Abstract
We report the diagnostic surgical pathology of two children who underwent multivisceral abdominal transplantation and survived for 1 month and 6 months. There is little relevant literature, and diagnostic criteria for the various clinical possibilities are not established; this is made more complicated by the simultaneous occurrence of more than one process. We based our interpretations on conventional histology, augmented with immunohistology, including HLA staining that distinguished graft from host cells in situ. In some instances functional analysis of T cells propagated from the same biopsies was available and was Used to corroborate morphological interpretations. A wide spectrum of changes was encountered. Graft-versus-host disease, a prime concern before surgery, was not seen. Rejection was severe in 1 patient, not present in the other, and both had evidence of lymphoproliferative disease, which was related to Epstein-Barr virus. Bacterial translocation through the gut wall was also feature in both children. This paper documents and illustrates the various diagonstic possibilities.
Keywords: lymphoproliferative disease, intestine, liver, transplantation, rejection
INTRODUCTION
Multiple abdominal organ transplantation has been attempted, with even short-term survival, in only a limited number of children whose own small intestines were missing or irreversibly damaged and who had secondary liver disease from the prolonged intravenous alimentation (1, 2). In addition to these previously described patients, another child subsequently underwent the same procedure at our institution, surviving for 37 days. During the 6 months that our first patient survived and the 5 weeks of the second, numerous biopsies of intestine, liver, lymphoid tissue, and skin were performed as part of the clinical diagnostic monitoring. Interpretation of some of the biological processes represented in the tissues was hampered at times by a lack of definitive criteria, by a dearth of prior experience in this or in similar clinical situations (3), and by the presence of more than one process at any particular time, each of which could be affected by the immunosuppressive therapy being administered.
We reinforced our morphologic interpretations by using immunohistochemistry that distinguished host from recipient cells and by using in-situ demonstration of lymphoid and viral markers. Although complete autopsies were denied in both instances, extensive postmortem sampling of the grafts was permitted. In this paper we set out to describe the morphology of the major processes observed in the grafted organs following multiple visceral transplantation in two children who survived for 912 and 37 days after operation.
CLINICAL HISTORIES
Case 1
A 3½-year-old black girl had a Christmas-tree deformity of the small intestine and lost all but 12.0 cm of proximal jejunum including the ileocecal junction and colon at 2 days of age. Intravenous hyperalimentation was instituted at age 5 months. Her growth was 5th percentile for height and 50th percentile for weight at 3½ years. Jaundice secondary to parenteral nutrition was progressive from the age of 2 years. At the time of surgery she was in frank liver failure, total bilirubin 27.5 mg/dl, serum albumin 2.0 g/dl, prothrombin time 18.2 seconds, and aspartate aminotransferase level 303 U/L. The surgery, immune suppression, and clinical course have been reported in detail elsewhere (1). Briefly, the donor was a 2-month-old female who suffered a head injury. The graft consisted of stomach, small and large intestine, liver, and pancreas (Fig. 1). To deplete the graft of T cells and thus obviate graft-versus-host disease, the donor was given 10 mg of OKT3 intravenously before removal of the graft, and the patient received 4.5 Gμ of radiation to the abdomen. Immunosuppression was with cyclosporine A and prednisone and a 1-week course of OKT3 was given from day 23 because of a clinical suspicion of rejection. Gram-negative bacteremia with Serratia marcescens and coagulase negative Staphylococcus aureus was documented on seven occasions during the first 12 days and was accompanied, on day 11, by bacterial cholangitis. Epstein-Barr virus (EBV) associated lymphoproliferative disease in the liver was diagnosed at 3 months after a relatively good clinical course, immune suppression was withdrawn, and the hepatic nodules regressed over the next month. After cyclosporine was restarted, a new hilar mass appeared that led to biliary obstruction, abscess formation, sepsis, and death.
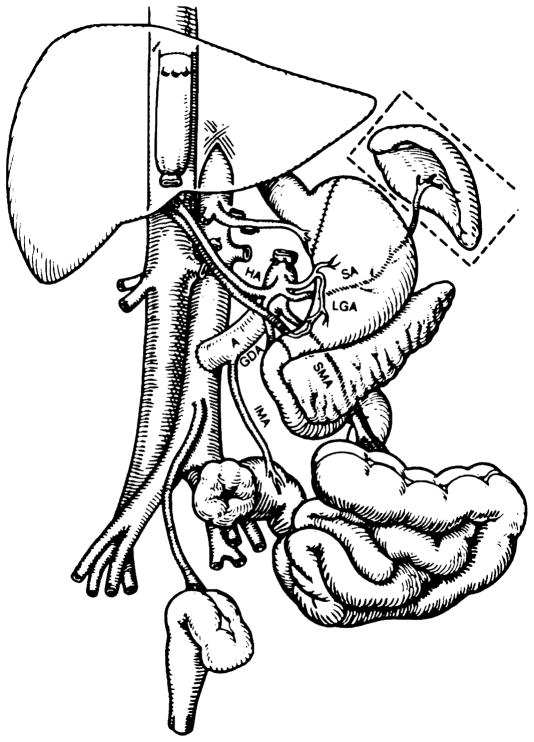
FIGURE 1.
The recipient operation of multivisceral transplantation. Note that the venous outflow of the graft was into a cloaca of the left and middle hepatic veins, leaving the recipient vena cava intact. (A: donor aorta; HA: hepatic artery; SA: splenic artery; LGA: left gastric artery; SMA: superior mesentric artery; IMA: inferior mesenteric artery; GDA: gastroduodenal artery.)
Case 2
A Navajo girl developed secretory diarrhea a few days after birth. Parenteral hyperalimentation was instituted and despite chronic hospitalization her initial development was normal. At age 39 months she was admitted to Children’s Hospital of Pittsburgh where a diagnosis of microvillus inclusion disease was made (4). Because of progressive chronic liver disease from hyperalimentation she underwent multivisceral transplantation.
In contrast to patient 1, the donor organs were not irradiated; the rest of the procedure was identical. Gastrointestinal continuity was restored by gastrogastrostomy and colocolostomy. Vascular connections were achieved by “piggyback” anastomosis of the donor aorta with its intact visceral branches onto the recipient aorta, and graft venous outflow was from the hepatic veins into the donor cava, which was interposed into the recipient vena cava.
The donor was an 18-month-old male who suffered a lethal head injury 7½ hours before organ removal. Cold ischemia time of the organs was 6 hours and preservation was by the “slush technique” after infusion of UW solution (5). Intravenous cyclosporine and prednisone were started by the time of operation and azathioprine was given on postoperative days 2 and 3 only. A 14-day course of OKT3 was begun on day 18.
During the first few days Bacteroides thetaiotamicron was cultured from blood, pleural fluid, and abdominal drainage; coagulase-negative Staphylococcus aureus was cultured from blood on three occasions after the Bacteroides had been eradicated, but clinical infection was never a serious problem. Bilirubin rose from 2 mg/dl to 11.9 mg/dl on day 18 and resolved promptly with the OKT3 administration. At the completion of the OKT3 cycle, the bilirubin rose once more, a capillary leak syndrome and progressive renal failure developed making fluid balance impossible, and she died on the 37th postoperative day. Biopsies had revealed a disseminated lymphoproliferative disease on day 33.
In the 37 days of postoperative life, the patient was submitted to laparotomy five times for suspected perforation of a viscus, although none was found. An ileostomy was made on day 24 to permit closer monitoring. Autopsy permission was refused, but extensive postmortem sampling of the graft was allowed.
MATERIALS AND METHODS
When sufficient quantities were available, biopsy (and postmortem) specimens were triaged for fixation in 10% neutral buffered formalin, snap-freezing in optimum cutting temperature compound (OCT, Miles, Inc., Elkhart, IN), bacterial and viral cultures. At selected times, tissue from patient 2 was also placed in tissue culture medium supplemented with recombinant interleukin-2 for selective expansion of graft-infiltrating lymphoid cells (6). Formalin-fixed, embedded bowel sections were exposed to an alkaline phosphatase substrate (Vector, Burlingame, California, Red #1) according to the suggestion of Lake (7). Fixed tissues were stained with an avidin-biotin complex technique (ABC, Vector, Burlingame, California); the primary antibodies used were rabbit polyclonal antibodies against immunoglobulin heavy and light chains, lysozyme, alpha-1-antichymotrypsin, and pancreatic peptide (DAKO, Santa Barbara, California). Monoclonal antibodies to cytomegalovirus (Chemicon, El Segundo, California), B-cells (L-26) and some T-cells (UCH-1) (DAKO, Santa Barbara, California) were also used with appropriate biological controls using the ABC method. Snap-frozen tissues were stained for the presence of lymphoid subset markers CD2, CD3, CD4, CD8; for IL-2 receptor (CD25), HLA-DR and HLA-ABC, CD-14 for monocytes and CD20 for B-cells (Becton-Dickinson, Mountain View, California) anti-kappa and anti-lambda light chains (DAKO, Santa Barbara, California).
For differentiating between donor and recipient cells in situ, monoclonal antibodies were used that had specificity against host and donor antigens, HLA-A3 versus Bw4 in Case 1; A25/32 and A24 versus Bw6 and A2 in Case 2 (Karin Nelson, Ph.D. Genetic Systems, Seattle, Washington). These alloantigens were demonstrated using an alkaline-phosphatase, antialkaline phosphatase procedure (8) or by an ABC method (Vector Elite, Burlingame, California). Amino-ethylcarbazol was used for detection, and endogenous peroxidase was quenched in some instances following the primary antibody step by immersion in 1 % hydrogen peroxide in Tris-buffer.
EBV DNA was detected in situ in formalin-fixed, paraffin-embedded tissue using 35S autoradiography by the methods of Unger et al. (9) for pretreatment and hybridization and of Weiss (10) for posthybridization washes. The EBV DNA probes were the two Bg1 II/Bam HI fragments of the Bam HI-W repeat unit, radiolabeled with α35S-αATP and α35S-dCTP by the random primer technique. Controls consisted of known EBV -positive and negative tissues, and the use of radiolabeled plasmid DNA in place of probe.
RESULTS
Bacterial Translocation
Bacterial translocation was documented in both patients. Serratia was cultured on seven occasions from the blood of patient 1 during the first 12 postoperative days, and Bacteroides was found in blood, pleural fluid and abdominal drainage in the first 4 days in patient 2. In both patients, Straphylococci were thereafter found in the blood on occasion but without clinical evidence of sepsis.
The practical consequence of this phenomenon is that cholangitis was seen as an early feature in both children. Gram-negative bacteria were demonstrable in the bile ducts in patient 1, whereas cholangitis without demonstrable organisms was seen on day 5 in patient 2. No bacteria were observed in a mesenteric lymph node sampled on day 5. In both instances the cholangitis had resolved, in the face of intensive antibiotic therapy, by day 17 or 18.
Rejection
Patient 1: Gastrointestinal Tract, Pancreas, and Liver
The donor colon was subjected to biopsy at 6 days, 3 months and at 6 months and was essentially normal (Fig. 2a). At postmortem examination a range of epithelial preservation was revealed (Fig. 2b, c, d). Staining for intestinal alkaline phosphatase in the brush border of the jejunum revealed a normal microvillous distribution. Ganglion cells were present throughout the bowel, and the intrinsic nerves were not unduly conspicuous. Sections of the stomach revealed only that the donor epithelium was slightly lower than the recipient gastric mucosa. No cellular infiltrate was present on the donor side of the anastomosis. The pancreas had very little interstitial fibrosis but patchy microcystic dilatations of pancreatic ducts were noted. The endocrine pancreas was intact; all cell types were represented in the islets.
FIGURE 2.
Intestinal sections, patient 1. (a) Donor colon; biopsy at 6 months. The epithelium is intact; there is no cellular infiltrate. × 240. (b) Donor jejunum; postmortem sample. Hyperplastic villi have intact epithelium. ×120. (c)Donor midileum; postmortem sample; dystrophic changes. The mucosa has partial villus atrophy, and the submucosa is fibrotic; no cellular infiltrate is present. × 120. (d) Donor terminal ileum. The surface is lined by a single layer of regenerating columnar cells. The submucosa and muscularis arc scarred. × 120.
The bowel in general was depleted of lymphoid tissue; no solitary follicles or Peyer’s patches were found. Immunostaining revealed only occasional UCH-1 and immunoglobulin-containing cells in the lamina propria, far fewer than would be age-appropriate for this site.
The liver, which had been biopsied on 15 separate occasions, had never displayed a pattern of cellular rejection, and at postmortem sampling none was evident. Steatosis and sinusoidal fibrosis was ascribed to parenteral hyperalimentation, and there was evidence of biliary obstruction. The major mesenteric vessels revealed no morphological changes.
Rejection
Patient 2: Gastrointestinal Tract, Pancreas, and Liver
The gastrointestinal tract, stomach, ileum, and colon were biopsied for the first time on day 24, 6 days after the patient had experienced a sharp and severe rise in bilirubin and had been treated empirically with OKT3 for rejection. The entire graft (the stomach, ileum, colon, and liver) showed evidence of mucosal rejection (Fig. 3). Lymphocytes were grown from a lymph node and the liver, and had strong reactivity against donor antigens.
FIGURE 3.
Intestinal and liver sections, patient 2, day 24. (a) Donor stomach; rejection. The gastric mucosa is tall, but the deep glands are being destroyed by a cellular infiltrate (arrows)× 120. (b) Donor liver; rejection. The portal area is expanded by a mixed cellular infiltrate rich in lymphocytes and eosinophils. The hepatic lobule is relatively spared. × 280. (c)Donor, terminal ileum; rejection. The mucosa is devoid of epithelium. A residual lymphoid follicle is recognizable. The lamina propria is populated by lymphoid cells. × 160. (d) Donor colon; rejection. Damaged and regenerating glands and surface epithelium are present, and an infiltrate separates the glands. ×160.
Frozen sections of the donor colon (Fig. 4) revealed, also on day 24, that the epithelium and most of the infiltrating cells expressed HLA-DR in large amounts. The predominant cells in the infiltrate were CD2 +, CD25 +, and CD4 > CD8 (after 6 days on OKT3), and the allospecific sera highlighted the fact that donor sera (A2, BW6) stained residual epithelial and endothelial cells, but the infiltrating lymphoid cells had the recipient phenotype (HLA-25/32 and HLA 24). Endoscopic biopsies of small bowel were performed 5 days later and, apart from more granulation tissue and macrophages, showed little difference from the preceding.
FIGURE 4.
Donor colon, frozen sections, patient 2, day 27. (a) Donor-specific antibody Bw6 stains epithelial and endothelial cells but not the cellular infiltrate. (b) Recipient specific antibody HLA 25/32 give a mirror image to (a); the infiltrating cells in the lamina propria are of recipient phenotype (c) HLA-DR staining reveals intense expression on epithelial as well as infiltrating cells. Surprisingly, some glands (arrow) are unstained. (d) Many of the infiltrating lymphoid cells bear CD25 (IL-2) receptors × 220.
The liver had previously been biopsied on day 18, when the cholangitis was subsiding. It did not reveal cellular rejection, although lymphocytes grown from this biopsy exhibited strong alloreactivity against donor antigens. Rebiopsy after 6 days of OKT3 therapy, however, revealed a classical picture of partially treated cellular rejection (Fig. 3b).
Frozen sections of liver revealed an increase in the intensity of HLA-DR and donor-specific HLA expression on bile duct cells from the first to the second biopsy, and an increase in CD2 + CD25 + infiltrating cells of recipient phenotype.
The pancreas was sampled postmortem and was intact but infiltrated extensively with the lymphoproliferative process to be described. It was not possible to detect cellular rejection in the presence of the heavy lymphoproliferative infiltrate.
Graft-versus-Host and Other Lymphoid Reactions
In neither patient was graft-versus-host disease documented. The recipient colon and transient skin rashes were biopsied on two occasions in patient 1, looking for evidence of graft-versus-host reaction. No morphologic features of graft-versus-host were found, and there was no epithelial damage to host epithelium; postmortem sampling did not disclose donor cells in the host colon or esophagus when the allospecific sera were used. Patient 2 had biopsies of the native colon at the same time that rejection was documented in the grafted colon. This material was used as control for the allospecific sera and failed to show donor cells or epithelial injury of any kind. Bone marrow was not included in the postmortem sampling permit and was not examined.
Mesenteric lymph nodes of donor origin were sampled at laparotomy because of their prominence on days 5, 13, 17, 24, and 33 in patient 2, and donor nodes from the mesentery were also sampled at the time of transplantation. The nodes revealed a progressive expansion of the paracortex, which pushed small follicles, devoid of germinal centers, up against the capsule (Fig. 5a). The center of the paracortical expansion was filled with large, pale immunoblasts with prominent nucleoli, lymphoblasts with pale cytoplasm, and many dendritic interdigitating cells best demonstrated with antibody to S100 (Fig. 5b). The periphery of the paracortical nodules had darker, large plasmacytoid cells. The medullary cords had plasma cells and lymphocytes, and the architectural landmarks were at all times clearly discernable. Frozen sections of lymph node on days 13, 17, and 24 showed that the paracortical expansion was predominantly T cell, CD2 + CD4 > CD8; almost all lymphoid cells were of recipient phenotype by day 13; only the cordal lining cells and endothelial cells preserved their donor HLA markers.
FIGURE 5.
Donor mesenteric node, patient 2, day 24. (a) Marked paracortical expansion compresses the small follicles against the capsule. Giemsa, ×160. (b) The exuberant proliferation of interdigitating dendritic cells is revealed in the paracortex by S-100 staining. × 440.
Lymphoproliferative Disease
Patient 1
Computed tomogram on day 91 revealed a Swiss-cheese appearance to the liver. Biopsy of the liver showed this to be a result of a cellular infiltrate composed almost entirely of plasmacytoid cells (Fig. 6). Frozen sections confirmed that kappa and lambda light chains were equally represented, and the cells stained for HLA-A3, not for Bw4, indicated that the proliferation was of probable host, not donor origin (Fig. 6b). UCH-1 staining cells, mainly T cells, were conspicuous between the immunoglobulin-producing cells (Fig. 6c). The process was thought to represent a polymorphous (polyclonal) lymphoproliferative reaction, and this was liable to involution.
FIGURE 6.
Lymphoproliferative disease, patient I. (a) Biopsy of the liver at 91 days reveals a fairly uniform population of cells that obscure all portal landmarks and spills over into the lobule. Giemsa, × 110. (b) The lymphoproliferative reaction was of recipient phenotype. Recipient specific antibody (R) HLA-A3 stains most cells, whereas the donor specific antibody (D) Bw4 leaves the cells unstained. × 200. (c) Although immunoglobulin cells predominate, T cells, as demonstrated by UCH-l, are well represented in the lesion. × 200. (d) The calcific rim is seen around the ghost cells of what appears to be infarcted lymphoproliferative disease at day 137. × 180.
EBV nuclear antigen was detected in frozen sections of liver biopsy, and EBV DNA was demonstrated using DNA probes. Serology had previously demonstrated a rise of EBV IgG capsid antigen from 1: 5 6 weeks before the diagnosis of lymphoproliferative disease, to 1: 160 3 weeks before diagnosis. IgM anti-EB viral capsid antigen was less than 1: 5 two weeks after diagnosis of the LPD.
Immune suppression was stopped for 15 days, and the lesions underwent necrosis by a process that appeared to be infarction. No host T cells were observed in the necrotic tissue (Fig. 6d). The lesions shrunk over the next month and calcified at the periphery. For the next 2 months only necrotic lymphoproliferative disease was seen on four successive biopsies, but at 5 months, the presence of active, viable lymphoproliferative disease coincided with the appearance of a new hilar lesion. The hilar lesion caused biliary obstruction; the obstruction became infected; the child became septic and died.
Postmortem sampling revealed the old, calcified lesions and the new hilar mass (Fig. 7a). The hilar mass consisted largely of infarcted liver, but at the edges and core were large blood vessels with a perivascular (angiocentric and angioinvasive) infiltrate (Fig. 7b, c). The angiocentric cells were not plasma-cytoid but pleomorphic (anaplastic) and could not be phenotyped. The lesion was thought to be related to the more typical LPD because the two processes merged in places, but the identity of the vascular lesions remains cryptic. EBV was not demonstrated in the angiocentric lesions, and the host phenotype could not be confirmed.
FIGURE 7.
Liver, postmortem, patient I. (a) A large juxtahilar mass consists in part of lymphoproliferative disease and infarction. (b) The presumably lymphoid proliferation is perivascular, angiocentric, and angioinvasive. × 150. (c) The wall of a hepatic artery is infiltrated and destroyed by the atypical cellular proliferation. × 350. (d) The cells did not react with B cell, T cell, or monocyte markers. S100 reveals a nerve, but the cells are unreactive. They are morphologically different from the plasmacytoid elements that were still recognizable elsewhere. × 660.
Sampling of other graft organs postmortem showed that the lymphoproliferative process was confined to the mass in the hilus, but it extended contiguously to a portion of colon adherent to the hilus and to the head of the pancreas, also in contiguity. In the pancreas, cells at the periphery of the reaction were plasmacytoid; those at the center had the anaplastic angiocentric appearance seen best in the hilar mass. A host lymph node was sampled and was uninvolved.
Lymphoproliferative Disease
Patient 2
On day 33 after transplantation, only two days after prior exploration that had revealed cellular rejection, biopsies of the donor intestine, liver, and lymph node revealed a diffuse lymphoproliferative disease.
Whereas the prior biopsies had shown epithelial destruction accompanied by a mixed cell infiltrate (Fig. 8a), the biopsy on day 33 had cohesive sheets of lymphoid cells with plasma cells, plasmacytoid cells, and large immunoblasts (Fig. 8b). There were few, if any, small lymphocytes, eosinophils, or neutrophils, and the cellular infiltrate now spilled over into the submucosa, obscuring all landmarks.
FIGURE 8.
Lymphoproliferative disease, patient 2. (a) Donor ileum, day 24; rejection. Severe cellular rejection is represented. Fragments of residual epithelium are seen, and a mixed cellular infiltrate is presented in the lamina propria, × 160. (b) Donor ileum, day 34; lymphoproliferative disease. The infiltrate is more exuberant, more monotonous, the cells are larger, and the infiltrate obscures anatomic boundaries such as the muscularis mucosae, × 160. (c) Ileum, day 34. Frozen section stained with donor specific antibody Bw6 stains endothelial cells and epithelial remnants, × 300. (d) An adjacent section stained with recipient-specific antibody HLA 25/32 highlights the infiltrating lymphoid cells. The pattern does not distinguish rejection (see Fig. 4) from the lymphoproliferative process, ×300.
Immunostains documented IgM in most cells and an equal distribution of kappa and lambda light chains. Allospecific sera showed the infiltrate to be of host phenotype (Fig. 8c, d); by this test the lymphoproliferative infiltrate was not distinguishable from rejection. Lymphocytes cultured from this biopsy still had marked antidonor activity, revealing that cellular rejection, although no longer morphologically recognizable, was still in progress.
Liver biopsy also differed from the previous specimen seen 9 days earlier in that a highly cellular portal infiltrate overran all architectural landmarks, spilling into the lobule, and the cells were the same as those seen in the gut. The lymph node differed from the one seen only 5 days before in that all architectural landmarks were obliterated by the diffuse proliferation (Fig. 9). Sinuses, cords, and follicles were no longer distinguishable. The cells in the lymph node marked as did those in the gut; IgM-predominated, kappa and lambda light chains were equally represented, and the infiltrate was of host phenotype.
FIGURE 9.
Lymphoproliferative disease; donor mesenteric lymph node, day 34, patient 2 (compare with day 24, Fig. 5). (a) Whereas the lymph node previously demonstrated an expanded paracortex, the lymph node architecture is now effaced by a diffuse lymphoid proliferation. × 120. (b) The proliferation is polymorphous; lymphocytes, plasma cells, plasmacytoid cells, and immunoblasts are represented. Giemsa, × 660. (c) Donor lymph node, day 34 (see Fig. 9). Most of the immunoglobulin-containing cells stain for IgM. Kappa and lambda chains were equally represented. Immunoperoxidase, × 160. (d) The lymphoproliferative process was not confined to donor organs, being seen here in the host kidney. × 280. (e) Recipient-specific antibody stains almost all infiltrating cells but not endothelium or sinus lining cells. Frozen section, immunoperoxidase. × 300. (f) Donor-specific antibody Bw6 stains sinus lining cells, endothelial cells, and occasional large (possibly dendritic) cells. × 300.
Sampling of host organs postmortem was limited to a lymph node and kidney. Diffuse infiltration by the lymphoproliferative process was present in both of these (Fig. 9). Donor and recipient lymph nodes were indistinguishable except for the greater amount of lipochrome and bile pigment in the host lymph nodes (Hamazaki-Wesenberg bodies).
EBV DNA was detected in large amounts in wide distribution in the node on day 33, but only in rare cells in the follicles on day 17 (Fig. 10). All other viral cultures, specifically for cytomegalovirus and adenovirus, were consistently negative in both patients.
FIGURE 10.
(a) Donor mesenteric lymph node at day 17. 35S label for EBV DNA is seen in a lymphoid follicle (arrow). (The large black object at center is artefact.) × 200. (b) Donor mesentric lymph node at day 34. There is now diffuse labeling for EBV DNA of many cells throughout the node. × 200.
DISCUSSION
Morphologic monitoring remains a mainstay after transplantation of various organs such as kidney or liver, even though there is an accumulated clinical experience that now stretches back over decades. When faced with an experimental clinical situation, such as the one we are describing, the pathologist is placed in an uncomfortable position; the transplant team is forced to resort to biopsy for clinical monitoring, yet there is no prior experience on which pathologists can base their diagnostic decisions. The 2 cases presented here, bolstered by functional studies in patient 2, have allowed us to describe the morphologic appearances of a number of clinical events.
The entire spectrum of graft rejection was represented. One patient had no documented rejection; the second had severe and profound cellular rejection involving intestine and to a lesser extent the liver. The biopsy diagnosis of rejection in the intestine was based on the following three criteria; the similarity of the rejection reaction to that described in other solid organs such as kidney and liver (11, 12), the similarity of the reaction to that described in animal models of small intestinal transplantation (13, 14), and by the confirmation obtained from the alloreactivity exhibited by lymphocyte cultures propagated from multiple liver biopsies and a single ileal biopsy (6).
Functional studies of lymphocytes grown from biopsies were used to gain confidence in the morphologic diagnosis of rejection. Although the cells that grow from the biopsies are a selected population of activated T cells, the correlation between morphology and antidonor reactivity of the alloreactive cells has been demonstrated in cardiac biopsies (15). Also, antidonor alloreactivity has been shown to predict rejection in a subsequent biopsy when the cells are grown from a biopsy that fails to reveal cellular rejection (16). This was demonstrated in the liver biopsy of patient 2 on day 17 when only resolving cholangitis was seen, but on day 24 classical rejection was observed. It also indicated to us, although we could not see it, that in the biopsy of the ileum demonstrating lymphoproliferative disease (Fig. 8), cellular rejection was occurring simultaneously.
The features of cellular rejection appear to include a cellular infiltration of the lamina propria with a mixed cell infiltrate; the effector cells are activated T-cells, CD2 + CD25 +, and intermixed with these are eosinophils, neutrophils, and monocyte-macrophages. HLA-DR hyperexpression is present on epithelium.
Teitelbaum et al. (14), using a rat model, make a number of important points that are pertinent here. Early villus destruction was seen after reperfusion, and this regenerates very rapidly within 2 days. When cellular rejection supervenes (day 6 in their model), the effect is seen first in the crypts with crypt cell damage leading to villus blunting and later, by 10 days, to mucosal destruction. Biopsy of the stomach in our patient 2 showed in similar fashion that the surface mucosa was quite intact but that the deep glands were severely damaged. Dilated lymphatics, which might have been anticipated because of interference with lymphatic connections, were never a feature. Teitelbaum et al. (14) warn that chronic ischemia, malnutrition, and enteritis in their model may produce histologic changes that mimic rejection. Caution in the interpretation of histologic changes is always prudent, but the characterization of the infiltrate as activated T cells, as in our patients, lends support to the diagnosis of rejection. Teitelbaum et al. (14) also point to the patchiness of their findings, suggesting that multiple biopsies should be performed at any procedure to prevent overlooking focal involvement. Our diagnostic biopsies in patient 2 were taken when the process was already advanced, so that changes were seen in all fragments, even though there was some variation in the amount of residual epithelium. This corresponds to the phase III rejection response described by Rosemurgy and Schraut (17), who were convinced that rejection in their rat model followed a defined and predictable morphologic course.
There has been debate over the adequacy of mucosal biopsy for the monitoring of small bowel rejection in animal models. Some authors claim that only full-thickness biopsy is reliable and that mucosal biopsies are unreliable (18, 19). Cohen et al. (20) found mucosal biopsies to be adequate, and Madara and Kirkman (21), in an elegant morphometric study, suggested that a mucosal microvascular lesion was the most characteristic. There does not seem to be an alternative to mucosal biopsy in humans, and the observations of Goulet et al. (3), as well as our own, reveal that mucosal biopsy, using the criteria previously described, is sufficient for the monitoring of rejection. The differential diagnosis of rejection from viral infection in a mucosal biopsy may still be difficult.
The effect of changes in immune suppression on the morphologic appearance of rejection, whether by augmentation of doses or by a change in the kinds of medications used, remains to be elucidated. We are mindful of the fact that our patients (or their grafts) had received, at one time or another, cyclosporine A, azathroprine, solumedrol, solucortef, antithymocyte globulin, OKT3, and irradiation.
Graft-versus-host disease (GVHD) has been of major concern since multiple visceral transplantation was first considered (22). In animal models of small bowel transplantation, the balance achieved between rejection on one hand, and graft-versus-host disease on the other is well demonstrated (23–26). Irradiation of the donor small bowel in rats abolished GVHD, but rejection was then more severe (27). In our first patient the graft was irradiated, there was no evidence of GVHD, and no rejection was demonstrated. In the second patient the graft was not irradiated, and there was no evidence of GVHD, but severe rejection occurred. It is likely that in humans, following multivisceral transplantation, GVHD and rejection can be modulated independently.
The definitive diagnosis of GVHD requires evidence of donor lymphoid engraftment and damage to host tissues. In the clinical situation, when there is no damage to host tissues (skin, native gastrointestinal tract, lung, or bone marrow), the pathologist may not pursue the search for cells of donor origin. Skin and esophagus showed no morphological evidence of GVH. We used allospecific sera on native colon and kidney to look for evidence of donor cell infiltration even in the face of a normal epithelium, but none was found. Donor engraftment in host lymph nodes and bone marrow was not sought in these 2 cases.
In the animal models of small bowel transplantation, rejection is associated with small and involuted lymph nodes, and graft-versus-host disease with the finding of large infiltrated mesenteric nodes (23–26). The morphologic pattern of paracortical expansion seen in successive samples of enlarging mesenteric nodes from patient 2, during periods of rejection, was associated with rapid replacement within 17 days of virtually all donor lymphoid cells by those of host origin. Only the sinusoidal lining cells, endothelial cells, and possibly some dendritic cells, retained the donor phenotype. We therefore interpreted the increase in paracortical cellularity in the mesenteric nodes as a hyperplastic reaction, much in the sense of a mixed lymphocyte culture, and not as a graft versus host reaction. Whether it represents the nodal manifestation of rejection we are not able to say, although propagated cells demonstrated strong antidonor reactivity.
Both patients developed a lymphoproliferative syndrome associated with EBV infection. In both instances the proliferation was polymorphous and plasmacytoid, polyclonal or polytypic by light chain assay (28), and confined to the liver in patient 1 but more systemic in patient 2. The puzzling nature of the terminal vasculitic and anaplastic lesion in patient 1 was never fully elucidated but may represent lymphomatous transformation of the LPD. We agree with Colby (29) that designating this angiocentric lymphomalike process as lymphomatoid granulomatosis is unhelpful in understanding its nature.
Lymphoproliferative disease was diagnosed on biopsy by the B-cell nature of the infiltrate, its plasmacytoid character, and by the absence of the pleocytotic infiltrate characteristic of rejection (28). The allospecific sera were of no assistance, since the lymphoproliferative reaction, like rejection, was of host origin. Likewise the lymphocyte cultures grown from the allograft biopsies taken at the time of active lmphoproliferative disease still showed some anti-donor activity indicating that the lymphoproliferative disease masks some ongoing cellular rejection. A similar event is described by Williams et al. (2), although the lymphoproliferative disorder in their multivisceral recipient was monoclonal.
Resolution of the lymphproliferative disorder in patient 1 was by a process that appears to be infarction of the lymphoid tissue. A similar process is observed in lymphoproliferative disorders involving Waldeyer’s ring in which extensive necrosis of tissue is common, often associated with thrombosis in high-walled venules (28). A small amount of venous thrombosis was seen in the liver. We assume that vascular endothelial swelling and proliferation, which is prominent during active EBV infection, involutes and regresses with thrombosis and tissue infarction as the viral infection passes. Local monokines may regulate these vascular effects, but the morphology of the involuting lesion does not support the idea that host cytotoxicity plays a major role. There is very little inflammatory response to the infarcted lymphoid proliferation, and regression is by granulation and macrophage activity from the periphery.
The intestine loses its impermeability to bacteria after being subjected to the ischemia associated with transplantation (30). Bacterial translocation, as the process is referred to, involves migration of endogeneous flora across the now permeable bowel into the lacteals then the blood. The same microorganisms that are found in the blood of these patients are found 1 or 2 days in advance in the stool (1). In general the bacterial translocation was not associated with clinical sepsis other than the bacterial cholangitis early on in patient 1. This was an unexpected finding, since the multivisceral operation, unlike a liver transplant, does not involve a biliary anastomosis.
There are a number of issues that remain unresolved. We are able, we believe, to recognize the broad outlines of the immunologic processes, rejection, graft-versus host disease, and the complication of lymphoproliferative disease. When these processes are present simultaneously we may not be able to determine the relative proportions or importance of each. We do not know the effect of the various immunologic manipulations on the tissue expression; for example the administration of OKT3, steroid bolus therapy, azathioprine, and changes in cyclosporine levels may have effects that we have not anticipated. Numerous other drugs are given in concert, antibiotics, antiviral agents, antihypertensives, and these may have toxic as well as their anticipated therapeutic effects. We were able to detect hyperalimentation effect in the liver in patient 1. Superimpose on the above the effects of the bacteremias that follow bacterial translocation, and the pathologist has a formidable task.
We recommend that protocol sampling of the various organs be part of the monitoring of experimental transplantation procedures; this is a learning process for everyone concerned, not least the pathologist.
Footnotes
The Children’s Hospital of Pittsburgh Histology Laboratory Supervisor, Helen Baginski, and staff handled unreasonable demands with their usual excellence. Our fellows came, often at midnight, to process tissue specimens: Kathryn Neilson, M.D., Deborah Schofield, M.D., Elizabeth Mroczek, M.D., Eduardo Ruchelli, M.D., and Susan Kennedy, M.D. Eduardo Yunis, M.D., and A. Jake Demetris, M.D. are my sounding boards. Joseph Locker, M.D., Ph.D., provided the EBV probe. Research in transplantation pathology is supported in part by the Pathology Education and Research Foundation, Pittsburgh, Pennsylvania.
Contributor Information
Ronald Jaffe, Department of Pathology, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Jonathan D. K. Trager, Department of Pathology, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Adriana Zeevi, Department of Pathology, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Eni Sonmez-Alpan, Department of Pathology, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Rene Duquesnoy, Department of Pathology, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Satoru Todo, Department of Surgery, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Marc Rowe, Department of Surgery, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
Thomas E. Starzl, Department of Surgery, University of Pittsburgh School of Medicine and Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania 15213.
References
- 1.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–57. [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JS, Sankary HN, Foster PF, Lowe J, Goldman GM. Splanchnic transplantation: An approach to the infant dependent on parenteral nutrition who develops irreversible liver disease. JAMA. 1989;261:1458–62. doi: 10.1001/jama.261.10.1458. [DOI] [PubMed] [Google Scholar]
- 3.Goulet OJ, Révillon Y, Cerf-Bensussan N, et al. Small intestinal transplantation in a child using cyclosporine. Transpl Proc. 1988;20:288–96. [PubMed] [Google Scholar]
- 4.Cutz E, Rhoads JM, Drumm B, Sherman PM, Durie PR, Forstner GG. Microvillus inclusion disease: An inherited defect of brush border assembly and differentiation. N Engl J Med. 1989;320:646–51. doi: 10.1056/NEJM198903093201006. [DOI] [PubMed] [Google Scholar]
- 5.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Trager JD, Zeevi A, Duquesnoy RJ, Jaffe R, Rowe MI, Todo S, Starzl TE. Functional characteristics of lymphocytes infiltrating a human multivisceral allograft. Submitted for publication. [PMC free article] [PubMed] [Google Scholar]
- 7.Lake BD. Microvillus inclusion disease: Specific diagnostic features shown by alkaline phosphatase histochemistry. J Clin Pathol. 1988;41 :880–2. doi: 10.1136/jcp.41.8.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordell JL, Falini B, Erben WN, et al. Immunoenzymatic labelling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (AP-AAP complexes) J Histochem Cytochem. 1984;32:219–29. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 9.Unger ER, Budgeon LR, Myerson D, Brigati DJ. Viral diagnosis by in-situ hybridization: Description of a rapid simplified colormetric method. Am J Surg Pathol. 1986;10:1–8. doi: 10.1097/00000478-198601000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Weiss LM, Movahed LA. In situ demonstration of Epstein-Barr viral genomes in viral-associated B cell lymphoproliferations. Am J Pathol. 1989;134 :651–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Porter KA. Renal transplant. In: Heptinstall RH, editor. Pathology of the Kidney. 3. Boston: Little, Brown; 1983. pp. 1455–547. [Google Scholar]
- 12.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric posttransplant liver pathology. Pathol Annu. 1987;22(pt.2):347–86. [PubMed] [Google Scholar]
- 13.Kirkman RL. Small bowel transplantation. In: Moms PJ, editor. Progress in Transplantation. London: Churchill Livingstone; 1987. pp. 31–75. [Google Scholar]
- 14.Teitelbaum DH, Wise WE, Sonnino RE, Dunaway DJ, Powers P, McClung HJ, Harmel RP., Jr Monitoring of intestinal transplant rejection. Am J Surg. 1989;157:318–22. doi: 10.1016/0002-9610(89)90561-8. [DOI] [PubMed] [Google Scholar]
- 15.Fung JJ, Zeevi A, Markus B, Zerbe TR, Duquesnoy RJ. Dynamics of allospecific T lymphocyte infiltration in vascularized human allografts. Immunological Research. 1986;5:149–63. doi: 10.1007/BF02917589. [DOI] [PubMed] [Google Scholar]
- 16.Weber T, Kaufman C, Zeevi A, et al. Lymphocyte growth from cardiac allograft biopsy specimens with no or minimal cellular infiltrates: Association with subsequent rejection episode. J Heart Transplant. 1989;8:233–40. [PubMed] [Google Scholar]
- 17.Rosemurgy AS, Schraut WH. Small bowel allografts. Sequence of histologic changes in acute and chronic rejection. Am J Surg. 1986;151:470–5. doi: 10.1016/0002-9610(86)90106-6. [DOI] [PubMed] [Google Scholar]
- 18.Millard PR, Dennison A, Hughes DA, Collin J, Morris PJ. Morphology of intestinal allograft rejection and the inadequacy of mucosal biopsy in its recognition. Br J Exp Pathol. 1986;67:687–98. [PMC free article] [PubMed] [Google Scholar]
- 19.Banner B, Dean P, William J. Morphologic features of rejection in long-surviving canine small bowel transplants. Transplantation. 1988;46:665–9. doi: 10.1097/00007890-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Cohen Z, Wassef R, Langer B. Transplantation of the small intestine. Surg Clin North Am. 1986;66:583–8. doi: 10.1016/s0039-6109(16)43941-1. [DOI] [PubMed] [Google Scholar]
- 21.Madara JL, Kirkman RL. Structural and functional evolution of jejunal allograft rejection in rats and the ameliorating effects of cyclosporine therapy. J Clin Invest. 1985;75:502–12. doi: 10.1172/JCI111726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–29. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen Z, MacGregor AB, Moore KT, Falk FE, Langer B, Cullen JB. Canine small bowel transplantation: A study of the immunological responses. Arch Surg. 1976;111:248–53. doi: 10.1001/archsurg.1976.01360210042008. [DOI] [PubMed] [Google Scholar]
- 24.Schraut WH, Lee KK, Davson PJ, Hurst RD. GVHD induced by small bowel allografts; clinical course and pathology. Transplantation. 1986;41 :286–90. doi: 10.1097/00007890-198603000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Deitz E, Ulrichs K, Schack T, Friedrichs B, Müller-Hermelink GK, Thiede A. Graft-versus-host reaction in small bowel transplantation and possibilities for its circumvention. Am J Surg. 1986;151 :379–85. doi: 10.1016/0002-9610(86)90473-3. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara H, Raju S, Grogan JB, Lewin RJ, Johnson WW. Total orthotopic small bowel allotransplantation in the dog: Features of atypical rejection and graft-versus-host reaction. Transplantation. 1987;44:747–54. doi: 10.1097/00007890-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Saat RE, deBruin RW, Marquet RL, Jeekel J. Total orthotopic allogeneic small bowel transplantation in rats: Attempts to ameliorate the graft-venus-host disease by irradiation and transfusion of the donor. Transplantation. 1989;47:451–3. [PubMed] [Google Scholar]
- 28.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Colby TV. Central nervous system. Lymphoid granulomatosis in AIDS. Hum Pathol. 1989;20:301–2. doi: 10.1016/0046-8177(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 30.Toledo-Pereyra LH, Raij L, Simmons RL, Najarian JS. Role of endotoxin and bacteria in long-term survival of preserved small bowel allografts. Surgery. 1974;73:474–81. [PubMed] [Google Scholar]