SUMMARY
Twenty patients were treated with antilymphocyte globulin (ALG) which was prepared from the serum of immunized horses. The ALG was used as an adjuvant to azathioprine and prednisone and its use limited to 4 months. The surviving patients are now 1 to 7 months postoperative. There was 1 death, the consequence of a technical accident. The function in the remaining 19 patients is excellent, despite reduced doses of azathioprine and especially prednisone. Biopsies were obtained in the first 8 consecutive cases from 108 to 145 days after operation. There was no evidence in the specimens of either Masugi-like or serum sickness nephritis.
Since June 1966, all patients receiving renal homotransplantation at the Colorado General and Denver Veterans Administration Hospitals have been treated with heterologous immune globulin, prepared from the serum of horses which had been immunized with human spleens, thymuses, and lymph nodes. The steps which led to the clinical use of antilymphocyte globulin (ALG) were based on the pioneering studies in rodents by Waksman (12), Woodruff (13), Gray (3), Monaco (8), Jeejeebhoy (6), and Levey and Medawar (7) and upon our own previously reported investigations in dogs (4, 5,10).
The postoperative follow-up of the first 20 cases in this test series is of course too brief to allow conclusions about the influence that treatment with ALG will have upon the ultimate course of these patients. Evidence has been obtained, however, that such foreign protein therapy is of at least short term value, and that it can be given without prohibitive toxicity for a period of several months as will be summarized in the present report. A far more detailed analysis of these cases has been published elsewhere, as well as an account of ALG therapy in patients with late failing homografts (11).
METHODS
ALG administration was planned within guidelines established from animal studies which included the following considerations: (1) ALG when used alone does not prevent or even delay rejection of all canine homografts (1, 10); (2) it can be advantageously combined with other commonly used immunosuppressive agents (7, 10); (3) its use carries an inherent risk of anaphylaxis and/or nephrotoxicity even though this is apparently less than with normal horse serum or globulin (1, 10) ; (4) its greatest effectiveness is with administration before as well as after transplantation (8, 10) ; and (5) its immunosuppression is not dependent upon the production of lymphopenia (1, 7,10).
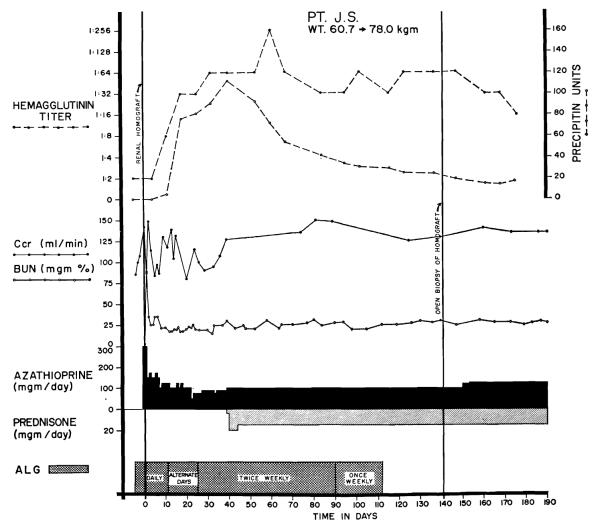
Because of the above information, ALG was used only as an adjuvant agent, in combination with azathioprine and prednisone. In the usual case, daily injections were started 5 days before operation, continued each day for 10 to 17 days afterwards, then every other day for 2 weeks, twice a week for 2 months and once a week for 1 month (Fig. 1). Two patients who had anaphylactic reactions had therapy stopped prematurely. The ALG had a leukoagglutinin titer of 1:4096 to 1:32, 768, and a protein concentration of 4.6 to 9.3 gm/100 ml. Individual intramuscular injections were 1 to 5 ml. The weekly protein dose per kg was 14–50 mg at the beginning and 2–6 mg at the end of therapy.
Figure 1.
Course of a patient who received ALG therapy before and after renal homotransplantation. The homograft was provided by a brother. There has never been rejection. The prednisone therapy was started after 40 days because of the increases in hemagglutinin and precipitin titers. Note the subsequent fall in these measures despite continuation of globulin injections.
Hemagglutinin titers against sheep red blood cells and precipitin titers were monitored as indices of antibody formation against the foreign protein. The first 8 patients had open homograft biopsies from 108 to 145 days after transplantation.
Azathioprine and prednisone were given as described in detail elsewhere (11), in a schedule which called for the maximum doses of the former drug which were possible without producing leukopenia, and the smallest steroid doses which were compatible with stable renal function. The effectiveness of therapy was judged by analyses of the death rate, the quality of homograft excretion, and the quantities of standard immunosuppressive agents necessary to retain this function.
RESULTS
Mortality
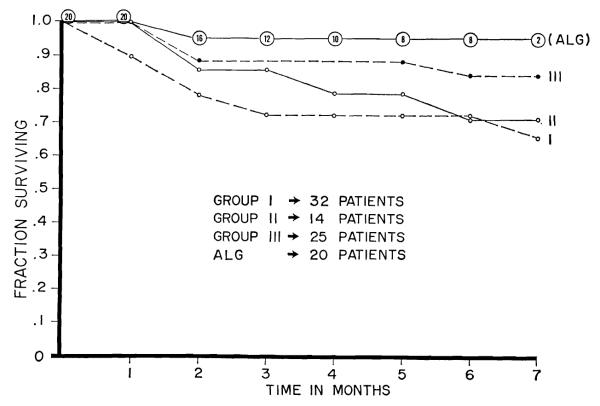
The 20 patients have been followed for 1 to 7 months. The operations were 5½ to 7 months ago in the first 8 cases, 2 to 4 months in the next 8, and 4 to 6 weeks in the last 4. There has only been 1 death (Fig. 2), the fourteenth patient in the group, from complications stemming directly from a technical accident.
Figure 2.
Mortality in patients treated with adjuvant ALG compared to that in 3 preceding series of consanguineous transplantations carried out in Denver.
Nineteen of the recipients were donated kidneys by blood relatives on essentially random selection basis; parents in 5 instances, siblings in 13, and a maternal uncle in the other. The twentieth patient received a cadaveric homograft from a donor who was shown by the teams of van Rood, Amos, and Terasaki, who were working in Denver at the time, to have a high degree of antigen incompatibility with the recipient. Because of the preponderance of related cases, the mortality was compared to that with 3 sequential past series of consanguineous transplantations at our institutions (Fig. 2). The early mortality in the globulin-treated series was the lowest, a finding which has become meaningful at least in the first 12 cases since the dangerous first 3 postoperative months have already passed.
Function
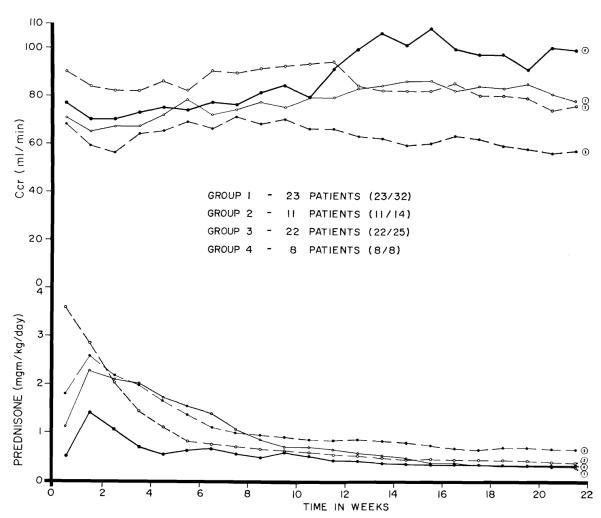
The 19 surviving patients all have good renal function. A week by week view of the average creatinine clearance of the first 8 consecutive cases for the first 5 months is shown in Figure 3. Function for the first 2 months was adequate, but after this, the clearances further improved. At the time of their biopsies from 108 to 145 days post-transplantation the various measures of renal function3 were blood urea nitrogen 26.7 ± 5.5 (S.D.) mg/100 ml, plasma creatinine 0.92 ± 0.2 (S.D.) mg/100 ml, creatinine clearance 97.4 ± 21 (S.D.) ml/min, inulin clearance 61 ± 12.6 (S.D.) ml/min and PAH clearance 349 ± 66 (S.D.) ml/min. The filtration fraction was 17.9% ± 3.3% (S.D.). Urine protein concentration was 5.4 ± 3.6 (S.D.) mg/100 ml.
Figure 3.
Week by week creatinine clearances and average daily prednisone doses during the first 22 weeks for 8 consecutive ALG treated patients, compared to the same information in 3 previously treated series of transplantations betwen blood relatives; in these retrospective control cases, patients who died before 22 weeks were excluded. See text for discussion.
For comparison, the average creatinine clearances are shown (Fig. 3) for those patients who lived for at least 5 months in each of the 3 preceding series of consanguineous transplantations; the elimination of early deaths in the retrospective control series created a bias since only data from the best cases were retained. Nevertheless, the mean clearances from the third month onward were greater in the globulin treated group.
Immunosuppression
The quantities of azathioprine per kg were less than in any of the previously treated series (11). However, the most striking declines were in the steroid doses, which were approximately half of those in the 3 previous groups of patients who were selected as controls by virtue of their ability to survive for at least 5 months (Fig. 3). These data, together with those from the preceding section, suggest that the reduction in the stringency of therapy with standard immunosuppressive drugs was not paid for by an increased injury to the homograft.
Effect of lymphocytes
There was no statistically significant difference in the degree of postoperative lymphopenia in the globulin-treated as opposed to the 3 retrospective control series.
Toxicity
Pain and tenderness at the injection sites and fever were observed in virtually every case. Four of the 20 patients have had anaphylactic reactions which lasted for only a few minutes; in 2 of these cases, injections were continued for an additional month or longer. Increases in precipitin titers and antisheep red cell titers eventually occurred in every patient, but tended to regress either spontaneously or with the addition of steroids.
The homograft biopsies from the first 8 cases are fully described elsewhere (11). None were completely normal, but there was evidence of permanent damage in only one case where narrowing of several interlobular arteries was found.
A portion of each of the biopsies was examined by Drs. Beatrice C. Seegal and Konrad C. Hsu of Columbia University, New York, and by Dr. Giuseppe A. Andres of the University of Rome, Italy, using fluorescein-labeled and ferritin-conjugated antihorse protein antibodies to detect the presence or absence of equine protein in these kidneys. None was found.
DISCUSSION
Experience with these cases has been distinctly encouraging in 2 respects. First, the early course after human renal homotransplantation seems to have been improved by the addition of ALG to the therapeutic protocol. There has only been 1 death in the 20 cases, and that fatality followed a serious technical accident. Renal function has been as good as in any comparable group of patients previously treated in Denver, and in the cases with the longest follow-up it is distinctly better. These results have been obtained with smaller quantities of standard immunosuppressive agents, particularly of prednisone.
Secondly, the use of ALG has been possible without prohibitive morbidity. Anaphylactic reactions were seen in 20% of the patients but these were relatively minor, and in 2 of the 4 cases a number of subsequent injections were given. Two other feared immunologic reactions, each with a distinctive immunologic mechanism, would have been detectable in the homograft biopsies since both cause intrarenal deposits of heterologous protein which can be identified for many months afterwards with specific labeled-antibody techniques (2, 9). However, there was no evidence either that the ALG antibodies had reacted directly with the renal tissue (Masugi nephritis) or that these kidneys were the passive receptacles of soluble antigen-antibody complexes (serum sickness nephritis).
Footnotes
Aided by Grants AM 06283, AM 06344, HE 07735, AM 07772, AI 04152, FR 00051 and FR 00069 from the United States Public Health Service, and by a grant from the Medical Research Council of Great Britain.
REFERENCES
- 1.Abaza HM, Nolan B, Watt JG, Woodruff MFA. Transplantatation. 1966;4:618. doi: 10.1097/00007890-196609000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Dixon FJ, Feldman JD, Vazquez JJ. J. Exp. Med. 1961;113:899. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray JG, Monaco AP, Russell PS. Surg. Forum. 1964;15:1942. [PubMed] [Google Scholar]
- 4.Huntley RT, Taylor PD, Iwasaki Y, Marchioro TL, Jeejeebhoy H, Starzl TE. Surg. Forum. 1966;17:230. [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki Y, Porter KA, Amend J, Marchioro TL, Zühlke V, Starzl TE. Surg. Gynec. Obst. 1967;124:1. [PMC free article] [PubMed] [Google Scholar]
- 6.Jeejeebhoy HF. Immunology. 1965;9:417. [PMC free article] [PubMed] [Google Scholar]
- 7.Levey RH, Medawar PB. Proc. National Acad. Sci. 56:1130. doi: 10.1073/pnas.56.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monaco AP, Wood ML, Gray JG, Russell PS. J. Immunol. 1966;96:229. [PubMed] [Google Scholar]
- 9.Seegel BC, Hsu KC, Rothenburg MS, Chapeau ML. Am. J. Path. 1962;41:183. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. Surg. Gynec. Obst. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Kashiwagi N. In: Antilymphocytic serum. Medawar PB, editor. J. & A. Churchill; London: 1967. In press. [Google Scholar]
- 12.Waksman BH, Arbouys S, Arnason BG. J. Exp. Med. 1961;114:997. doi: 10.1084/jem.114.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodruff MFA, Anderson NF. Nature (London) 1963;200:702. doi: 10.1038/200702a0. [DOI] [PubMed] [Google Scholar]