Abstract
Aims
Natriuretic peptides (NPs) inhibit cardiomyocyte hypertrophy through a cyclic GMP (cGMP)-dependent process, although these effects are associated with substantial vasodilatation. In this study, we used CU-NP, a non-vasodilatating novel NP synthesized from the ring structure of human C-type NP (CNP) and both C- and N-termini of urodilatin, and investigated whether it can directly modulate cardiomyocyte hypertrophy.
Methods and results
Experiments were carried out in cultured neonatal rat ventricular myocytes exposed to phenylephrine, angiotensin II, or endothelin-1 in the absence or presence of CU-NP. CU-NP produced a concentration- and time-dependent increase in intracellular cGMP levels. The hypertrophic responses to all agonists were abrogated by 10 nM CU-NP. CU-NP treatment also prevented increased activity, gene and protein expression of sodium–hydrogen exchanger-1 (NHE-1) as well as elevations in intracellular Na+ concentrations caused by hypertrophic agents. In addition, these effects were associated with a more than two-fold increase in activity of the Ca2+-dependent protein phosphatase calcineurin that peaked 6 h after addition of hypertrophic stimuli. Early (1–3 h) calcineurin activation was unaffected by CU-NP, although activation at 6 and 24 h was prevented by CU-NP as was the resultant translocation of the transcriptional factor NFAT into nuclei.
Conclusion
Our study demonstrates a direct anti-hypertrophic effect of the chimeric peptide CU-NP via NHE-1 inhibition, thereby preventing calcineurin activation and NFAT nuclear import. Thus, CU-NP represents a novel fusion peptide of CNP and urodilatin that has the potential to be developed into a therapeutic agent to treat cardiac hypertrophy and heart failure.
Keywords: Natriuretic peptides, Sodium–hydrogen exchanger 1, Hypertrophy, Cardiomyocytes, Calcineurin
1. Introduction
Natriuretic peptides (NPs) are a group of structurally similar hormones that play a crucial role in cardiovascular homeostasis. Biological activity of naturally occurring NPs—atrial NP (ANP), B-type NP (BNP), and C-type NP (CNP)—is determined by a 17-amino acid ring structure formed by a disulfide bridge. ANP and BNP are of cardiac origin, whereas CNP is synthesized and released from endothelial cells. ANP and BNP activate guanylyl cyclase A (GC-A) and cause vasodilatation, natriuresis, growth suppression, and inhibition of the sympathetic nervous as well as the renin–angiotensin–aldosterone systems (RAAS).1,2 Another NP named urodilatin is a 32-amino acid peptide generated from alternative processing of ANP expressed in the kidney,3,4 which plays an important role in the regulation of sodium and water homeostasis in the kidney.5 The production and secretion of both ANP and BNP are increased under cardiac-loading conditions.1,2,4 In contrast to ANP and BNP, CNP activates GC-B and fails to exert natriuretic properties due to a lack of a C-terminus extension. Plasma CNP concentrations are not changed in patients with congestive heart failure.4,6
Although synthetic NPs such as carperitide and nesiritide are used to treat congestive heart failure, vasodilatation and reductions in renal perfusion pressures and the potential for reflex sympathetic responses limit their clinical effectiveness. Recently, an emerging innovative therapeutic strategy has evolved involving the engineering of proteins that fuse structural elements unique for one peptide with active structures from separate peptides to create chimeras, which possess attractive therapeutic properties.7 One of these peptides, CU-NP, consists of the ring structure and disulfide bond of CNP in combination with the N-terminus and the C-terminus of urodilatin.7,8 CU-NP activates both GC-B and GC-A.9,10 CU-NP exerts cyclic GMP (cGMP)-activating, diuretic, natriuretic, glomerular filtration rate-enhancing, RAAS-suppressing, and cardiac-unloading properties without lowering blood pressure,8 although whether it exerts direct anti-hypertrophic effects on cardiomyocytes has not been demonstrated previously. Accordingly, the present study was carried out to determine whether CU-NP exerts direct anti-hypertrophic effects in cultured cardiomyocytes and to assess the possible underlying mechanisms for such actions.
2. Methods
2.1. Neonatal cardiomyocyte culture
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and procedures followed are in accordance with the University of Western Ontario animal care guidelines, which conform to the guidelines of the Canadian Council on Animal Care (Ottawa, ON, Canada). Neonatal cardiomyocytes were prepared from the ventricles of 1- to 3-day-old Sprague–Dawley rats (Charles River Canada, Montreal, Quebec, Canada), as described previously.11
2.2. Experimental protocol
Cells were first serum-starved for 24 h and then were treated with either 10 µM phenylephrine, 100 nM angiotensin II, or 10 nM endothelin-1 (all from Sigma-Aldrich, Oakville, ON, Canada) as the hypertrophic stimuli for a further 24 h. To assess the effect of different interventions, the cells were treated with CU-NP (10 nM), ANP (100 nM), or CNP (100 nM) 30 min before agonist addition. For some experiments, the NP receptor blocker lysophosphatidic acid (LPA, 10 µM) was added 15 min prior to NP administration.
2.3. Measurement of cell surface area
Cell surface area was measured and analysed as described previously.12 Briefly, cardiomyocytes were plated at a density of 1 × 106 cells and cultured for 48 h in serum-containing media followed by 24 h serum starvation. Cell surface area was captured using a Leica inverted microscope equipped with a Polaroid digital camera at ×200 magnification and measured using SigmaStat Pro 5.0 software (Systat, Inc., Richmond, CA, USA).
2.4. Leucine incorporation
The rate of protein synthesis was examined by analysis of [3H]-leucine incorporation as described previously.13 Briefly, cardiomyocytes were plated at 1 × 106 cells per well in 24-well Primaria culture plates and cultured for 48 h in serum-containing media. After incubation in serum-free medium for 24 h, cardiomyocytes were treated with the indicated treatments in the presence of 1 µCi of [3H]-leucine for 24 h. After three washes with ice-cold PBS, proteins were precipitated with 5% trichloroacetic acid (TCA) for 30 min at 4°C. Following two washes with ice-cold 5% TCA, precipitates were then dissolved in 0.5 N NaOH. After neutralizing with 0.5 N HCl, the total radioactivity of incorporated [3H]-leucine into proteins was measured by liquid scintillation counter.
2.5. Western blotting
Western blots for phosphorylated forms of ERK-1/2, total and phosphorylated forms of p38 MAPK, actin (antibodies from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and sodium–hydrogen exchanger-1 (NHE-1; Chemicon, Temecula, CA, USA) were performed as described previously.12 Cell lysates were transferred to 1.5 mL Eppendorf tubes, homogenized, and centrifuged at 10 000 g for 5 min at 4°C. The supernatant was transferred to a fresh tube and the protein concentration determined by the Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). Thirty micrograms of protein were resolved through a 10% SDS–polyacrylamide gel and transferred to nitrocellulose membranes (Amersham Biosciences Inc., Piscataway, NJ, USA). The membranes were blocked in 5% milk for 1 h and incubated with primary antibody for 1 h followed by secondary antibody for 1 h and then detected by enhanced chemiluminescence reagent (Amersham Biosciences Inc.).
2.6. Calcineurin phosphatase activity assay
The Biomol Green calcineurin assay kit (Biomol, Plymouth Meeting, PA, USA) was used to determine the calcineurin phosphatase activity according to the manufacturer's instructions. Briefly, cardiomyocytes were washed with cold TBS, lysed with 70 µL of lysis buffer and 3 µg protein was used for the assay. Calcineurin phosphatase activity was measured spectrophotometrically by detecting free-phosphate released from the calcineurin-specific RII substrate peptide.
2.7. Radioimmunoassay
Myocytes were treated with increasing concentrations of CU-NP (0.1–100 nM) for 5 min to 24 h in cell culture medium containing 20 mM HEPES, 0.1% BSA, and 0.5 mM 3-isobutyl-1-methylzanthine. The reaction was stopped with ice-cold 70% (v/v) ethanol. The dishes were frozen at −80°C, thawed, and scraped. After centrifugation (3000 g, 5 min, 4°C), the supernatants were dried in a speed vacuum concentrator and then resuspended in sodium acetate buffer (50 mM, pH 6.0) and acetylated, and cGMP contents were quantified using a competitive RIA cGMP kit (Perkin-Elmer, Boston, MA, USA). Briefly, samples and standards were incubated with 100 µL anti-human cGMP polyclonal antibody and I125-antigen for 18 h. cGMP assay buffer was added to the samples which were then centrifuged for 20 min at 2500 g. The free fraction was aspirated off and the bound fraction was counted and concentrations were determined. Samples were corrected for dilution factors and protein concentration. There is no cross-reactivity with ANP, BNP, CNP, and endothelin and <0.001% cross-reactivity with cAMP, GMP, GDP, ATP, and GTP.11
2.8. RNA isolation, reverse transcription, and real-time PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. RNA (5 µg) was used to synthesize first strand of cDNA using SuperScript II RNase H-Reverse Transcriptase (Invitrogen) according to the manufacturer's protocol and used as a template in the following PCRs. The expression levels of ANP, α-skeletal actin, NHE-1, MCIP, and 18S rRNA genes were determined in 10 µL reaction volume using SYBR Green Jumpstart Taq ReadyMix DNA polymerase (Sigma-Aldrich), and fluorescence was measured and quantified using a DNA Engine Opticon 2 System (MJ Research, Waltham, MA, USA). PCR conditions and cell cycle number were optimized for each set of primers. Melting curve analysis showed a single PCR product for each gene amplification. PCR conditions to amplify all genes were 30 s at 94°C followed by annealing at 54°C for 20 s for 18S rRNA and 60°C for 25 s for all other genes, with a further elongation at 72°C for 30 s. 18S rRNA gene expression was used as a control.
2.9. Immunofluorescent cell staining
NFAT3 translocation was assessed as described previously.13 In brief, cardiomyocytes were grown on collagen-pre-coated glass cover slips (2 µL/mL) and maintained in serum-containing medium for 48 h followed by incubation in serum-free media for 24 h. Following treatments, cardiomyocytes were fixed for 10 min with freshly prepared 4% (w/v) paraformaldehyde in PBS (pH 7.2) or with cold acetone/methanol (20:80). Cells were then permeabilized with 0.2% (v/v) Triton X-100 in PBS for 15 min and washed twice with PBS. Non-specific-binding sites were blocked with blocking solution (1% BSA and 0.1% Triton X-100 in PBS) for 10 min and washed twice with PBS. The cardiomyocytes were then incubated with antibodies (1:100) for 1 h at room temperature or at +4°C overnight. Cardiomyocytes were then washed three times with PBS and incubated for 1 h at room temperature with Alexa Fluor 488-conjugated goat anti-mouse and/or Alexa 594-conjugated goat anti-rabbit secondary antibodies (1:250; Invitrogen). Immunofluorescence was assessed with a Zeiss LSM 510 microscope (Carl Zeiss, Oberkochen, Germany). The nuclear translocation of NFAT was assessed quantitatively by determining the ratio of the fluorescence intensity of the nuclear region to that of the entire region of the cell on a confocal plane.
2.10. Measurement of intracellular pH
Intracellular pH (pHi) was measured in cultured cardiomyocytes using the pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (2 μM, Invitrogen) as described earlier.14 Cells were loaded with the dye at 37°C for 30 min and placed on the stage of an inverted Leica microscope. Cardiomyocytes were continuously perfused at 1 mL/min with HCO3-free HEPES buffer solution. After equilibration with the superfusion buffer and stabilization of myocytes, cells were superfused with buffer containing phenylephrine, angiotensin II, or endothelin-1 in the absence or presence of CU-NP for 15 min at 37°C. The pHi of individual cardiomyocytes was recorded by photometry at 502.5 and 440 nm for excitation and 528 nm for emission using a monochromator Deltascan-4000 system (PTI, Lawrenceville, NJ, USA). After each experiment, an in situ calibration of the fluorescent signal was performed for each cell. Cardiomyocytes were calibrated by superfusion with calibration buffers containing the ionophore nigericin. The pHi for each cell was determined from a linear regression of fluorescence ratio vs. the pH value of the calibration buffer.
The NH4Cl prepulse technique was used to determine the activity of NHE-1 and the effect of treatments on NHE-1 activity in cardiomyoyctes. The cells were subjected to intracellular acidification by exposure to 25 mM NH4Cl for 5 min followed by perfusion with NH4+-free buffer. Drug addition was performed as described above.
2.11. Measurement of cytosolic sodium concentration
Cells were plated in 24-well Primaria plates. After 24 h treatments, cells were incubated with CoroNa-Red dye (1 µM, Invitrogen) for 20 min at 37°C. The loaded cells were then washed twice with PBS. The fluorescence intensity was measured in a spectral fluorimeter (SpectraMax M5, MDS Analytical Technologies, Sunnyvale, CA, USA) at a wavelength between 554 and 578 nm. The fluorescence intensity was normalized against control (CoroNa-Red-loaded cells) after subtraction of baseline (CoroNa-Red without cells).
2.12. Statistical analysis
All values in the figures and text are presented as mean ± SEM. For all studies ‘n’ refers to the number of litters used, although for cell surface area, at least 50 randomly selected cells per litter were first measured and averaged to provide an n-value of 1.
Multiple comparisons between groups were determined by one- and two-way ANOVA with repeated measurements. An unpaired two-tailed Student's t-test was used to compare mean differences between groups. Differences were considered to be statistically significant at a level of P < 0.05.
3. Results
3.1. Effects of CU-NP on cGMP production in cardiac myocytes
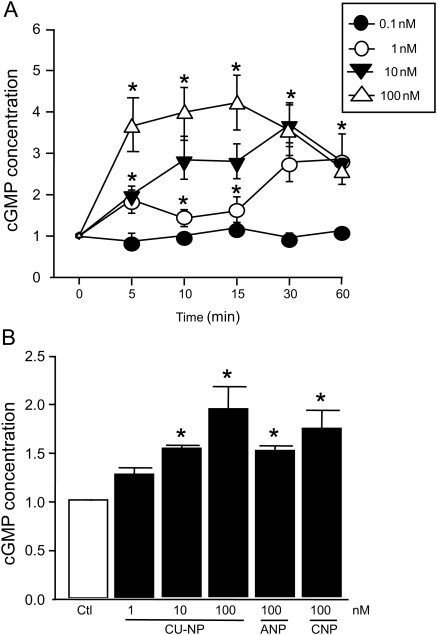
We first confirmed that CU-NP increases cGMP production in cardiomyocytes. As shown in Figure 1A, CU-NP (1, 10, and 100 nM) produced a concentration- and time-dependent increase in intracellular cGMP levels with peak stimulation seen 10–15 min after CU-NP addition. In addition, the effect of CU-NP on cGMP levels was comparable to that produced by equimolar concentrations of either ANP or CNP after 24 h treatment (Figure 1B).
Figure 1.
CU-NP increases cGMP concentrations in myocytes. (A) Time- and concentration-dependent increase in cGMP concentrations in neonatal cardiomyocytes after treatment with increasing concentrations of CU-NP. (B) cGMP levels after treatment with CU-NP (1, 10, and 100 nM), ANP or CNP (100 nM for both) for 24 h. Values indicate means ± SEM and indicate fold increase. n = 8. *P < 0.05 from untreated cells. Ctl, control.
3.2. Effect of CU-NP on hypertrophic responses to phenylephrine, angiotensin II, or endothelin-1
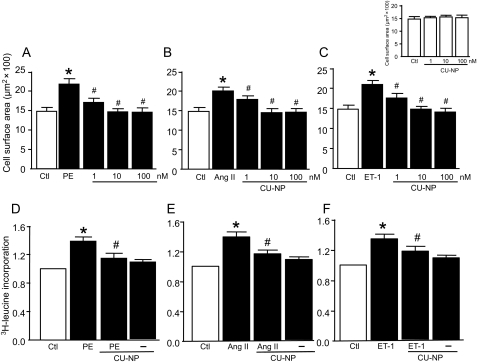
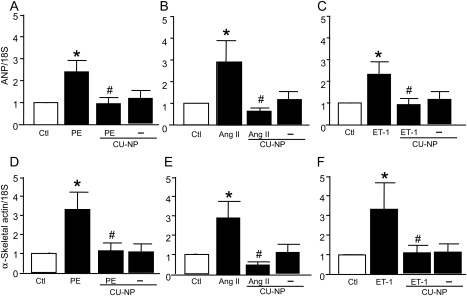
As shown in Figure 2A–C, all agents induced cardiomyocyte hypertrophy as determined by cell surface area with increases of 47, 35, and 41% produced by phenylephrine, angiotensin II, and endothelin-1, respectively (P < 0.05 for all). These effects were attenuated by increasing concentrations of CU-NP with complete abrogation seen with both 10 and 100 nM of the peptide, although CU-NP had no direct effect on cell surface area in the absence of hypertrophic stimuli (Figure 2, inset). As shown in Figure 2D–F, the increased cell surface area was accompanied by increased leucine incorporation (38, 33, and 39% with phenylephrine, angiotensin II, and endothelin-1, respectively, P < 0.05 for all) which was significantly inhibited by CU-NP. The anti-hypertrophic effect of CU-NP was confirmed by the ability of the peptide (10 nM) to completely abrogate hypertrophic responses to all three agonists as assessed by gene expression of two molecular markers of hypertrophy, ANP, and α-skeletal actin (Figure 3).
Figure 2.
CU-NP inhibits cardiomyocyte hypertrophy. (A–C) The concentration-dependent effects of CU-NP on cardiomyocyte surface area. (D–F) Leucine incorporation after 24 h treatment under various experimental conditions. Values for leucine incorporation indicate fold increase. Final PE, Ang II, and ET-1 concentrations were 10 µM, 100, and 10 nM, respectively. Inset (top right) indicates a lack of direct effect of CU-NP on cell surface area in the absence of hypertrophic stimuli. Values indicate means ± SEM. n = 6–9 per group; *P < 0.05 vs. control, #P < 0.05 vs. hypertrophic stimuli. Ctl, control; PE, phenylephrine; Ang II, angiotensin II; ET-1, endothelin-1.
Figure 3.
CU-NP suppresses molecular markers of hypertrophy. Effects of CU-NP on gene expression of ANP (A–C) and α-skeletal actin (D–F) after 24 h treatment with PE, Ang II, or ET-1. All drug concentrations as for Figure 2. Values indicate fold increase and are given as means ± SEM. n = 6–9 per group; *P < 0.05 vs. control, #P < 0.05 vs. hypertrophic stimuli. Ctl, control; PE, phenylephrine; Ang II, angiotensin II; ET-1, endothelin-1.
3.3. Effect of CU-NP on NHE-1 expression and activity
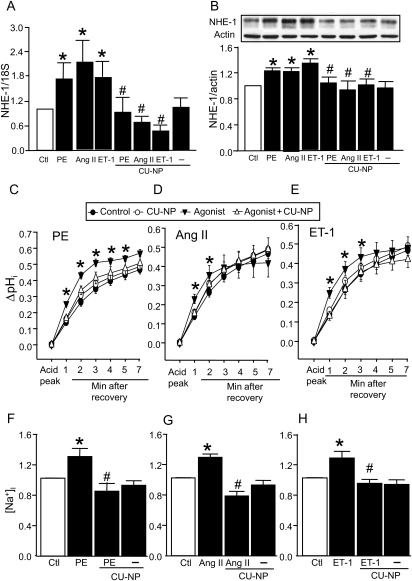
As shown in Figure 4A and B, 10 nM CU-NP prevented the increase in both gene and protein expression of NHE-1 by each of the three hypertrophic stimuli. Moreover, CU-NP prevented the increased NHE-1 activity seen in hypertrophic myocytes treated with phenylephrine, angiotensin II, or endothelin-1 for 24 h (Figure 4C–E). CU-NP had no direct effect on either NHE-1 expression or activity in the absence of hypertrophic stimuli.
Figure 4.
CU-NP inhibits NHE-1 expression and activity and reduces [Na+]i in hypertrophied cardiomyocytes. (A and B) The effect of CU-NP on NHE-1 mRNA and protein expression, respectively, after treatments, whereas (C) to (E) show pHi recoveries after NH4Cl pulsing. (F–H) show [Na+]i. All values were determined after 24 h agonist treatment. All drug concentrations as for Figure 2. Values indicate fold increase, except (C–E), and are given as means ± SEM. n = 7–9 per group; *P < 0.05 vs. control, #P < 0.05 vs. hypertrophic stimuli. Ctl, control; PE, phenylephrine; Ang II, angiotensin II; ET-1, endothelin 1.
3.4. Effect of CU-NP on intracellular Na+ levels
All three agonists produced a significant elevation in intracellular Na+ concentrations ([Na+]i) by ∼30% which was completely prevented by CU-NP, although the peptide was without direct effect on [Na+]i (Figure 4F–H).
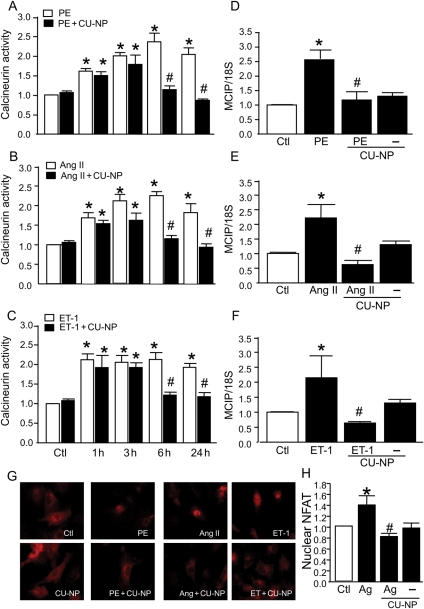
3.5. Effect of CU-NP on calcineurin activity
The next question we addressed is whether CU-NP could affect calcineurin activation as a potential mechanism underlying the anti-hypertrophic effect of the peptide. As shown in Figure 5A–C, all three hypertrophic stimuli increased calcineurin activity within 1 h of stimulation. Although CU-NP on its own had no effect on calcineurin activity, it was able to prevent calcineurin activation after 6 h of treatment with any of the hypertrophic stimuli with the inhibition of calcineurin activation persisting for the 24 h treatment period. The increased activity of calcineurin was also reflected in increased mRNA expression of MCIP which has been shown to be a sensitive indicator of calcineurin activation.15 As demonstrated in Figure 5D–F, CU-NP completely prevented the increase in MCIP expression, thus reflecting decreased calcineurin activity. The inhibition of calcineurin activity was not associated with changes in protein expression levels of calcineurin (data not shown).
Figure 5.
CU-NP abrogates calcineurin activation. (A–C) The effect of CU-NP on calcineurin activity in myocytes at various time points after agonist addition as determined by phosphatase activity assay, whereas (D) to (F) show gene expression levels of MCIP 24 h after agonist administration. (G) NFAT immunofluorescence staining for NFAT, whereas (H) shows the ratio of the fluorescence intensity of the nuclear region to that of the entire region of the cell on a confocal plane. As results were identical, data were pooled for all agonists for fluorescence data. Values indicate fold increase and are given as means ± SEM. n = 6. *P < 0.05 vs. control, #P < 0.05 vs. hypertrophic stimuli. Ctl, control; PE, phenylephrine; Ang II, angiotensin II; ET-1, endothelin-1; Ag, agonists.
We further determined the contribution of calcineurin as a potential downstream target for the anti-hypertrophic effect of CU-NP by measuring the calcineurin-dependent NFAT translocation into nuclei. As shown in Figure 5G and H, the inhibition of agonist-induced calcineurin activation by CU-NP was paralleled by concomitant prevention of NFAT nuclear translocation.
3.6. Effect of CU-NP on MAPK activity
To further characterize the molecular pathways driving cardiac hypertrophy and the potential site targeted by CU-NP, we examined the expression and phosphorylation of the ERK-1/2 and p38 MAPKs (data not shown). Peak activation of both MAPKs occurred 5 min after agonist addition. Activation of ERK-1/2 was markedly more robust that p38 and the effect persisted throughout the 60 min treatment period. In contrast, the phosphorylation of p38 was relatively subtle with values returning to control levels after 60 min. Increased phosphorylation of either of these MAPKs was unaffected by treatment with CU-NP.
3.7. Comparison of CU-NP with CNP and ANP and effect of natriuretic receptor blockade
The ability of CU-NP to induce hypertrophy, up-regulate NHE-1, stimulate calcineurin, and increase NFAT translocation into nuclei was mimicked by the naturally occurring NPs ANP and CNP (see Supplementary material online, Figures S1–S4). Moreover, the NP receptor blocker LPA completely abrogated all effects seen with CU-NP administration (see Supplementary material online, Figures S5–S7).
4. Discussion
The development of chimeric NPs with reduced hypotensive properties represents a new approach for optimizing pharmacological profiles of current NPs, thus improving therapeutic strategies. However, whether these compounds can directly modify cardiomyocyte hypertrophy in the absence of their natriuretic and antihypertensive effects has not been established. Accordingly, we used CU-NP, a novel NP synthesized from the ring structure of human CNP and both C- and N-termini of urodilatin, and investigated whether it can modulate cardiomyocyte hypertrophy. CU-NP exerts extensive natriuretic, diuretic, and GFR-enhancing effects while possessing a less potent vasodilatating influence compared with BNP, thus resulting in a potentially more favourable therapeutic profile in the clinical setting.9,16 The overall major novel finding of the present study is that CU-NP potently inhibits hypertrophy in cardiomyocytes exposed to either of the three hypertrophic stimuli (phenylephrine, angiotensin II, or endothelin-1). Moreover, we show that the underlying mechanism for this effect involves the inhibition of NHE-1 with subsequent inhibition of calcineurin activation. This direct effect of CU-NP is likely mediated via NP receptor activation as its effects were effectively blocked by LPA and mimicked by both ANP and CNP.
We first confirmed that CU-NP increases intracellular cGMP levels in a time- and concentration-dependent manner and thus shares a similar effect in this regard with both ANP and CNP.17 It has been previously shown that CU-NP activates both GC-A and GC-B in contrast to CNP which preferentially activates GC-B and can activate GC-A at high concentrations.17 Activation of GCs increases cGMP which is an important second messenger regulating varied cellular functions. In the heart, cGMP/cGMP-dependent protein kinase I (PKG I) can counteract the effects of growth hormones on cultured neonatal cardiomyocytes and cardiac fibroblasts.2
NHE-1 has been demonstrated to play a key role in the mediation of cardiomyocyte hypertrophy, although its precise mechanisms of action are not known with certainty.18 It has been shown that NPs exert receptor-dependent modulation of cardiac growth response by preventing excessive activation of NHE-1.19–21 Interestingly, it has been recently reported that NHE-1 activation is sufficient to initiate cardiac hypertrophy via activation of Ca2+-dependent pro-hypertrophic signalling pathways including calcineurin, resulting in transcriptional modification.22 On the basis of the above observations, we considered it of interest to determine whether CU-NP could exert its anti-hypertrophic effect, at least in part, via modulation of the NHE-1/calcineurin pathway. Indeed, treatment with CU-NP led to a marked reduction in cardiac mRNA and protein levels of NHE-1. In addition, reduced agonist-stimulated NHE-1 activity was also observed which could reflect diminished NHE-1 protein abundance in myocytes treated with CU-NP. The mechanism by which CU-NP, and indeed other NPs, modulates NHE-1 expression levels is not known with certainty; however, various transcriptional factors including the cAMP response element-binding protein, the serum response factor, as well as NFAT have been linked to cGMP.23 It is possible that CU-NP stimulation of cGMP modulates NHE-1 expression, although the exact nature of transcriptional factor involvement needs to be determined. The possibility that CU-NP also directly inhibits NHE-1 cannot be ruled out with certainty. Indeed, in isolated cardiomyocytes, NPs were shown to inhibit NHE-1 activity in a GC-A/B/cGMP-dependent manner possibly via PKG I-dependent phosphorylation of NHE-1.19–21 Furthermore, in a recent study using non-cardiac tissue, the ability of nitric oxide to inhibit NHE-1 activity was shown to be cGMP-dependent.24 However, we cannot exclude other potential mechanisms for the anti-hypertrophic action of CU-NP related to the NHE-1 system. One possibility stems from the work of Garciarena et al.25 who proposed the beneficial effects of inhibiting mitochondrial NHE-1 and prevention of mitochondrial permeability transition pore opening in hypertrophied ventricular myocytes from spontaneously hypertensive rats. Accordingly, the possibility that CU-NP exerts its effect by inhibiting mitochondrial NHE-1 activity via cGMP generation is worthy of further study. Irrespective of the precise mechanisms, CU-NP down-regulation of the NHE-1 pathway appears to be critically linked to the anti-hypertrophic effect of the peptide and possibly NPs in general.
Numerous signalling pathways are known to coordinate pathological hypertrophy and heart failure, including activation of MAPKs and calcineurin-dependent transcriptional factor modulation.26 NHE-1 activation has been linked to a Ca2+-dependent activation of calcineurin and the resultant hypertrophic response22 and a recent report has suggested that calcineurin is involved in endothelin-1-induced hypertrophy in rat myocytes, secondary to NHE-1 activation.27 The present study supports the concept of NHE-1-dependent calcineurin-mediated hypertrophic responses to endothelin-1 but also to both phenylephrine and angiotensin II. We further show that calcineurin activation occurs as early as 1 h after stimulation of cells with any of the hypertrophic agonists and that this activation persists for up to 24 h of treatment. Moreover, our study shows that the anti-hypertrophic effect of CU-NP occurs independently of any effect on early calcineurin activation but is associated with a complete abrogation of calcineurin activation 6 and 24 h after addition of the hypertrophic stimuli with identical effects being seen with phenylephrine, endothelin-1, and angiotensin II. These effects were also associated with diminished NFAT translocation into nuclei. Thus, these findings suggest that early inhibition of calcineurin activation is not a prerequisite for the anti-hypertrophic effects of CU-NP and that sustained calcineurin activation may be required for materialization of the hypertrophic programme. The inhibition of the calcineurin pathway by CU-NP likely reflects a GC-dependent phenomenon which is supported by a previous report that cardiac hypertrophy in GC-deficient mice is dependent on the calcineurin pathway.28
We also determined the possible contributing role of the MAPK family as a target for the anti-hypertrophic effect of CU-NP. Indeed, phenylephrine, endothelin-1, and angiotensin II have all been shown to activate various components of the MAPK family in cardiac cells,29,30 a finding which was clearly observed in our study. However, in our study, the activation of MAPKs by any of hypertrophic agents was unaffected by CU-NP, thus demonstrating a dissociation between hypertrophy and MAPK activation, at least with respect to the anti-hypertrophic effect of CU-NP. Dissociation between MAPK activation has been previously reported with respect to other pro-hypertrophic factors.31–33
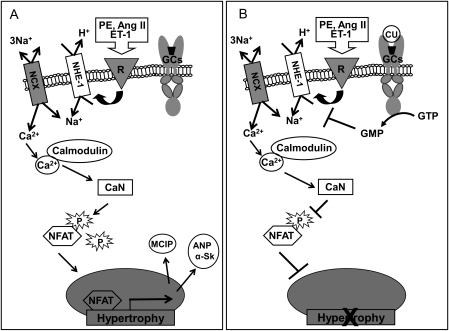
In summary and as presented in Figure 6, our study demonstrates a direct anti-hypertrophic effect of the novel designer peptide CU-NP which acts by inhibition of NHE-1 and subsequent activation of calcineurin. CU-NP as well as other novel chimeric NPs represent potentially effective therapeutic modalities for the treatment of cardiac hypertrophy and heart failure via natriuretic effects as well as a direct influence on cardiomyocyte hypertrophy. However, whether inhibition of the NHE-1/calcineurin axis as a mechanism for anti-hypertrophic effects is shared by all such chimeric compounds needs to be determined with further studies.
Figure 6.
Potential pathway underlying the anti-hypertrophic effect of CU-NP. As illustrated in (A), hypertrophic stimuli including PE, Ang II, and ET-1 activate NHE-1 via stimulation of their respective receptors (R). NHE-1 activation and the resultant increase in intracellular Na+ will elevate intracellular Ca2+ levels via the 3Na+–Ca2+ exchanger (NCX) either by slowing the removal of intracellular Ca2+ or driving the NCX in reverse. Elevated intracellular Ca2+ and the resulting Ca2+/calmodulin complex will activate calcineurin (CaN) which dephosphorylates the transcriptional factor NFAT resulting in its translocation into nuclei and subsequent up-regulation of genes related to hypertrophy including MCIP, ANP, and α-Sk actin (α-Sk). As proposed in (B), CU-NP (CU) acting via GCs/cGMP inhibits NHE-1, thus abrogating Ca2+/calmodulin-dependent calcineurin activation, dephosphorylation/translocation of NFAT, and the resultant hypertrophy.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: CU-NP has been licensed by the Mayo Clinic to Nile Therapeutics and J.C.B. Jr. is Chair of its Scientific Advisory Board.
Funding
This work was supported by the Canadian Institutes of Health Research to M.K. and National Institutes of Health (HL 83231) and the Mayo Foundation to J.C.B. Jr. A.K. was supported by Fellowships from the Heart and Stroke Foundation of Canada and the Tailored Advanced Collaborative Training in Cardiovascular Science (TACTICS) program. C.Y.L. was supported by a 2007 Heart Failure Society of America Research Fellowship Award. M.K. holds a Tier 1 Canada Research Chair in Experimental Cardiology.
Supplementary Material
References
- 1.Burnett JC., Jr Natriuretic peptides and remodelling in heart failure. Heart Fail Clin. 2005;1:129–139. doi: 10.1016/j.hfc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn M. Structure, regulation and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–709. doi: 10.1161/01.RES.0000094745.28948.4D. doi:10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 3.Yandle TG. Biochemistry of natriuretic peptides. J Intern Med. 1994;235:561–576. doi: 10.1111/j.1365-2796.1994.tb01263.x. doi:10.1111/j.1365-2796.1994.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 4.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors and cyclic guanosine monophosphate-dependent signalling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. doi:10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch JR, Meyer M, Forssmann WG. ANP and urodilatin: who is who in the kidney. Eur J Med Res. 2006;11:447–454. [PubMed] [Google Scholar]
- 6.Maack T. Receptors of atrial natriuretic factor. Annu Rev Physiol. 1992;54:11–27. doi: 10.1146/annurev.ph.54.030192.000303. doi:10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- 7.Lee CYW, Burnett JC., Jr . ANP, BNP and CNP: physiology and pharmacology of the cardiorenal axis. In: Singh A, Williams GH, editors. Textbook of Nephro-endocrinology. San Diego, CA: Academic Press; 2009. pp. 289–307. [Google Scholar]
- 8.Lee CYW, Burnett JC., Jr Discovery of a novel synthetic natriuretic peptide, CU-NP. J Card Fail. 2007;13(6 Suppl. 2):S74. (Abstract) [Google Scholar]
- 9.Lee CYW, Chen HH, Sandberg SM, Harty GJ, Burnett JC., Jr Renal mechanisms of action of a novel designer natriuretic peptide, CU-NP. J Card Fail. 2008;14(6 Suppl. 1):S79–S80. (Abstract) [Google Scholar]
- 10.Lee CYW, Huntley BK, Sandberg SM, Chen HH, Burnett JC., Jr A novel designer natriuretic peptide, CU-NP, in human aortic endothelial cells: evidence for NPR-B involvement in cyclic GMP response. Can J Clin Pharmacol. 2008;15:e517. (Abstract) [Google Scholar]
- 11.Huntley BK, Sandberg SM, Noser JA, Cataliotti A, Redfield MM, Matsuda Y, et al. BNP-induced activation of cGMP in human cardiac fibroblasts: interactions with fibronectin and natriuretic peptide receptors. J Cell Physiol. 2006;209:943–949. doi: 10.1002/jcp.20793. doi:10.1002/jcp.20793. [DOI] [PubMed] [Google Scholar]
- 12.Javadov S, Baetz D, Rajapurohitam V, Zeidan A, Kirshenbaum LA, Karmazyn M. Antihypertrophic effect of Na+/H+ exchanger isoform 1 inhibition is mediated by reduced mitogen-activated protein kinase activation secondary to improved mitochondrial integrity and decreased generation of mitochondrial-derived reactive oxygen species. J Pharmacol Exp Ther. 2006;317:1036–1043. doi: 10.1124/jpet.105.100107. doi:10.1124/jpet.105.100107. [DOI] [PubMed] [Google Scholar]
- 13.Zeidan A, Javadov S, Chakrabarti S, Karmazyn M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc Res. 2008;77:64–72. doi: 10.1093/cvr/cvm020. doi:10.1093/cvr/cvm020. [DOI] [PubMed] [Google Scholar]
- 14.Kilić A, Javadov S, Karmazyn M. Estrogen exerts concentration-dependent pro- and anti-hypertrophic effects on adult cultured ventricular myocytes. J Mol Cell Cardiol. 2009;46:360–369. doi: 10.1016/j.yjmcc.2008.11.018. doi:10.1016/j.yjmcc.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, et al. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. doi:10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–68. doi: 10.1016/j.jacc.2008.02.077. doi:10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickey DM, Burnett JC, Jr, Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–35009. doi: 10.1074/jbc.M804538200. doi:10.1074/jbc.M804538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karmazyn M, Kilić A, Javadov S. The role of NHE-1 in myocardial hypertrophy and remodelling. J Mol Cell Cardiol. 2008;44:647–653. doi: 10.1016/j.yjmcc.2008.01.005. doi:10.1016/j.yjmcc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Kilić A, Velic A, De Windt LJ, Fabritz L, Voss M, Mitko D, et al. Enhanced activity of the myocardial Na+/H+ exchanger NHE-1 contributes to cardiac remodeling in atrial natriuretic peptide receptor-deficient mice. Circulation. 2005;112:2307–2317. doi: 10.1161/CIRCULATIONAHA.105.542209. doi:10.1161/CIRCULATIONAHA.105.542209. [DOI] [PubMed] [Google Scholar]
- 20.Tajima M, Bartunek J, Weinberg EO, Ito N, Lorell BH. Atrial natriuretic peptide has different effects on contractility and intracellular pH in normal and hypertrophied myocytes from pressure-overloaded hearts. Circulation. 1998;98:2760–2764. doi: 10.1161/01.cir.98.24.2760. [DOI] [PubMed] [Google Scholar]
- 21.Fidzinski P, Salvador-Silva M, Choritz L, Geibel J, Coca-Prados M. Inhibition of NHE-1 Na+/H+ exchanger by natriuretic peptides in ocular nonpigmented ciliary epithelium. Am J Physiol Cell Physiol. 2004;287:C655–C663. doi: 10.1152/ajpcell.00552.2003. doi:10.1152/ajpcell.00552.2003. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res. 2008;103:891–899. doi: 10.1161/CIRCRESAHA.108.175141. doi:10.1161/CIRCRESAHA.108.175141. [DOI] [PubMed] [Google Scholar]
- 23.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res. 2003;93:1034–1046. doi: 10.1161/01.RES.0000103311.52853.48. doi:10.1161/01.RES.0000103311.52853.48. [DOI] [PubMed] [Google Scholar]
- 24.Shahidullah M, Mandal A, Delamere NA. Nitric oxide inhibits sodium hydrogen exchange in porcine cultured non-pigmented ciliary epithelium. Invest Ophthalmol Vis Sci. 2009;50:5851–5858. doi: 10.1167/iovs.09-3453. doi:10.1167/iovs.09-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garciarena CD, Caldiz CI, Correa MV, Schinella GR, Mosca SM, et al. Na+/H+ exchanger-1 inhibitors decrease myocardial superoxide production via direct mitochondrial action. J Appl Physiol. 2008;105:1706–1713. doi: 10.1152/japplphysiol.90616.2008. doi:10.1152/japplphysiol.90616.2008. [DOI] [PubMed] [Google Scholar]
- 26.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. doi:10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 27.Ennis IL, Garciarena CD, Escudero EM, Pérez NG, Dulce RA, Camilión de Hurtado MC, et al. Normalization of the calcineurin pathway underlies the regression of hypertensive hypertrophy induced by Na+/H+ exchanger-1 (NHE-1) inhibition. Can J Physiol Pharmacol. 2007;85:301–310. doi: 10.1139/y06-072. doi:10.1139/Y06-072. [DOI] [PubMed] [Google Scholar]
- 28.Tokudome T, Horio T, Kishimoto I, Soeki T, Mori K, Kawano Y, et al. Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides. Circulation. 2005;111:3095–3104. doi: 10.1161/CIRCULATIONAHA.104.510594. doi:10.1161/CIRCULATIONAHA.104.510594. [DOI] [PubMed] [Google Scholar]
- 29.Yue TL, Gu JL, Wang C, Reith AD, Lee JC, Mirabile RC, et al. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:37895–37901. doi: 10.1074/jbc.M007037200. doi:10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]
- 30.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. doi:10.1161/01.RES.0000038488.38975.1A. [DOI] [PubMed] [Google Scholar]
- 31.Post GR, Goldstein D, Thuerauf DJ, Glembotski CC, Brown JH. Dissociation of p44 and p42 mitogen-activated protein kinase activation from receptor-induced hypertrophy in neonatal rat ventricular myocytes. J Biol Chem. 1996;271:8452–8457. doi: 10.1074/jbc.271.14.8452. [DOI] [PubMed] [Google Scholar]
- 32.Irukayama-Tomobe Y, Miyauchi T, Kasuya Y, Sakai S, Goto K, Yamaguchi I. Activation of peroxisome proliferator-activated receptor-alpha decreases endothelin-1-induced p38 mitogen-activated protein kinase activation in cardiomyocytes. J Cardiovasc Pharmacol. 2004;44:S358–S361. doi: 10.1097/01.fjc.0000166303.33313.01. doi:10.1097/01.fjc.0000166303.33313.01. [DOI] [PubMed] [Google Scholar]
- 33.Azuma M, Takahashi K, Fukuda T, Ohyabu Y, Yamamoto I, Kim S, et al. Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eur J Pharmacol. 2000;403:181–188. doi: 10.1016/s0014-2999(00)00483-0. doi:10.1016/S0014-2999(00)00483-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.