Tacrolimus has been shown to be an effective and well tolerated agent in primary solid organ transplantation.1–3 We have previously reported that an additional important feature of this drug is its ability to salvage renal allografts in patients experiencing ongoing acute rejection resistant to conventional antirejection therapy.4,5 Salvage can be defined as a return to a defined percentage of baseline renal function or histologic reversal of acute rejection, or both. In our initial experience with 77 patients who developed ongoing rejection while receiving cyclosporine-based immunosuppression, 57 (74%) had a successful outcome after conversion to tacrolimus after a follow-up of 13.9 ± 9.1 months.4
A total of 169 patients (median age 33 years; range 2 to 75 years; 98 males, 71 females) with ongoing rejection after failing baseline cyclosporine therapy have now been converted to tacrolimus in an attempt to reverse rejection.6,7 We herein report the long-term follow-up in this group of patients.
PATIENTS
Indications for conversion to tacrolimus were ongoing biopsy-confirmed rejection in all patients after ruling out other causes of allograft dysfunction, as previously described.4,5 There were 138 (82%) primary transplant patients, and 31 (18%) had undergone retransplantation; 132 (78%) received cadaveric renal transplants and 37 (22%) received grafts from living donors. The median time to tacrolimus conversion was 2 months (range 2 days to 55 months; mean 4.3 ± 2.6 months) after transplantation. Conversion was undertaken 12 hours after the last dose of cyclosporine, and cyclosporine levels continued to be monitored for 1 week. The initial dose of tacrolimus was 0.25 to 0.30 mg/kg/d to achieve trough blood levels of 20 to 25 ng/mL in week 1.20 ng/mL by week 2.15 ng/mL by week 4.10 to 15 ng/mL between weeks 5 to 12, and 10 ng/mL thereafter. Of the 169 patients, 144 (85%) had received at least one course of antilymphocyte therapy (OKT3: n = 89; antilymphocyte globulin [ALG] or antithymocyte globulin [ATG]: n = 4: OKT3 and ALG/ATG: n = 51) plus high-dose corticosteroid therapy prior to conversion. Forty-seven of these patients had received two or more courses of OKT3. All other patients (n = 25) had failed high-dose corticosteroid therapy alone. Twenty-eight patients (17%) were dialysis-dependent at the time of conversion owing to the severity of rejection.
RESULTS
Renal Function
At a mean follow-up of 30.0 ± 2.4 months (median 36.5, range 12 to 62 months), tacrolimus therapy was successful in 125 of 169 (74%) patients, who continue to have functioning grafts with a mean serum creatinine level of 2.3 ± 1.1 mg/dL (Fig 1). Renal function had significantly improved (P < .01) compared with preconversion values as early as 1 month after initiation of tacrolimus therapy, and serum creatinine levels remained significantly reduced throughout the follow-up period. There was no significant difference in success rates between those converted from dual therapy (cyclosporine/prednisone, 67% success) or triple therapy (cyclosporine/azathioprine/prednisone, 77% success). Similarly, renal function at the time of conversion (excluding the 28 patients on dialysis) had no significant effect on successful outcome. Successful conversion was achieved in 84% of the 81 patients with preconversion serum creatinine levels <3 mg/dL and in 73% of the 60 patients with levels ≥3 mg/dL.
Fig 1.
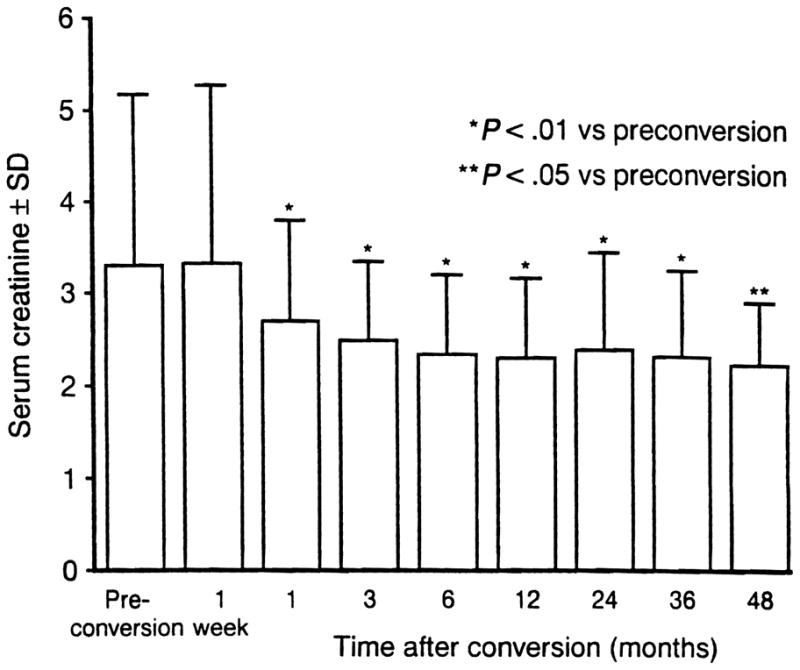
Renal function after tacrolimus conversion.
Of the 144 patients treated with antilymphocyte preparations prior to conversion, 117 (81%) were successfully salvaged with tacrolimus. Furthermore, of the 28 patients who were dialysis-dependent at the time of conversion, 13 (46%) had functioning grafts (mean serum creatinine 2.15 ± 0.37 mg/dL) at a mean follow-up of 37.3 ± 16.7 months.
Conversion Interval
One of the factors that significantly affected outcome was the time to conversion, with a higher chance of success in those converted early. The mean time to conversion was 4.3 ± 2.6 months after transplantation (median 2 months, range 2 days to 55 months). Patients who were converted to tacrolimus within the first 6 months after transplantation had a significantly higher success rate than those converted later than 6 months (77% versus 50% success; P = .006).
Type of Rejection
Analysis of the 169 patients according to preconversion biopsy diagnosis of rejection revealed successful conversion in 77% of the 91 patients with acute cellular rejection alone, 75% of the 62 patients with acute cellular rejection with vascular involvement, and 50% of the 16 patients with acute cellular rejection and primary nonfunction. Although the latter group fared the worst, there was no statistically significant difference in outcome compared with the other two groups.
Concomitant Therapy
In the 125 patients successfully converted to tacrolimus, prednisone doses were reduced from a mean of 28.0 ± 9.0 mg/d (range 4 to 60 mg/d) before conversion to 8.5 ± 4.1 mg/d (range 2.5 to 20 mg/d) postconversion. A total of 28 of 125 (22%) patients are currently receiving no corticosteroids, and the dose was reduced in a further 87 (70%) patients. Furthermore, in the 87 patients who were receiving azathioprine preconversion, 39 (45%) are no longer receiving this agent, and the dose has been reduced in a further 24 patients. The mean dose of tacrolimus in the successful cases was 19.3 ± 9.1 mg/d at the time of conversion, which was reduced to 11.3 ± 6.8 mg/d (P < .01) at the latest follow-up.
High-Versus Low-Risk Patients
Treatment of ongoing rejection was found to be effective in high-risk (n = 99) as well as low-risk patients (n = 70). High-risk patients included those on dialysis at the time of conversion, patients with baseline serum creatinine levels >5.0 mg/dL, those who had undergone >2 retransplants, patients >6 months posttransplant at the time of conversion, and pediatric patients (<13 years). As expected, there were more retransplantations and a trend toward reduced 3-year graft survival in the high-risk group (Table 1), but given the high-risk circumstances at the time of conversion, this outcome can be regarded as very acceptable compared with the low-risk group.
Table 1.
Outcome After Conversion to Tacrolimus in High- and Low-Risk Patients
| Low-Risk (n = 70) | High-Risk (n = 99) | Total (n = 169) | |
|---|---|---|---|
| Retransplantation | 6 (9%) | 25 (25%) | 31 (18%) |
| Patient survival (%) | |||
| 6 months | 96 | 95 | 95 |
| 1 year | 96 | 94 | 95 |
| 3 years | 96 | 93 | 94 |
| Graft survival (%) | |||
| 6 months | 87 | 76 | 80 |
| 1 year | 84 | 70 | 76 |
| 3 years | 83 | 68 | 74 |
| Cytomegalovirus infection (%) | 2 | 5 | 4 |
DISCUSSION
‘Rescue’ or ‘conversion’ therapy from one baseline immunosuppressive regimen to another to salvage grafts with ongoing rejection is a new concept since the cyclosporine era. The aims of optimal conversion therapy should be to preserve renal function, maintain patient safety, avoid overimmunosuppression, and achieve satisfactory long-term graft outcome. Since our initial reports of successful treatment of rejecting renal allografts with tacrolimus, other single- and multicenter studies have confirmed our original observations.8–11 This long-term experience demonstrates that tacrolimus has sustained efficacy as conversion therapy for ongoing renal allograft rejection with salvage rates identical to those reported earlier in a smaller group of patients and is effective in both low- and high-risk patients. Furthermore, corticosteroid tapering and tacrolimus monotherapy are achievable over the long-term. Based on these data, we recommend that conversion to tacrolimus be performed prior to increasing conventional antirejection treatment of renal transplants under primary cyclosporine-based therapy.
References
- 1.Starzl TE, Fung J, Jordan ML, et al. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 2.Pirsch JD, Miller J, Deierhoi MH, et al. Transplantation. 1997;63:977. doi: 10.1097/00007890-199704150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro R, Jordan ML, Scantlebury V, et al. Transplantation. 1995;59:485. doi: 10.1097/00007890-199559040-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan ML, Shapiro R, Vivas C, et al. Transplantation. 1994;57:860. doi: 10.1097/00007890-199403270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan ML, Shapiro R, Jensen CWB, et al. Transplant Proc. 1991;23:3078. [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan ML, Naraghi R, Shapiro R, et al. Transplantation. 1997;63:223. doi: 10.1097/00007890-199701270-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan ML, Naraghi R, Shapiro R, et al. Transplant Proc. 1998;30:1257. doi: 10.1016/s0041-1345(98)00233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felldin M, Backman L, Brattstrom C, et al. Transpl Int. 1997;10:13. doi: 10.1007/BF02044336. [DOI] [PubMed] [Google Scholar]
- 9.Woodle ES, Thistlethwaite JR, Gordon JH, et al. Transplantation. 1996;62:594. [PubMed] [Google Scholar]
- 10.Mathew A, Talbot D, Minford EJ, et al. Transplantation. 1995;60:1182. doi: 10.1097/00007890-199511270-00022. [DOI] [PubMed] [Google Scholar]
- 11.Woodle ES, Jordan ML, Facklam D, et al. Transplant Proc. 1998;30:1297. doi: 10.1016/s0041-1345(98)00249-8. [DOI] [PubMed] [Google Scholar]


