in situ rabbit hearts decreases by 8% of the risk zone for every 1°C decrement.21 The infarct-limiting property of mild hypothermia has been confirmed in several species including dogs,19 rabbits,12,21–25 pigs,20,26–28 and rats.29
3.2. What is the optimal target temperature?
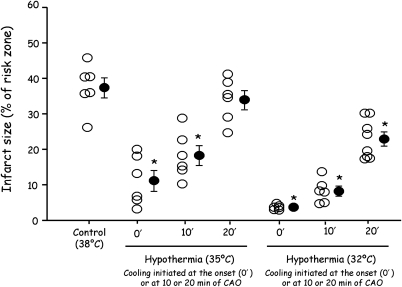
Importantly, there is no threshold temperature for the cardioprotective effect of hypothermia.19–21 In fact, warming the heart above normothermia extends infarct size by the same degree as cooling reduces it.21 For example, Hamamoto et al.30 demonstrated in sheep submitted to 60 min of coronary artery occlusion that infarct size was progressively reduced when cardiac temperature during ischaemia and reperfusion was lowered by 1°C decrements from 39.5 to 35.5°C. As shown in Table 1, temperature decrement during ischaemia within the mild hypothermic range has demonstrated a powerful anti-infarct effect in many studies. Figure 1 illustrates cooling to only 35°C at the onset of ischaemia elicits ∼70% decrease in infarct size following 30 min of regional ischaemia in the open-chest rabbit.12 Moreover, protection is still realized if the onset of cooling is delayed for 10 min. Cooling the heart to 32°C at the onset of ischaemia essentially prevented infarction, and a protective effect was still evident when cooling was initiated as long as 20 min after the onset of ischaemia and, therefore, 10 min before reperfusion.12 Furthermore, even when the ischaemic period was extended to 60–120 min and cooling to 30–32°C was initiated 20–30 min after the onset of ischaemia, the infarction process appeared to be halted when cooling was started and significant myocardial salvage was realized.12,22 The heart continued to beat strongly and support the rabbit's circulation.
Table 1.
Summary of in vivo experimental studies investigating the infarct-limiting effect of myocardial cooling during ischaemia, i.e. with cooling initiated before ischaemia or at least 5 min before the end of coronary artery occlusion
| Species | Ref. | Cooling procedure | Duration of CAO (min)/CAR (h) | Target heart temperature (°C) | Time of coolinga | IS with Cooling vs. Control groups (% decrease) |
|---|---|---|---|---|---|---|
| Rabbit | 36 | Topical epicardial cooling | 30/3 | ∼33 | 10 min CAO → 15 min CAR | 23 ± 4 vs. 44 ± 4 (−48%) |
| Rabbit | 22 | Topical epicardial cooling | 120/3 | ∼30 | 30 min CAO → 15 min CAR | 59 ± 3 vs. 72 ± 3 (−18%) |
| Rabbit | 49 | Closed pericardioperfusion circuit | 30/3 | ∼34 | −30 → 25 min CAO | 18 ± 3 vs. 35 ± 6 (−49%) |
| Rabbit | 24 | Topical epicardial cooling | 30/3 | ∼32 | 20 min CAO → 120 min CAR | 27 ± 4 vs. 51 ± 5 (−47%) |
| Rabbit | 12 | Blood cooling through heat exchanger | 30/3 | ∼35 | 0 → 30 min CAO | 11 ± 3 vs. 37 ± 3 (−70%) |
| 10 → 30 min CAO | 18 ± 3 vs. 37 ± 3 (−51%) | |||||
| 20 → 30 min CAO | 34 ± 2 vs. 37 ± 3 (NS) | |||||
| ∼32 | 0 → 30 min CAO | 4 ± 1 vs. 37 ± 3 (−89%) | ||||
| 10 → 30 min CAO | 8 ± 1 vs. 37 ± 3 (−78%) | |||||
| 20 → 30 min CAO | 23 ± 2 vs. 37 ± 3 (−38%) | |||||
| Rabbit | 25 | Total liquid ventilation | 30/3 | ∼32 | 0 → 30 min CAO | 4 ± 1 vs. 38 ± 1 (−89%) |
| Rabbit | 48 | Total liquid ventilation | 30/72 | ∼32 | 5 → 30 min CAO | 4 ± 1 vs. 39 ± 2 (−90%) |
| 15 → 30 min CAO | 11 ± 5 vs. 39 ± 2 (−72%) | |||||
| Rabbit | 37 | Surface cooling (water blankets) | 30/3 | ∼37.0 | Before CAO→ 180 min CAR | 30 ± 5 vs. 59 ± 1 (−48%) |
| 0 min CAO → 180 min CAR | 33 ± 5 vs. 59 ± 1 (−43%) | |||||
| 15 min CAO→ 180 minCAR | 42 ± 1 vs. 59 ± 1 (−28%) | |||||
| Rabbit | 52 | Topical epicardial cooling | 30/3 | ∼35 | −20 min before CAO → 15 min CAR | 16 ± 3 vs. 46 ± 4 (−65%) |
| Rabbit | 44 | Surface cooling | 60/4 | ∼32 | 5 → 60 min CAO | 78 ± 10 vs. 82 ± 7 (NS) |
| Total liquid ventilation | 5 → 60 min CAO | 45 ± 18 vs. 82 ± 7 (−45%) | ||||
| 20 → 60 min CAO | 58 ± 5 vs. 82 ± 7 (−29%) | |||||
| Pig | 26 | Endovascular cooling | 60/3 | ∼34 | 20 min CAO → 15 min CAR | 9 ± 6 vs. 45 ± 8 (−80%) |
| Pig | 27 | Topical epicardial cooling | 40/3 | ∼29 | 0 → 40 min CAO | 25 ± 2 vs. 62 ± 5 (−60%) |
| Pig | 28 | Intracoronary cold saline infusion | 60/3 | ∼33 | 15 min CAO → 15 min CAR | 9 ± 2 vs. 36 ± 4 (−75%) |
| Pig | 35 | I.V. cold saline + endovascular cooling | 40/∼4.3 | ∼33 | 25 min CAO → 25 min CAR | 46 ± 8 vs. 75 ± 5 (−39%) |
| Sheep | 30 | Surface cooling (ice bags) | 60/3 | ∼38.5 | 0 min CA0 → 180 min CAR Control Group at 39.5°C | 63 ± 2 vs. 72 ± 3 (−12%) |
| ∼37.5 | 49 ± 1 vs. 72 ± 3 (−31%) | |||||
| ∼36.5 | 39 ± 1 vs. 72 ± 3 (−46%) | |||||
| ∼35.5 | 22 ± 2 vs. 72 ± 3 (−70%) | |||||
| Dog | 32 | Hypothermic retroperfusion of autologous blood | 210/3 | ∼28–30 | 30 → 210 min CAO | 6 ± 3 vs. 24 ± 7b (−75%) |
CAO, coronary artery occlusion; CAR, coronary artery reperfusion; IS, infarct size (expressed as % of area at risk); Ref, reference number.
aThe time of the application of the cooling strategy and not the actual time at which the target temperature was reached. In several studies, a delay was inevitable between the onset of the cooling protocol and the time of achievement of the target temperature (e.g. with low rate cooling strategy such as surface cooling).
bThe Control value corresponds to infarct sizes observed with normothermic retroperfusion.
Figure 1.
Infarct sizes (expressed as % of the area at risk) in different groups of anaesthetized rabbits submitted to 30 min of coronary artery occlusion and 3 h of reperfusion. In the different groups, rabbits were either subjected to a normothermic protocol (Control group) or to extracorporeal blood cooling to 35 or 32°C from the onset (0′) or from the 10th or the 20th minute of ischaemia (10′ and 20′ ischaemia). Open circles represent the individual infarct size of each animal and closed circles represent the mean ± SEM of each group. Data adapted from Miki et al.12 *P < 0.05 vs. Control.
3.3. What is the window of protection?
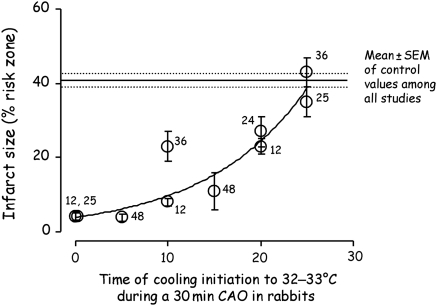
Cooling the heart during the ischaemic period reduces infarct size. Very early studies from the Corday Laboratory31,32 clearly showed that if the dog heart were retrogradely perfused with cooled arterial blood through the coronary sinus beginning 30 min after coronary occlusion and persisting for the remaining 2 1/2–3 h of the ischaemic period, infarct size was decreased by 65–90%. Table 1 reveals that the degree of protection afforded by cooling is not only determined by the depth of cooling but also by its duration during the ischaemic period. Miki et al.12 demonstrated, for example, that the magnitude of infarct size reduction decreased when the onset of hypothermia was delayed from the beginning to the 20th minute of a 30 min period of ischaemia in rabbits. As illustrated in Figure 1, cooling during just the last 10 min of ischaemia still afforded significant cardioprotection when the target temperature was 32°C but not when it was 35°C. Figure 2 merges the results of several studies investigating the effect of hypothermia to 32°C initiated at different times during a 30 min period of ischaemia in rabbits, again illustrating that cardioprotection is attenuated when the onset of cooling is delayed. This raises a major logistical problem in the clinical setting since these patients with acute myocardial infarction present with ischaemia already in progress. To significantly shorten the normothermic ischaemic time cooling would have to be accomplished very soon after arrival in the hospital and efforts to effect early cooling must not come at the expense of increasing the time to reperfusion. That schedule has been difficult to implement.
Figure 2.
Infarct sizes (expressed as % of the area at risk) obtained from several studies in anaesthetized rabbits subjected to 30 min of coronary artery occlusion and cooled to 32°C starting at different times after the initiation of ischaemia. Closed circles represent the mean ± SEM of the cooled groups for each study. Numbers next to the data points are reference citations.
Cooling the body at the time of reperfusion or beyond seems to be protective in the central nervous system.3,33 That has led investigators to ask whether mild hypothermia might be cardioprotective when instituted only at the onset of the reperfusion phase, i.e. a postconditioning manoeuvre. Cooling to 10°C at the onset of reoxygenation and glucose resupply of chick cardiomyocytes subjected to simulated ischaemia and reperfusion improved cell viability, and initiation of cooling towards the end of the simulated ischaemia further diminished cell necrosis.34 These observations strongly suggested that cooling during the peri-reperfusion period might be successful as well. Table 2 shows the results of several intact animal studies that have investigated this possibility.25,28,35–39 Unfortunately, all but one37 failed to see any effect on infarct size, and in the one positive study, cooling still included that last 5 min of a 30 min ischaemic period. Either the much lower temperature or the non-mammalian cell type was likely responsible for the protection in the chick cardiomyocyte study.34 Of course, cooling a patient to 10°C is not feasible because that would stop the heart. However, it was found that hypothermia during reperfusion prevents microvascular damage and thereby offers some protection against the no-reflow phenomenon.24,35 One might, therefore, speculate that hypothermia at reperfusion protects against vascular alteration and subsequent microvascular obstruction35 but cannot protect the cardiomyocytes. This could mean that one should maintain cooling during the first hours of reperfusion even if cooling had been instituted early in the ischaemic period. Surprisingly, the question of whether prolonging hypothermia after reperfusion measurably adds to the anti-infarct effect has yet to be addressed. It should be noted that most patients spend ∼90 min in hospital prior to revascularization because of delays associated with admitting, diagnosis, and preparation for intervention.40 Whole-body cooling during that period could produce significant reduction of the resulting infarct.
Table 2.
Summary of in vivo experimental studies investigating the infarct-limiting effect of myocardial cooling during the reperfusion phase, i.e. with cooling started only 5 min before reperfusion or later yielding normothermic ischaemia and hypothermic reperfusion
| Species | Ref. | Cooling procedure | Duration of CAO (min)/CAR (h) | Target heart temperature (°C) | Time of coolinga | IS with Cooling vs. Control groups (% decrease) |
|---|---|---|---|---|---|---|
| Rabbit | 36 | Topical epicardial cooling | 30/3 | ∼33 | 25 min CAO →15 min CAR | 43 ± 4 vs. 44 ± 4 (NS) |
| Rabbit | 25 | Total liquid ventilation | 30/3 | ∼32 | 25 min CAO →30 min CAR | 35 ± 4 vs. 38 ± 1 (NS) |
| Rabbit | 37 | Surface cooling (water blankets) | 30/3 | ∼37.0 | 25 min CAO → 180 min CAR | 44 ± 2 vs. 59 ± 1 (−25%) |
| 30 min CAO → 180 min CAR | 51 ± 2 vs. 59 ± 1 (NS) | |||||
| Pig | 38 | Regional blood cooling through heat exchanger | 45/3 | ∼33 | 43 min CAO → 120 min CAR | 71 ± 8 vs. 68 ± 1 (NS) |
| Pig | 28 | Intracoronary cold saline infusion | 60/3 | ∼33 | 0 min CAR → 30 min CAR | 33 ± 2 vs. 45 ± 5 (NS) |
| Pig | 35 | Intravenous infusion of cold saline + endovascular cooling | 40/∼4.3 | ∼33 | 0 min CAR →30 min CAR | 80 ± 6 vs. 75 ± 5 (NS) |
CAO, coronary artery occlusion; CAR, coronary artery reperfusion; IS, infarct size (expressed as % of area at risk); Ref, reference number.
aThe time of the application of the cooling strategy and not the actual time at which the target temperature was reached. In several studies, a delay was inevitable between the onset of the cooling protocol and the time of achievement of the target temperature (e.g. with low rate cooling strategy such as surface cooling).
3.4. How can myocardium be cooled?
As emphasized earlier, the sooner hypothermia is achieved following the onset of ischaemia, the more cardioprotective it would be. Experimentally, some studies have been performed using topical epicardial cooling to quickly lower cardiac temperature, but this method would be difficult to implement in patients. In patients with acute myocardial infarction, the critical parameters determining benefit would be the time when cooling could be started and also the rate at which cooling could be achieved. As explained earlier, the benefit would be expected to be small or absent if the target temperature is only reached far into the reperfusion phase. Obviously, the least invasive strategy for implementing therapeutic hypothermia in patients is external cooling. This can be done using ice packs or with specific medical devices designed to promote heat exchange by a more efficient contact between the skin and the cooling medium.2,41,42 Unfortunately, the cooling rate afforded by these strategies is rather low, averaging only 1–2°C/h in humans.43 That is because the cutaneous microcirculation tends to constrict when cold in an attempt to thermally insulate the body from the environment. Small laboratory animals cool faster with skin cooling than humans since their ratio of body mass to surface area is much smaller than in man. The time required to cool a rabbit weighing 2.0–3.0 kg to 32°C is still ∼45 min, and this does not significantly limit infarct size after 60 min of ischaemia.44 Although it is unlikely that patients with acute myocardial infarction would experience any anti-infarct benefit from conventional external cooling, this strategy is reported to be beneficial following cardiac arrest when even delayed hypothermia improves overall survival and neurological recovery after resuscitation.2,41,42
Other strategies have been proposed to induce therapeutic hypothermia. Some examples include endovascular cooling with intravenous thermodes, infusion of cold intravenous fluids, gastric lavage with cold fluid through a nasogastric catheter, and even urinary bladder lavage.42 Most of those techniques have been investigated in the clinics for their neuroprotective abilities.42 The one that has been investigated for a cardioprotective therapy is endovascular cooling.26,45,46 In human-sized pigs, this strategy afforded promising results when cooling was initiated early during ischaemia.26,45 A clinical trial in patients with ST-segment elevation myocardial infarction (STEMI) clearly demonstrated the feasibility of that strategy,46 but disappointingly did not show a significant cardioprotective benefit,8 probably because of an insufficient cooling rate that resulted in normothermia during most of the ischaemic period.47
3.5. Ultra-fast cooling
Accordingly, other strategies have been proposed that elicit a much faster rate of cooling which should increase the degree of cardioprotection in the clinical setting. Examples include extracorporeal blood cooling12 or total liquid ventilation with temperature-controlled perfluorocarbons.25,48 These techniques can decrease cardiac temperature to 32°C within 3–5 min in rabbits. A pericardial perfusion circuit has also been proposed.49 Unfortunately, all these strategies would be challenging to implement in patients presenting with myocardial infarction since they are very invasive and would significantly delay reperfusion by angioplasty or thrombus extraction. A promising technique could be peritoneal lavage, which, although still invasive, should be easier to institute than any of the above-mentioned methods. As seen in patients with malignant hyperthermia, it can cool a patient very quickly.50 Whether any of these ultra-fast cooling strategies would be beneficial in humans with STEMI remains to be investigated.
4. Mechanism of cooling-induced cardioprotection
The mechanism of hypothermia-induced cardioprotection has mostly been investigated in experimental models receiving cold cardioplegia. The temperature of the heart is reduced to very low levels, which, among other things, arrests it.13,51 However, mechanisms are likely to be quite different for mild hypothermia of an in vivo beating heart and an arrested heart exposed to cold cardioplegia. In beating hearts, one could, for example, argue that the cardioprotection might be related to the bradycardia elicited during ischaemia. However, this is unlikely because the relationship between infarct size and temperature was unchanged when normothermic heart rate was restored by pacing in hypothermic rabbits.21,52 It is therefore commonly assumed that hypothermia protects the heart, at least in part, through reduced cardiac metabolism. This assumption has been amply supported by studies performed during cold cardioplegia (<20°C).51,53–60 Most enzymes have a Q10 of ∼2, which means the reaction rate doubles for every 10°C increase in temperature. Reducing the cardiac temperature by 20°C should therefore decrease metabolism by a factor of 4. Mild hypothermia (>30°C) also decreases the rate of high energy phosphate11,61,62 and glucose63 utilization as well as lactate accumulation63 but to a lesser extent than deep hypothermia.
Deep hypothermia may also alter ion exchange because it inhibits Na+/Ca2+ and Na+/K+ sarcoplasmic exchangers, although it paradoxically activates the Na+/H+ exchanger.64 Interestingly, hibernating hypothermic frogs increase their resistance to hypoxia through a decreased demand for ATP by reduced Na+/K+-ATPase activity.65 An NMR study in isolated newborn rabbit hearts further confirmed that deep cooling (12°C) with cold crystalloid cardioplegia limited acidosis and calcium and sodium cellular overload during ischaemia/reperfusion.13 Deep hypothermia (17°C) also limited mitochondrial calcium overload in Langendorff guinea pig hearts undergoing ischaemia.66 Although deep hypothermia tends to increase baseline reactive oxygen species formation during normoxia, it limits the burst following ischaemia–reperfusion.66 Using electron spin resonance spectroscopy in isolated reperfused rat heart, investigators have observed reduced free-radical generation at reperfusion following ischaemia at 4°C.67
Deep hypothermia reduces several modulators of the mitochondrial permeability transition pore (mPTP), i.e. ATP depletion, calcium overload, and generation of reactive oxygen species. Mild hypothermia (32°C) inhibited calcium-induced mPTP opening in ventricular samples from rabbit hearts subjected to ischaemia alone or to ischaemia followed by 10 min of reperfusion.48 Suppression of mPTP formation at reperfusion is thought to be the mode of action of ischaemic preconditioning68 and ischaemic postconditioning.69 Thus it is reasonable to speculate that hypothermia acts, at least in part, through the inhibition of MPTP formation. However, the manner of modulation of mPTP opening is probably different from that afforded by pre- and postconditioning since the latter strategies are believed to trigger signal transduction pathways that determine the fate of the previously ischaemic myocardium during the first minutes of reperfusion,69–73 whereas hypothermia seems to exert its protection during ischaemia.48 Opening of mPTP at reperfusion only occurs if the heart has been injured by a period of prolonged ischaemia. The nature of that injury is poorly understood, but Honda et al.74 referred to it as ‘priming’. Mild hypothermia may act to lessen that ischaemic injury.
The protection from mild hypothermia is proportional to the decrease in temperature which argues against any off/on type of mechanism. A direct effect of mild hypothermia on enzyme kinetics seems unlikely to be the protective mechanism since most enzymes are not so temperature-dependent. Hearts function quite well over the entire range of mild hypothermia (32–38°C). An intriguing possibility is that mild hypothermia might somehow activate cardioprotective signal transduction pathways. Ning et al.75 demonstrated that cold cardioplegia (30°C) preserves mitochondrial protein gene expression during hypoxia, including genes coding for HSP70, ANT1, and β-F1-ATPase.76 They observed that neither ATP levels nor anaerobic metabolism is linked to mRNA expression of these latter proteins.77 Ning et al.77 also showed that moderate hypothermia (30°C) promotes expression of proteins involved in cell survival, although it inhibits induction of p53 protein. It is, therefore, reasonable to hypothesize that mild hypothermia triggers its protection through thermal sensors. This hypothesis is supported by studies from Halestrap's group, which reported that a short period of perfusion at 26°C in isolated rat hearts induced a preconditioning-like protection which increased protein kinase C-ε translocation to the particulate fraction (an index of its activation) and phosphorylated AMP-activated protein kinase.78 In one recent study in chick cardiomyocytes undergoing simulated ischaemia, cooling to 25°C just before reoxygenation protected the cells.34 More importantly that protection could be blocked by either a PKC or a nitric oxide synthase (NOS) blocker indicating signal transduction. Protection in the latter study, however, seemed to mimic that of postconditioning rather than that of mild hypothermia since the critical time for cooling was during reoxygenation rather than simulated ischaemia, and PKC and NOS are well-known components of the signal transduction pathways of pre- and postconditioning. Also cooling was much more severe. All chemical reactions are at some point temperature-dependent, but the temperature coefficients (Q10) for the various mammalian enzymes vary widely. The possibility that one particular enzyme might be very sensitive to temperature and could serve as a sensor to implement the cardioprotective effect of mild hypothermia through cell signalling is an attractive hypothesis, but, of course, that enzyme could be very difficult to find.
Hypothermia seems to protect against injury during ischaemia, whereas ischaemic preconditioning protects against injury during reperfusion. Because of the different mechanisms, it is possible to add the two together to produce additive protection.12 It is currently possible to pharmacologically postcondition a heart79,80 and such an intervention is thought to protect by a mechanism identical to that of preconditioning. Thus, it should be possible to combine mild hypothermia during ischaemia with a postconditioning agent at reperfusion. The primary impediment to using mild hypothermia clinically has been the logistics of implementing it quickly after the onset of ischaemia. However, if signalling pathways turn out to be responsible for the protection of mild hypothermia, then pharmacological activation of those pathways would likely be simpler to implement and such an agent could possibly even be given by EMS personnel.
5. Cooling and cardioprotection: STEMI clinical data
Soon after the turn of the twenty-first century, the promising experimental results regarding the cardioprotective effect of cooling inspired several clinical trials testing the feasibility of mild hypothermia and safety in STEMI patients.46,81,82 As shown in Table 3, two large-scale clinical trials (COOL-MI8 and ICE-IT10) were conducted using endovascular cooling. Both studies assessed infarct size using single-photon emission computed tomography (SPECT). The results of these studies have not yet been published in peer-reviewed journals, but they have been summarized in several reviews.41,83,84
Table 3.
Infarct size assessed by single-photon emission computed tomography in patients presenting with STEMI and treated by revascularization alone or in conjunction with endovascular cooling in the COOL-MI8 and the ICE-IT10 studies
| Infarct size (% left ventricle) |
P-value | ||
|---|---|---|---|
| Cooled group | Normothermic group | ||
| COOL-MI (n = 392 patients) | |||
| Overall population | 13.8 | 14.1 | 0.86 |
| Subgroup with anterior STEMI cooled to <35°C at the time of revascularization | 9.3 | 18.2 | 0.05 |
| ICE-IT (n = 228 patients) | |||
| Overall population | 10.2 | 13.2 | 0.14 |
| Subgroup with anterior STEMI cooled to <35°C at the time of revascularization | 12.9 | 22.7 | 0.09 |
In the COOL-MI study (COOLing as an adjunctive therapy to percutaneous intervention in patients with acute Myocardial Infarction), 392 STEMI patients were enrolled within 6 h following the onset of symptoms.8 Patients were assigned to either treatment with a percutaneous coronary intervention alone or to cooling with an endovascular cooling device (Reprieve Temperature Therapy System, Radiant Medical, Redwood City, CA, USA) prior to revascularization. The percutaneous coronary intervention was accomplished a mean of 18 min after cooling was instituted, with a mean temperature of 35.0°C at the moment of reperfusion. As shown in Table 3, cooling did not provide an overall significant reduction in infarct size, except in a subgroup of patients with anterior myocardial infarction who were cooled to <35°C at the time of revascularization.8 The overall negative result of that study might have been biased since the average door-to-balloon time in cooled patients was 18 min longer than in control patients (110 vs. 92 min).
In the ICE-IT study (Intravascular Cooling Adjunctive to Primary Coronary Intervention), the design was similar to COOL-MI with the inclusion of 228 patients randomized to either normothermic revascularization or to revascularization with mild hypothermia using another endovascular device (Innercool by Celsius Control System, San Diego, CA, USA).10 Hypothermia again did not provide a significant benefit regarding infarct size in the whole population (Table 3). A trend for a benefit was observed in the subgroup with anterior infarction with a body temperature of <35°C at the time of reperfusion. Interestingly, a subanalysis of the six sites with the best protocol compliance out of the 22 participating sites also demonstrated a significant reduction in infarct size with cooling compared with conventional revascularization (−44%). These data suggest, as emphasized above, that the overall negative result of the COOL-MI and ICE-IT trials was related to a delay in the institution of hypothermia and/or to an insufficient cooling rate which resulted in little shortening of the normothermic ischaemic period. A further study (COOL-MI II) was accordingly recently conducted with an earlier, deeper, and faster cooling protocol, with cooling started in the emergency room rather than in the catheterization laboratory. The results of this last trial are not yet available.
It has conversely been suggested that hyperthermia may worsen the clinical outcome in patients with myocardial infarction.85 In experimental conditions, Chien et al.21 found not only that hypothermia could diminish infarct size but also that hyperthermia could increase it. One would, therefore, speculate that therapeutic hypothermia would also be indicated to reverse the adverse effects of hyperthermia. A general relationship between clinical outcome and body temperature may well exist.
5.1. Cardiac resuscitation
Another setting in which the protective effect of cooling would be relevant is cardiopulmonary resuscitation. Cooling after the heart is restarted can indeed protect the central nervous system, as has been previously shown,5 but it may also protect the heart since myocardial ischaemia is a common cause of cardiac arrest.41,84 Theoretically, the cardiac benefit would even be greater for those patients than for ‘typical’ STEMI as the time before revascularization is prolonged by the resuscitation time. There is controversy as to whether resuscitated patients should be immediately submitted to a coronary intervention41,84 since the use of hypothermia is basically recommended for comatose survivors after out-of-hospital cardiac arrest with ventricular fibrillation.6,7 Importantly, the combination of cooling and percutaneous intervention is at least feasible and should be safe in those patients.41,84
6. Conclusion
In conclusion, cooling the myocardium with whole-body mild hypothermia is a very potent cardioprotective manoeuvre, at least in the experimental setting. The benefit depends upon the rapidity with which cooling is instituted and by how much it shortens the normothermic ischaemic time. To afford a clinical benefit, a cooling strategy should accordingly be designed to achieve the target temperature well before the time of revascularization. The depth of cooling is also important. Temperatures in the range of 32–35°C are considered safe. Yet these temperatures exert a powerful anti-infarct effect in animal studies. Finally, more studies of mild hypothermia are needed to determine its actual mechanism, compared with deep hypothermia that acts through energy preservation.
Funding
This study was supported by grants HL-20648 and HL-50688 from the Heart, Lung and Blood Institute of the National Institutes of Health, grant TLVenCool (06-JCJC-0078) from the French ‘Agence Nationale pour la Recherche’, grant TLV-CARDAREST (R10028JS) from the INSERM-transfert and the ITMO ‘Technologies pour la Santé’, and grant ET7-460 from the ‘Fondation de l'Avenir’. M.C. was supported by a grant from the ‘Groupe de Reflexion sur la Recherche Cardiovasculaire’ and by a ‘Poste d'accueil INSERM’.
Acknowledgements
We are greatly indebted to Fanny Lidouren and Alain Bizé for their excellent support.
Conflict of interest: none declared.
References
- 1.Liss HP. A history of resuscitation. Ann Emerg Med. 1986;15:65–72. doi: 10.1016/s0196-0644(86)80490-5. doi:10.1016/S0196-0644(86)80490-5. [DOI] [PubMed] [Google Scholar]
- 2.Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006;70:369–380. doi: 10.1016/j.resuscitation.2006.01.017. doi:10.1016/j.resuscitation.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Varon J, Acosta P. Therapeutic hypothermia: past, present, and future. Chest. 2008;133:1267–1274. doi: 10.1378/chest.07-2190. doi:10.1378/chest.07-2190. [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. doi:10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. doi:10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WGJ, Billi J, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. doi:10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 7.Nolan JP, Deakin CD, Soar J, Böttiger BW, Smith G. European Resuscitation Council Guidelines for Resuscitation 2005. Section 4. Adult advanced life support. Resuscitation. 2005;67(Suppl. 1):S39–S86. doi: 10.1016/j.resuscitation.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill WW on behalf of the COOL-MI Investigators. A prospective randomized trial of mild systemic hypothermia during PCI treatment of ST elevation myocardial infarction. Presented at the 15th Annual Transcatheter Cardiovascular Therapeutics; September 2003; Washington, DC. O'Neill WW, Dixon SR. The year in interventional cardiology. J Am Coll Cardiol 2004;43:875–890. [Google Scholar]
- 9.Stone GW, Dixon SR, Grines CL, Cox DA, Webb JG, Brodie BR, et al. Predictors of infarct size after primary coronary angioplasty in acute myocardial infarction from pooled analysis from four contemporary trials. Am J Cardiol. 2007;100:1370–1375. doi: 10.1016/j.amjcard.2007.06.027. doi:10.1016/j.amjcard.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Grines CL on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at the 16th Annual Transcatheter Cardiovascular Therapeutics; September 2004; Washington, DC. O'Neill WW, Dixon SR, Grines CL: The year in interventional cardiology. J Am Coll Cardiol 2005;45:1117–1134. [DOI] [PubMed] [Google Scholar]
- 11.Jones RN, Reimer KA, Hill ML, Jennings RB. Effect of hypothermia on changes in high-energy phosphate production and utilization in total ischemia. J Mol Cell Cardiol. 1982;14(Suppl. 3):123–130. doi: 10.1016/0022-2828(82)90140-7. [DOI] [PubMed] [Google Scholar]
- 12.Miki T, Liu GS, Cohen MV, Downey JM. Mild hypothermia reduces infarct size in the beating rabbit heart: a practical intervention for acute myocardial infarction? Basic Res Cardiol. 1998;93:372–383. doi: 10.1007/s003950050105. doi:10.1007/s003950050105. [DOI] [PubMed] [Google Scholar]
- 13.Anderson SE, Liu H, Beyschau A, Cala PM. Effects of cold cardioplegia on pH, Na, and Ca in newborn rabbit hearts. Am J Physiol. 2006;290:H1090–H1097. doi: 10.1152/ajpheart.00776.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bernard SA, Jones BMC, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med. 1997;30:146–153. doi: 10.1016/s0196-0644(97)70133-1. doi:10.1016/S0196-0644(97)70133-1. [DOI] [PubMed] [Google Scholar]
- 15.Zeiner A, Sunder-Plassmann G, Sterz F, Holzer M, Losert H, Laggner AN, et al. The effect of mild therapeutic hypothermia on renal function after cardiopulmonary resuscitation in men. Resuscitation. 2004;60:253–261. doi: 10.1016/j.resuscitation.2003.11.006. doi:10.1016/j.resuscitation.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;179:85–88. doi: 10.1152/ajplegacy.1954.179.1.85. [DOI] [PubMed] [Google Scholar]
- 17.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(suppl.):S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 18.Lehot JJ, Piriz H, Villard J, Cohen R, Guidollet J. Glucose homeostasis. Comparison between hypothermic and normothermic cardiopulmonary bypass. Chest. 1992;102:106–111. doi: 10.1378/chest.102.1.106. doi:10.1378/chest.102.1.106. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz LM, Verbinski SG, Vander Heide RS, Reimer KA. Epicardial temperature is a major predictor of myocardial infarct size in dogs. J Mol Cell Cardiol. 1997;29:1577–1583. doi: 10.1006/jmcc.1997.0391. doi:10.1006/jmcc.1997.0391. [DOI] [PubMed] [Google Scholar]
- 20.Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol. 1996;270:H1189–H1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- 21.Chien GL, Wolff RA, Davis RF, van Winkle DM. ‘Normothermic range’ temperature affects myocardial infarct size. Cardiovasc Res. 1994;28:1014–1017. doi: 10.1093/cvr/28.7.1014. doi:10.1093/cvr/28.7.1014. [DOI] [PubMed] [Google Scholar]
- 22.Hale SL, Kloner RA. Myocardial temperature reduction attenuates necrosis after prolonged ischemia in rabbits. Cardiovasc Res. 1998;40:502–507. doi: 10.1016/s0008-6363(98)00191-6. doi:10.1016/S0008-6363(98)00191-6. [DOI] [PubMed] [Google Scholar]
- 23.Hale SL, Kloner RA. Myocardial hypothermia: a potential therapeutic technique for acute regional myocardial ischemia. J Cardiovasc Electrophysiol. 1999;10:405–413. doi: 10.1111/j.1540-8167.1999.tb00689.x. doi:10.1111/j.1540-8167.1999.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 24.Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res. 2003;59:715–722. doi: 10.1016/s0008-6363(03)00456-5. doi:10.1016/S0008-6363(03)00456-5. [DOI] [PubMed] [Google Scholar]
- 25.Tissier R, Hamanaka K, Kuno A, Parker JC, Cohen MV, Downey JM. Total liquid ventilation provides ultra-fast cardioprotective cooling. J Am Coll Cardiol. 2007;49:601–605. doi: 10.1016/j.jacc.2006.09.041. doi:10.1016/j.jacc.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Dae MW, Gao DW, Sessler DI, Chair K, Stillson CA. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am J Physiol. 2002;282:H1584–H1591. doi: 10.1152/ajpheart.00980.2001. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz DS, Bremner RM, Baker CJ, Uppal KM, Barr ML, Cohen RG, et al. Regional topical hypothermia of the beating heart: preservation of function and tissue. Ann Thorac Surg. 2001;72:804–809. doi: 10.1016/s0003-4975(01)02822-3. doi:10.1016/S0003-4975(01)02822-3. [DOI] [PubMed] [Google Scholar]
- 28.Otake H, Shite J, Paredes OL, Shinke T, Yoshikawa R, Tanino Y, et al. Catheter-based transcoronary myocardial hypothermia attenuates arrhythmia and myocardial necrosis in pigs with acute myocardial infarction. J Am Coll Cardiol. 2007;49:250–260. doi: 10.1016/j.jacc.2006.06.080. doi:10.1016/j.jacc.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 29.van den Doel MA, Gho BCG, Duval SY, Schoemaker RG, Duncker DJ, Verdouw PD. Hypothermia extends the cardioprotection by ischaemic preconditioning to coronary artery occlusions of longer duration. Cardiovasc Res. 1998;37:76–81. doi: 10.1016/s0008-6363(97)00222-8. doi:10.1016/S0008-6363(97)00222-8. [DOI] [PubMed] [Google Scholar]
- 30.Hamamoto H, Sakamoto H, Leshnower BG, Parish LM, Kanemoto S, Hinmon R, et al. Very mild hypothermia during ischemia and reperfusion improves postinfarction ventricular remodeling. Ann Thorac Surg. 2009;87:172–177. doi: 10.1016/j.athoracsur.2008.08.015. doi:10.1016/j.athoracsur.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haendchen RV, Corday E, Meerbaum S, Povzhitkov M, Rit J, Fishbein MC. Prevention of ischemic injury and early reperfusion derangements by hypothermic retroperfusion. J Am Coll Cardiol. 1983;1:1067–1080. doi: 10.1016/s0735-1097(83)80109-0. doi:10.1016/S0735-1097(83)80109-0. [DOI] [PubMed] [Google Scholar]
- 32.Wakida Y, Haendchen RV, Kobayashi S, Nordlander R, Corday E. Percutaneous cooling of ischemic myocardium by hypothermic retroperfusion of autologous arterial blood: effects on regional myocardial temperature distribution and infarct size. J Am Coll Cardiol. 1991;18:293–300. doi: 10.1016/s0735-1097(10)80251-7. doi:10.1016/S0735-1097(10)80251-7. [DOI] [PubMed] [Google Scholar]
- 33.Kollmar R, Schäbitz WR, Heiland S, Georgiadis D, Schellinger PD, Bardutzky J, et al. Neuroprotective effect of delayed moderate hypothermia after focal cerebral ischemia: an MRI study. Stroke. 2002;33:1899–1904. doi: 10.1161/01.str.0000019603.29818.9c. doi:10.1161/01.STR.0000019603.29818.9C. [DOI] [PubMed] [Google Scholar]
- 34.Shao Z-H, Chang W-T, Chan KC, Wojcik KR, Hsu C-W, Li C-Q, et al. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol. 2007;292:H1995–H2003. doi: 10.1152/ajpheart.01312.2005. [DOI] [PubMed] [Google Scholar]
- 35.Götberg M, Olivecrona GK, Engblom H, Ugander M, van der Pals J, Heiberg E, et al. Rapid short-duration hypothermia with cold saline and endovascular cooling before reperfusion reduces microvascular obstruction and myocardial infarct size. BMC Cardiovasc Disord. 2008;8:7. doi: 10.1186/1471-2261-8-7. doi:10.1186/1471-2261-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hale SL, Dave RH, Kloner RA. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res Cardiol. 1997;92:351–357. doi: 10.1007/BF00788947. [DOI] [PubMed] [Google Scholar]
- 37.Kanemoto S, Matsubara M, Noma M, Leshnower BG, Parish LM, Jackson BM, et al. Mild hypothermia to limit myocardial ischemia-reperfusion injury: importance of timing. Ann Thorac Surg. 2009;87:157–163. doi: 10.1016/j.athoracsur.2008.08.012. doi:10.1016/j.athoracsur.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeng M, Mortensen UM, Kristensen J, Kristiansen SB, Andersen HR. Hypothermia during reperfusion does not reduce myocardial infarct size in pigs. Basic Res Cardiol. 2006;101:61–68. doi: 10.1007/s00395-005-0550-7. doi:10.1007/s00395-005-0550-7. [DOI] [PubMed] [Google Scholar]
- 39.Schwiebert C, Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, et al. Postconditioning by xenon and hypothermia in the rat heart in vivo. Eur J Anaesthesiol. 2010;27:000–000. doi: 10.1097/EJA.0b013e328335fc4c. [DOI] [PubMed] [Google Scholar]
- 40.Magid DJ, Wang Y, Herrin J, McNamara RL, Bradley EH, Curtis JP, et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute ST-segment elevation myocardial infarction. JAMA. 2005;294:803–812. doi: 10.1001/jama.294.7.803. doi:10.1001/jama.294.7.803. [DOI] [PubMed] [Google Scholar]
- 41.Holzer M, Behringer W. Therapeutic hypothermia after cardiac arrest and myocardial infarction. Best Pract Res Clin Anaesthesiol. 2008;22:711–728. doi: 10.1016/j.bpa.2008.02.001. doi:10.1016/j.bpa.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Kimberger O, Kurz A. Thermoregulatory management for mild therapeutic hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22:729–744. doi: 10.1016/j.bpa.2007.11.002. doi:10.1016/j.bpa.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care. 2007;11:R91. doi: 10.1186/cc6104. doi:10.1186/cc6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chenoune M, Lidouren F, Ghaleh B, Couvreur N, Dubois-Rande J-L, Berdeaux A, et al. Rapid cooling of the heart with total liquid ventilation prevents transmural myocardial infarction following prolonged ischemia in rabbits. Resuscitation. 2010;81:359–362. doi: 10.1016/j.resuscitation.2009.12.005. doi:10.1016/j.resuscitation.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Dae MW, Gao DW, Ursell PC, Stillson CA, Sessler DI. Safety and efficacy of endovascular cooling and rewarming for induction and reversal of hypothermia in human-sized pigs. Stroke. 2003;34:734–738. doi: 10.1161/01.STR.0000057461.56040.FE. doi:10.1161/01.STR.0000057461.56040.FE. [DOI] [PubMed] [Google Scholar]
- 46.Dixon SR, Whitbourn RJ, Dae MW, Grube E, Sherman W, Schaer GL, et al. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40:1928–1934. doi: 10.1016/s0735-1097(02)02567-6. doi:10.1016/S0735-1097(02)02567-6. [DOI] [PubMed] [Google Scholar]
- 47.Wolfrum S, Radke PW, Schunkert H, Kurowski V. The authors reply to Ramaraj R: mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction. Crit Care Med. 2008;36:3280–3281. doi: 10.1097/CCM.0b013e31818f28d0. doi:10.1097/CCM.0b013e31818f2fd4. [DOI] [PubMed] [Google Scholar]
- 48.Tissier R, Couvreur N, Ghaleh B, Bruneval P, Lidouren F, Morin D, et al. Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left ventricular dysfunction. Cardiovasc Res. 2009;83:345–353. doi: 10.1093/cvr/cvp046. doi:10.1093/cvr/cvp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dave RH, Hale SL, Kloner RA. Hypothermic, closed circuit pericardioperfusion: a potential cardioprotective technique in acute regional ischemia. J Am Coll Cardiol. 1998;31:1667–1671. doi: 10.1016/s0735-1097(98)00129-6. doi:10.1016/S0735-1097(98)00129-6. [DOI] [PubMed] [Google Scholar]
- 50.Gjessing J, Barsa J, Tomlin PJ. A possible means of rapid cooling in the emergency treatment of malignant hyperpyrexia. Br J Anaesth. 1976;48:469–473. doi: 10.1093/bja/48.5.469. doi:10.1093/bja/48.5.469. [DOI] [PubMed] [Google Scholar]
- 51.Stowe DF, Varadarajan SG, An J, Smart SC. Reduced cytosolic Ca2+ loading and improved cardiac function after cardioplegic cold storage of guinea pig isolated hearts. Circulation. 2000;102:1172–1177. doi: 10.1161/01.cir.102.10.1172. [DOI] [PubMed] [Google Scholar]
- 52.Hale SL, Kloner RA. Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am J Physiol Heart Circ Physiol. 1997;273:H220–H227. doi: 10.1152/ajpheart.1997.273.1.H220. [DOI] [PubMed] [Google Scholar]
- 53.van der Vusse GJ, van der Veen FH, Flameng W, Coumans W, Borgers M, Willems G, et al. A biochemical and ultrastructural study on myocardial changes during aorto-coronary bypass surgery: St. Thomas Hospital cardioplegia versus intermittent aortic cross-clamping at 34 and 25 degrees C. Eur Surg Res. 1986;18:1–11. doi: 10.1159/000128499. doi:10.1159/000128499. [DOI] [PubMed] [Google Scholar]
- 54.Smolenski RT, Lachno DR, Yacoub MH. Adenine nucleotide catabolism in human myocardium during heart and heart-lung transplantation. Eur J Cardiothorac Surg. 1992;6:25–30. doi: 10.1016/1010-7940(92)90094-e. doi:10.1016/1010-7940(92)90094-E. [DOI] [PubMed] [Google Scholar]
- 55.Sukehiro S, Dyszkiewics W, Minten J, Wynants J, Van Belle H, Flameng W. Catabolism of high energy phosphates during long-term cold storage of donor hearts: effects of extra- and intracellular fluid-type cardioplegic solutions and calcium channel blockers. J Heart Lung Transplant. 1991;10:387–393. [PubMed] [Google Scholar]
- 56.Bical O, Gerhardt M-F, Paumier D, Gaillard D, Comas J, Landais P, et al. Comparison of different types of cardioplegia and reperfusion on myocardial metabolism and free radical activity. Circulation. 1991;84(Suppl. III):III-375–III-379. [PubMed] [Google Scholar]
- 57.Möllhoff T, Sukehiro S, Hendrickx M, Van Belle H, Flameng W. Effects of hypothermic ischemia on purine catabolism in canine, primate, and human myocardium. Thorac Cardiovasc Surg. 1991;39:187–192. doi: 10.1055/s-2007-1022706. doi:10.1055/s-2007-1022706. [DOI] [PubMed] [Google Scholar]
- 58.Shirakura R, Matsuda H, Nakano S, Nakata S, Kaneko M, Takami H, et al. Myocardial energy metabolism in asphyxiated canine hearts preserved for 24 h. Transplantation. 1992;53:1215–1218. doi: 10.1097/00007890-199206000-00009. doi:10.1097/00007890-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Chong YS, Cottier DS, Gavin JB. Myocardial protection during prolonged ischaemic cardiac arrest: experimental evaluation of three crystalloid cardioplegic solutions. J Cardiovasc Surg. 1994;35:35–44. [PubMed] [Google Scholar]
- 60.Minten J, Flameng W, Dyszkiewicz W. Optimal storage temperature and benefit of hypothermic cardioplegic arrest for long-term preservation of donor hearts: a study in the dog. Transpl Int. 1988;1:19–25. doi: 10.1007/BF00337844. doi:10.1111/j.1432-2277.1988.tb01774.x. [DOI] [PubMed] [Google Scholar]
- 61.Simkhovich BZ, Hale SL, Kloner RA. Metabolic mechanism by which mild regional hypothermia preserves ischemic tissue. J Cardiovasc Pharmacol Ther. 2004;9:83–90. doi: 10.1177/107424840400900203. doi:10.1177/107424840400900203. [DOI] [PubMed] [Google Scholar]
- 62.Ning X-H, Xu C-S, Song YC, Xiao Y, Hu Y-J, Lupinetti FM, et al. Hypothermia preserves function and signaling for mitochondrial biogenesis during subsequent ischemia. Am J Physiol. 1998;274:H786–H793. doi: 10.1152/ajpheart.1998.274.3.H786. [DOI] [PubMed] [Google Scholar]
- 63.Ichihara K, Robishaw JD, Vary TC, Neely JR. Protection of ischemic myocardium from metabolic products. Acta Med Scand Suppl. 1981;651:13–18. doi: 10.1111/j.0954-6820.1981.tb03627.x. [DOI] [PubMed] [Google Scholar]
- 64.Martineau Knerr SM, Lieberman M. Ion transport during hypothermia in cultured heart cells: implications for protection of the immature myocardium. J Mol Cell Cardiol. 1993;25:277–288. doi: 10.1006/jmcc.1993.1034. doi:10.1006/jmcc.1993.1034. [DOI] [PubMed] [Google Scholar]
- 65.Boutilier RG. Mechanisms of cell survival in hypoxia and hypothermia. J Exp Biol. 2001;204:3171–3181. doi: 10.1242/jeb.204.18.3171. [DOI] [PubMed] [Google Scholar]
- 66.Riess ML, Camara AKS, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc Res. 2004;61:580–590. doi: 10.1016/j.cardiores.2003.09.016. doi:10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Gambert S, Bès-Houtmann S, Vandroux D, Tissier C, Vergely-Vandriesse C, Rochette L, et al. Deep hypothermia during ischemia improves functional recovery and reduces free-radical generation in isolated reperfused rat heart. J Heart Lung Transplant. 2004;23:487–491. doi: 10.1016/S1053-2498(03)00211-0. doi:10.1016/S1053-2498(03)00211-0. [DOI] [PubMed] [Google Scholar]
- 68.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. doi:10.1016/S0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 69.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. doi:10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 70.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KHH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. doi:10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gateau-Roesch O, Argaud L, Ovize M. Mitochondrial permeability transition pore and postconditioning. Cardiovasc Res. 2006;70:264–273. doi: 10.1016/j.cardiores.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 72.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. doi:10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 73.Cohen MV, Yang X-M, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. doi:10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 74.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann NY Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. doi:10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 75.Ning X-H, Chen S-H, Xu C-S, Hyyti OM, Qian K, Krueger JJ, et al. Hypothermia preserves myocardial function and mitochondrial protein gene expression during hypoxia. Am J Physiol. 2003;285:H212–H219. doi: 10.1152/ajpheart.01149.2002. [DOI] [PubMed] [Google Scholar]
- 76.Ning X-H, Xu C-S, Portman MA. Mitochondrial protein and HSP70 signaling after ischemia in hypothermic-adapted hearts augmented with glucose. Am J Physiol. 1999;277:R11–R17. doi: 10.1152/ajpregu.1999.277.1.R11. [DOI] [PubMed] [Google Scholar]
- 77.Ning X-H, Chi EY, Buroker NE, Chen S-H, Xu C-S, Tien Y-T, et al. Moderate hypothermia (30°C) maintains myocardial integrity and modifies response of cell survival proteins after reperfusion. Am J Physiol Heart Circ Physiol. 2007;293:H2119–H2128. doi: 10.1152/ajpheart.00123.2007. doi:10.1152/ajpheart.00123.2007. [DOI] [PubMed] [Google Scholar]
- 78.Khaliulin I, Clarke SJ, Lin H, Parker J, Suleiman M-S, Halestrap AP. Temperature preconditioning of isolated rat hearts—a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007;581:1147–1161. doi: 10.1113/jphysiol.2007.130369. doi:10.1113/jphysiol.2007.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez L, Thibault H, Gharib A, Dumont J-M, Vuagniaux G, Scalfaro P, et al. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- 80.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. doi:10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 81.Kandzari DE, Chu A, Brodie BR, Stuckey TA, Hermiller JB, Vetrovec GW, et al. Feasibility of endovascular cooling as an adjunct to primary percutaneous coronary intervention (results of the LOWTEMP pilot study) Am J Cardiol. 2004;93:636–639. doi: 10.1016/j.amjcard.2003.11.038. doi:10.1016/j.amjcard.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 82.Ly HQ, Denault A, Dupuis J, Vadeboncoeur A, Harel F, Arsenault A, et al. A pilot study: the Noninvasive Surface Cooling Thermoregulatory System for Mild Hypothermia Induction in Acute Myocardial Infarction (the NICAMI Study) Am Heart J. 2005;150:933.e9–933.e13. doi: 10.1016/j.ahj.2005.02.049. doi:10.1016/j.ahj.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 83.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. doi:10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 84.Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med. 2008;36:1780–1786. doi: 10.1097/CCM.0b013e31817437ca. doi:10.1097/CCM.0b013e31817437ca. [DOI] [PubMed] [Google Scholar]
- 85.Naito K, Anzai T, Yoshikawa T, Maekawa Y, Sugano Y, Kohno T, et al. Increased body temperature after reperfused acute myocardial infarction is associated with adverse left ventricular remodeling. J Card Fail. 2007;13:25–33. doi: 10.1016/j.cardfail.2006.09.006. doi:10.1016/j.cardfail.2006.09.006. [DOI] [PubMed] [Google Scholar]