Abstract
A recent theory of pigeons' equivalence-class formation (Urcuioli, 2008) predicts that reflexivity, an untrained ability to match a stimulus to itself, should be observed after training on two “mirror-image” symbolic successive matching tasks plus identity successive matching using some of the symbolic matching stimuli. One group of pigeons was trained in this fashion; a second group was trained similarly but with successive oddity (rather than identity). Subsequently, comparison-response rates on novel matching versus mismatching sequences with the remaining symbolic matching stimuli were measured on nonreinforced probe trials. Higher rates were observed on matching than on mismatching probes in the former group. The opposite effect—higher rates on mismatching than matching probes—was mostly absent in the latter group, despite being predicted by the theory. Nevertheless, the ostensible reflexivity effect observed in former group may be the first time this phenomenon has been demonstrated in any animal.
Keywords: reflexivity, emergent oddity, stimulus equivalence, stimulus classes, successive matching, pigeons, key peck
Human subjects explicitly trained on A–B and B–C arbitrary or symbolic conditional discriminations (where the letters of each pair denote sets of sample and comparison stimuli, respectively) will often subsequently exhibit a variety of untaught or emergent performances (e.g., Sidman & Tailby, 1982). For example, after such training, subjects can match the A samples to the C comparisons (transitivity = A–C matching). They can also do the reverse of what they had explicitly learned: matching B samples to A comparisons and C samples to B comparisons (symmetry = B–A and C–B matching, respectively). Finally, they can match each stimulus to itself (reflexivity: A–A, B–B, and C–C matching). These results, when obtained, demonstrate that subjects have learned that the respective A, B, and C stimuli “go together”, as evidenced by their interchangeability with one another (Dougher & Markham, 1996; Sidman, 1992; Spradlin & Saunders, 1986; Zentall, 1996, 1998). Stated otherwise, conditional discrimination training has yielded n-member stimulus categories or equivalence classes.
Investigations of emergent effects have been conducted with nonhuman populations as well (Lionello-DeNolf, 2009; Schusterman & Kastak, 1993; Urcuioli, 2008; Zentall, Wasserman, Lazareva, Thompson, & Rattermann, 2008), prompted in part by questions regarding the origin(s) of such effects: Do they require the capacity for language or are they simply a product of reinforcement and discrimination (Horne & Lowe, 1996, 1997; Sidman, 2000)? Moreover, researchers have long recognized that a comprehensive account of behavior must be able to explain why “… a stimulus will sometimes evoke a reaction with which it has never been associated” (Hull, 1939, p. 353) and that “…physically dissimilar stimuli can have similar and apparently interrelated effects on behavior” (McIlvane, 1992, pp. 76–77).
The literature on categorization and stimulus-class formation (cf. Zentall et al. 2008) clearly shows that non-language-capable animals exhibit certain emergent effects like the phenomenon of acquired equivalence in which stimuli occasioning the same reinforced response or associated with a common (albeit distinctive) reinforcer become interchangeable with one another in new contexts (e.g., Astley & Wasserman, 1999; Spradlin, Cotter, & Baxley, 1973; Urcuioli, Zentall, Jackson-Smith, & Steirn, 1989). For example, pigeons taught to make the same reinforced comparison response to two dissimilar samples in two-alternative matching-to-sample and, later, a new comparison response to just one of those samples, preferentially make that new response to the remaining sample (e.g., Urcuioli et al., 1989, Experiment 2; Urcuioli & Lionello-DeNolf, 2005; Wasserman, DeVolder, & Coppage, 1992; see also Bovet & Vauclair, 1998; Delamater & Joseph, 2000; Honey & Hall, 1989; von Fersen & Delius, 2000). Echoing Hull's (1939) comment, the “remaining” (tested) sample evokes a response with which it was never associated.
Despite demonstrations of this sort, some have suggested that nonhuman animals may lack the capacity for other emergent effects like the aforementioned three that define stimulus equivalence (Horne & Lowe, 1996; Saunders, Williams, & Spradlin, 1996). One reason for this suggestion is the relative paucity of nonhuman animal data demonstrating some or all of these effects (e.g., D'Amato, Salmon, Loukas, & Tomie, 1985; Dugdale & Lowe, 2000; Sidman, Rauzin, Lazar, Cunningham, Tailby, & Carrigan, 1982; Yamamoto & Asano, 1995; although see Schusterman & Kastak, 1993). Another is that the hypothesized process used to explain acquired equivalence—viz., mediated or secondary generalization (Hall, Mitchell, Graham, & Lavis, 2003; Hull, 1939; Urcuioli & Lionello-DeNolf, 2001)—appears to be limited in what emergent effects it can support (Saunders et al., 1996). These points lend credence to the position that there are fundamental human versus nonhuman differences in categorization (Devany, Hayes, & Nelson, 1986; Dugdale & Lowe, 1990; Hayes, 1989; Horne, Hughes, & Lowe, 2006).
Recently, however, there have been two compelling demonstrations of symmetry not explicable in any straightforward fashion by mediated generalization. Using multidimensional, color clip-art stimuli, Frank and Wasserman (2005) found that concurrently training pigeons on A–B symbolic matching and two identity tasks involving the symbolic matching stimuli (i.e., A–A and B–B matching) yielded the symmetrical (B–A) relations. A notable feature of their study was the use of successive (rather than n-alternative) matching. In successive matching, only one comparison appears after each sample, with both appearing at the same spatial location for extended periods of time (Wasserman, 1976; see also Cullinan, Barnes, & Smeets, 1998). Some sample–comparison sequences end in reinforcement, others do not, and comparison-response rates are the main dependent measure. Learning is apparent when comparison-response rates are substantially higher on reinforced than on nonreinforced trials. After acquiring all baseline conditional relations, Frank and Wasserman's pigeons were given infrequent, nonreinforced probe trials involving sample–comparison sequences that were the reverse (B–A) of the explicitly trained symbolic (A–B) ones. Symmetry was evident in the fact that comparison-response rates were higher on the reverse of the reinforced training relations than on the reverse of the nonreinforced training relations.
Urcuioli (2008, Experiment 3) replicated these findings using simple color stimuli (homogeneous red and green hues) and forms (white inverted triangle and horizontal lines on black backgrounds) as the A and B sets of stimuli. In addition, Urcuioli (2008, Experiment 2) showed that symmetry did not emerge after similar concurrent training using two-alternative matching (see also Lionello-DeNolf & Urcuioli, 2002; Lipkens, Kop, & Matthijs, 1988; Sidman et al., 1982). These contrasting results led Urcuioli (2008) to propose that the continual juxtaposition of nonreinforced with reinforced sample–comparison sequences throughout successive matching training generates stimulus classes which contain the elements of the latter (reinforced) sequences. Class formation and differentiation are ostensibly facilitated in successive matching because half of all trials end without reinforcement independently of the subject's level of learning or performance. Thus, if red–horizontal and green–triangle sequences never end in reinforcement, but red–triangle and green–horizontal sequences always do, the red sample and triangle comparison become members of one class and the green sample and horizontal comparison become members of a separate class.
Urcuioli's (2008) theory also proposes that the functional matching stimuli consist of their nominal (e.g., visual) features plus their temporal or ordinal position within a trial1. This assumption captures the idea that pigeons discriminate whether a particular stimulus serves as a sample or as a comparison. Consequently, a red sample–triangle comparison sequence should be represented as R1–T2, where each number designates the ordinal position of each stimulus within a trial. Finally, the theory assumes that classes sharing a common member merge. Combined with the other assumptions, this helps to explain why concurrent A–A and B–B identity training is crucial for obtaining symmetry (Frank 2007; Frank & Wasserman, 2005). For instance, if the identity sequences of red sample–red comparison and triangle sample–triangle comparison are reinforced in training along with the red sample–triangle comparison symbolic sequence, the resulting three stimulus classes—[R1, T2], [R1, R2], and [T1, T2]—have certain elements in common: namely, R1 (a member of each of the first two classes) and T2 (a member of the first and third classes). Class merger should thus yield the 4-member class [R1, R2, T1, and T2]. This larger class contains the elements of each reinforced baseline sequence (e.g., R1–T2) as well as the untrained symmetrical sequence, triangle sample–red comparison (T1–R2). If pigeons respond more to a comparison in the same class as its preceding sample, comparison-response rates should be higher on the symmetrical versions of the reinforced baseline relations (e.g., T1–R2) than on the symmetrical versions of the nonreinforced baseline relations (e.g., H1–R2), precisely what Urcuioli (2008, Experiment 3) and Frank and Wasserman (2005, Experiment 1) observed.
Urcuioli's (2008) theory predicts other emergent relations, too, such as reflexivity, the untrained ability to match a stimulus to itself. The current experiment was designed to test for reflexivity by training a set of baseline relations that, according to the assumptions described above, should yield this emergent relation. Specifically, pigeons were concurrently trained on two “mirror-image” symbolic successive matching tasks (A–B and B–A) plus identity (B–B) matching. Afterwards, they were tested on the untrained A–A relations. The theory predicts that comparison-response rates should be higher on matching test trials (i.e., trials on which an A comparison is nominally identical to the preceding A sample) than on nonmatching test trials (i.e., trials on which an A comparison differs from the preceding A sample). A second, control group of pigeons was also run to evaluate the possibility that training the two mirror-image symbolic tasks might be sufficient to yield the same pattern of test results. For this group, A–B and B–A symbolic training was accompanied by concurrent training on successive B–B oddity in which comparison responding was reinforced only when a B comparison did not match the preceding B sample. If A–B and B–A training suffices to yield A–A reflexivity, pigeons in this group should also respond relatively more in testing to an A comparison that matches the preceding A sample. By contrast, the theory predicts precisely the opposite pattern of results— namely, relatively more responding to an A comparison that differs from the preceding A sample (i.e., emergent oddity).
METHOD
Subjects
Subjects were 12 male White Carneau pigeons from the Palmetto Pigeon Plant (Sumter, SC). Four were experimentally naïve, and the remaining 8 had two-alternative forced-choice experience on tasks unrelated to the successive matching contingencies of this experiment. Prior to the start of the experiment, they were randomly divided into two groups of 6, equated for numbers of experimentally naïve and experienced pigeons. The pigeons were housed individually in stainless-steel, wire-mesh cages in a colony room on a 14h–10h light–dark cycle with lights on at 07:00. They were kept at 80% of their free-feeding weights during their experimental participation by adjusting reinforcement durations across sessions as needed. Access to food was limited to the experimental sessions except on the one day/week they were not run. Pigeons had continuous access to water and grit in the home cages.
Apparatus
The experiment was run in two standard operant chambers (BRS/LVE, Laurel MD) with Model PIP-016 three-key response panels inside Model SEC-002 enclosures. Only the 2.5-cm-diameter center response keys were used. These keys were illuminated via back-mounted, inline projectors (Model IC-901-IDD) that could display a white homogeneous field, an inverted white triangle on a black background, three white horizontal lines on a black background, and red and green homogeneous hues (BRS/LVE Pattern 692). A GE No. 1829 bulb mounted 7.6 cm above the center key served as the house light. The bulb was partially covered so that its light was directed toward the chamber ceiling. Access to the food hopper was through a 5.8 cm × 5.8 cm opening in the response panel, located approximately 13 cm below the center key. A miniature bulb (ESB-28) illuminated the food hopper when elevated. A blower fan attached to the outside of each experimental chamber provided ventilation and also masked extraneous noise. IBM-compatible 386 computers were interfaced to each box and controlled stimulus presentation and recording of all experimental events.
Procedure
Preliminary training
After shaping to peck at an illuminated (white) center key and eat out of the food hopper, all birds received reinforcement for single pecks to the center-key stimuli that would later appear in successive matching. These 60-trial sessions consisted of either form (triangle and horizontal lines) or hue (red and green) trials, each stimulus appearing equally often and in randomized order in a session. After two sessions with each stimulus set, pigeons received four sessions of fixed interval (FI) training with the form center-key stimuli followed by four additional FI training sessions with the hue center-key stimuli. These sessions also lasted 60 trials and involved a gradual increase in the FI value across sessions: one session of FI 2 s, one of FI 3 s, and two of FI 5 s. The intertrial interval (ITI) in all sessions was 15 s. During FI training, the first 14 s of the ITI was spent in darkness. The house light then came on for the last 1 s of the ITI and remained on until the end of the reinforcement cycle.
Successive matching acquisition
Next, pigeons began concurrent training on three successive matching discriminations. Each matching trial consisted of a two-stimulus sequence on the center key. A single peck to the first (sample) stimulus initiated a FI 5-s schedule ending with the offset of the sample, a 500-ms blank interval, and then onset of the second (comparison) stimulus. For reinforced sequences, the first peck to the comparison stimulus after 5 s turned off the comparison and produced access to food for a duration constant within a session but varying from 1.8 to 6.0 s across sessions as needed. For nonreinforced sequences, the comparison and the house light automatically went off after 5 s. The next trial then commenced following a 15-s ITI during which the house light was off. The house light came on 1 s prior to appearance of the sample stimulus and remained on until the end of the reinforcement cycle (reinforced sequences) or comparison offset (nonreinforced sequences).
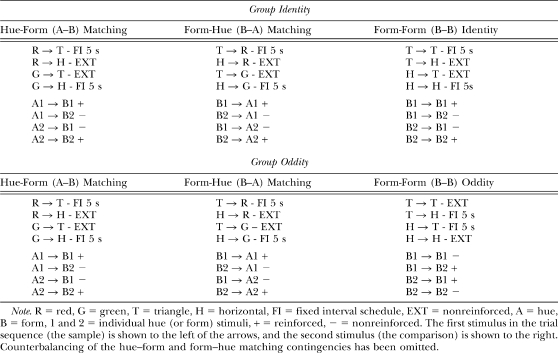
Table 1 shows the three successive matching discriminations for the two groups (Identity and Oddity). Both were trained on hue–form (A–B) and form–hue (B–A) symbolic matching in which the nominal samples for one task served as the nominal comparisons for the other and vice versa. Moreover, the reinforcement contingencies for these two sets of baseline relations were the mirror images of one another. Specifically, for half of the pigeons in each group, responding to the triangle comparison after the red sample (R→T) and to the horizontal-lines comparison following the green sample (G→H) was reinforced on a FI 5-s schedule in the hue–form (A–B) task. Likewise, responding to the red comparison after the triangle sample (T→R) and to the green comparison after the horizontal sample (H→G) was reinforced in the form–hue (B–A) task. Conversely, responding to the horizontal comparison after a red sample (R→H) and to the triangle comparison after a green sample (G→T) was not reinforced (EXT) as was responding to the red comparison after the horizontal sample (H→R) and to the green comparison after the triangle sample (T→G). Shown below these contingencies is the corresponding equivalence notation in which A and B denote the hue and form stimuli, respectively, 1 and 2 denote individual stimuli within each set, and “+” and “−” indicate reinforced and nonreinforced trials, respectively. For the other half of the pigeons in each group, these symbolic contingencies were reversed (not shown).
Table 1.
Successive Matching Training Contingencies for the Two Groups.
The groups differed on their form–form (B–B) training trials. For Group Identity, comparison responding in the B–B task was reinforced only when a form comparison was nominally identical to the preceding form sample (viz., on T→T and H→H trials). For Group Oddity, comparison responding was reinforced only when a form comparison differed from the preceding sample (viz., on T→H and H→T trials).
Acquisition sessions contained 96 trials divided equally among these three baseline tasks. The 12 possible sample-comparison sequences appeared eight times in random order in each session, with the limitation that none occur more than twice in a row. Acquisition of each task was measured using a discrimination ratio (DR) which was computed by dividing the total number of pecks to the comparisons on reinforced trials by the total number of comparison pecks on both reinforced and nonreinforced trials. Only pecks during the first 5 s of each comparison presentation were recorded. The DR is approximately 0.50 when there is little or no discrimination between reinforced and nonreinforced sequences (i.e., when comparison response rates are roughly the same on reinforced and nonreinforced trials). As a task is acquired, the DR approaches 1.0 (i.e., most or all comparison responding is confined to the reinforced trials). The acquisition criterion was a DR ≥ .80 on all three matching tasks for five of six consecutive sessions. If pigeons reached this level of performance for only one (or two) of the tasks, concurrent training on all three continued until the DRs for the remaining task(s) also met or exceeded .80. A minimum of 10 sessions of overtraining followed the last session at criterion and ended when the same criterion was met for 5 of the last 6 overtraining sessions. One pigeon in Group Oddity was dropped from the experiment because it did not meet criteria after 138 training sessions.
Reflexivity testing I
Following acquisition, each bird was given eight reflexivity test sessions conducted in two-session blocks separated by a minimum of five baseline sessions. Test sessions consisted of 104 trials, 96 baseline trials distributed equally across all three baseline tasks, and eight probe trials, two of each of the following (A–A) sample–comparison sequences: R→R, R→G, G→R, and G→G. All probe trials were nonreinforced; the comparison and house light went off automatically after 5 s. Probe trials never occurred within six baseline trials of each other, and the first probe trial in a test session did not occur until each baseline trial had been presented at least once. Of interest were the comparison-response rates on the matching (R→R and G→G) versus the nonmatching (R→G and G→R) hue sequences. The baseline sessions intervening between pairs of test sessions continued until DRs ≥ .80 for all three baseline tasks for five of six consecutive sessions. One pigeon in Group Identity (IREF 2) died after completing only two test sessions.
Reflexivity testing II
Because most pigeons continued to respond at an appreciable rate on the nonreinforced probe trials even after 8 test sessions, 10 more sessions were run consecutively for all pigeons except one (OREF4) that died before the additional tests began. These tests were preceded by a minimum of 20 baseline sessions run at various times following the last of each pigeon's initial 8 test sessions.
Theoretical predictions
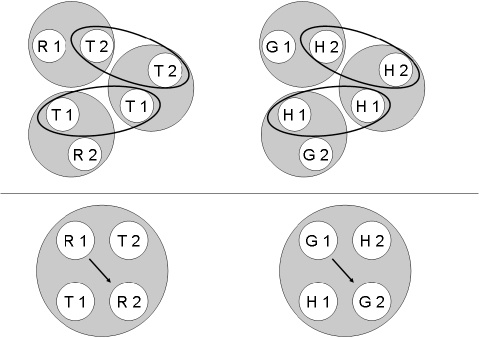
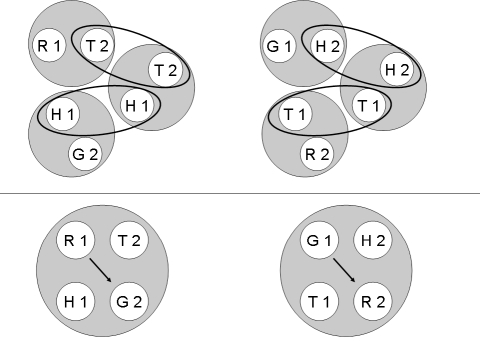
According to Urcuioli's (2008) theoretical assumptions, each individual successive matching task should yield two stimulus classes, both containing a particular sample stimulus (the nominal stimulus in the first ordinal position) and its reinforced comparison (the reinforced nominal stimulus in the second ordinal position). For the hue–form symbolic matching contingencies depicted in Table 1, the classes should be [R1, T2] and [G1, H2] for both groups. For the “mirror-image” form–hue symbolic matching task, the classes should be [T1, R2] and [H1, G2] for both groups. The classes arising from form–form successive matching, however, should differ: For Group Identity, they should be [T1, T2] and [H1, H2], whereas for Group Oddity, they should be [T1, H2] and [H1, T2].
The top half of Figure 1 provides a pictorial representation of these six classes for Group Identity with ellipses connecting stimuli common to more than one class. The bottom half of the figure shows the corresponding two 4-member classes that should result from class merger with arrows denoting the reflexivity prediction—namely, more frequent comparison responding to the red comparison (R2) after the red sample (R1) and to the green comparison (G2) after the green sample (G1). Figure 2 provides the corresponding representations for Group Oddity, with the arrow in each merged class denoting the prediction of emergent oddity—namely, more frequent comparison responding to the green comparison (G2) after the red sample (R1) and to the red comparison (R2) after the green sample (G1).
Fig 1.
Top panel: The six stimulus classes hypothesized to develop from the reinforced sample-comparison sequences for the two symbolic and form-identity baseline matching tasks for Group Identity. Ellipses highlight common class members. Bottom panel: Two 4-member stimulus classes hypothesized to arise from the merger of the stimulus classes shown in the top panel via their common elements. Arrows indicate sample-comparison sequences to which the Group Identity pigeons should preferentially respond in a reflexivity test. R = red, G = green, T = triangle, H = horizontal, 1 = first ordinal position within a matching trial, 2 = second ordinal position within a matching trial.
Fig 2.
Top panel: The six stimulus classes hypothesized to develop from the reinforced sample-comparison combinations for the two symbolic and form-oddity baseline matching tasks for Group Oddity. Ellipses highlight common class members. Bottom panel: Two 4-member stimulus classes hypothesized to arise from the merger of the stimulus classes shown in the top panel via their common elements. Arrows indicate sample-comparison sequences to which the Group Oddity pigeons should preferentially respond in a reflexivity test. R = red, G = green, T = triangle, H = horizontal, 1 = first ordinal position within a matching trial, 2 = second ordinal position within a matching trial.
RESULTS
Acquisition and baseline performances
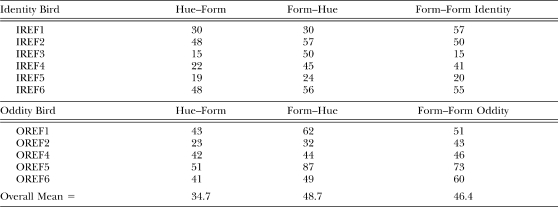
Table 2 shows the number of acquisition sessions required for pigeons to reach a DR of .80 on each successive matching task. The data represent the first five of six consecutive sessions at or above that performance level. Generally, acquisition to a .80 DR was fastest for hue–form matching, with form–hue and form–form matching acquired more slowly and at roughly the same average rate. Moreover, with only one exception, after pigeons met criterion on a particular task, they maintained that level of performance as discriminative performances on the remaining tasks improved to criterion levels. The exception was pigeon IREF6 whose form–hue DR fell below .80 for two successive sessions (.76 and .79) before recovering to criterion levels. Analysis of variance (ANOVA) on the data in Table 2 showed no overall between-group difference, F(1, 9) = 2.19, and no Group × Task interaction, F(2, 18 ) = 0.22. Consequently, the sessions-to-.80 results were averaged across all pigeons for further analyses and are shown at the bottom of the table. Post-hoc contrasts (Rodger, 1975) on these data confirmed that a .80 DR was reached in fewer sessions on hue–form matching than on the other two successive matching tasks, F(2, 20) = 7.17, which were acquired to criterion at comparable rates, F(2, 20) = .06.
Table 2.
The Number of Acquisition Sessions Needed to Reach a 0.80 Discrimination Ratio for Each Successive Matching Task.
DRs for the last five baseline sessions preceding the first test session were uniformly high and showed no between-group difference or a Group × Task interaction. Averaged over all pigeons, the DR for hue–form symbolic matching for these sessions was .93; for form–hue symbolic matching and form–form identity/oddity, the DRs were .89 and .90, respectively. Discriminative performance was significantly higher on former task than on the latter two, F(2, 18) = 3.92, which did not differ from one another, F(2, 18) = .13. The difference was not a concern, however, given the very high level of discriminative performance on all tasks.
Testing
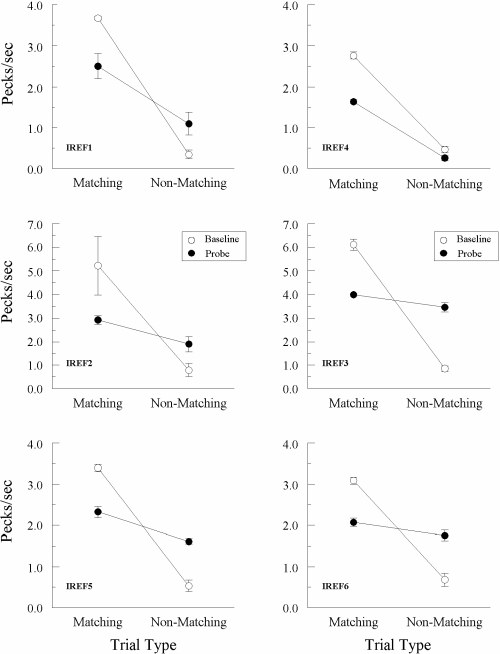
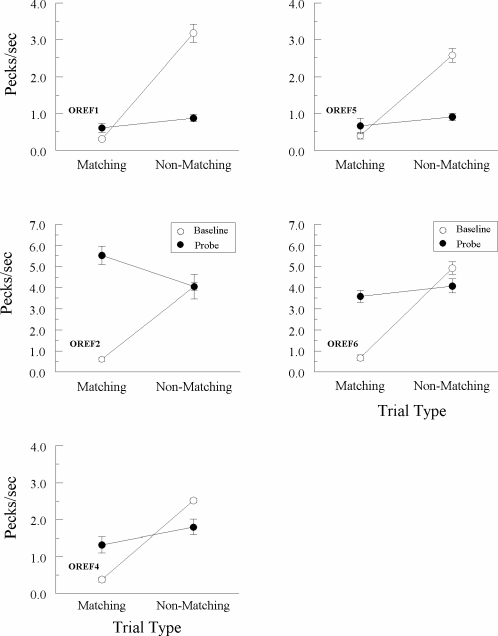
The reflexivity test results for each Group Identity pigeon are shown in Figure 3, which plots comparison-response rates (in pecks/s) on the matching and nonmatching (A–A) probe trials with red and green as samples and as comparisons (filled circles) and on the corresponding form–identity (B–B) baseline trials with the triangle and horizontal lines (open circles). The data are averaged across all eight reflexivity test sessions, except for pigeon IREF 2 whose results are averaged across the only two test sessions completed prior to its demise. Figure 4 plots the corresponding test results for each Group Oddity pigeon, for which form oddity served as a baseline task. In both figures, note the different ordinate scales, reflecting the large differences in pigeons' baseline response rates; each row, however, depicts data from pigeons with comparable baseline rates.
Fig 3.
Comparison pecks/sec (± 1 SEM) on form-identity baseline trials (open circles) and nonreinforced reflexivity probe trials (filled circles) averaged over the eight initial test sessions for each Group Identity pigeon. Matching = trials on which the comparison physically matched the preceding sample. Non-matching = trials on which the comparison did not physically match the preceding sample. Note that the ordinate for 2 of the pigeons (IREF2 and IREF3) differs from the other 4 pigeons.
Fig 4.
Comparison pecks/sec (± 1 SEM) on form-oddity baseline trials (open circles) and nonreinforced reflexivity probe trials (filled circles) averaged over the eight initial test sessions for each Group Oddity pigeon. Matching = trials on which the comparison physically matched the preceding sample. Non-matching = trials on which the comparison did not physically match the preceding sample. Note that the ordinate for 2 of the pigeons (OREF2 and OREF6) differs from the other 3 pigeons.
Every pigeon continued to respond appropriately on its baseline task: Comparison-response rates were considerably higher on form–form matching than on form–form nonmatching trials in Group Identity, and vice versa in Group Oddity. Of greater interest, however, are the comparison-response rates on the nonreinforced probe trials with red and green sample and comparison stimuli. Figure 3 shows that every Group Identity pigeon responded at a higher rate on matching (R–R and G–G) probes than on nonmatching (R–G and G–R) probes. The numerical difference was most noticeable for pigeons IREF1, IREF2, IREF4, and IREF5. ANOVA on these test results2 showed that the difference in probe-trial rates was significant for every pigeon except IREF6: Fs(1, 62) = 11.48 (IREF1), 6.62 (IREF3), 35.42 (IREF4), 10.77 (IREF5), and 3.72 (IREF6), and F(1, 14) = 7.27 for IREF2 (tested only twice).
The differences in matching versus nonmatching response rates on these hue–hue (A–A) probe trials were obviously not as large as on the explicitly reinforced form–form (B–B) baseline trials. This was true for all Group Identity pigeons, all Fs(1, 7) ≥ 12.53 (excluding IREF2 for which there were insufficient data for statistical evaluation.)
Figure 4 shows a different pattern of test results in Group Oddity. Four of the 5 pigeons in this group responded at a higher rate on nonmatching (R–G and G–R) probes than on matching (R–R and G–G) probes. Interestingly, the remaining pigeon (OREF2) showed the opposite result. ANOVA on the test results for Group Oddity, however, showed no significant difference for any pigeon in comparison-response rates on matching and nonmatching probes, Fs(1, 62) = 2.77, 3.69, 2.42, 3.64, and 1.21 for pigeons OREF1, 2, 4, 5, and 6, respectively. Not surprisingly, then, the response-rate differences on probe trials were smaller than on the explicitly reinforced form–form baseline trials, all Fs(1, 7) ≥ 38.84.
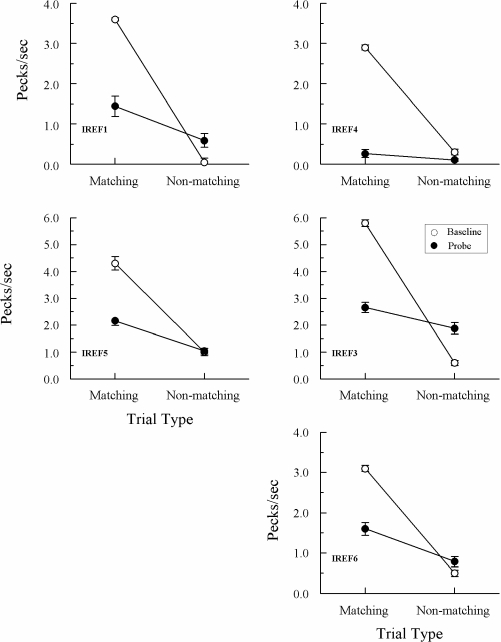
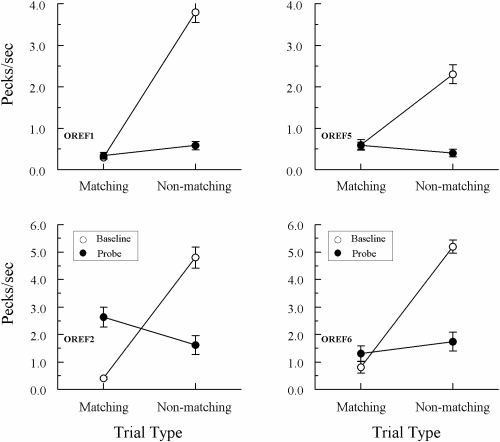
Figures 5 and 6 show the average results from the 10 consecutive test sessions run after the initial 8 tests for Groups Identity and Oddity, respectively. All pigeons again maintained highly differential performances on their baseline (form–form) trials throughout these tests. And, once again, each Group Identity pigeon responded at a higher rate to the comparisons on matching than on nonmatching probe trials. Although overall probe-trial rates were lower than during the initial tests (undoubtedly reflecting the cumulative effects of nonreinforcement on these trials), ANOVAs nevertheless showed that the difference in comparison-response rates on matching versus nonmatching probes was significant for every pigeon, Fs(1, 78) = 8.08, 24.79, 5.20, 25.95, and 15.80 for IREF1, IREF3, IREF4, IREF5, and IREF6, respectively.
Fig 5.
Comparison pecks/sec (± 1 SEM) on form-identity baseline trials (open circles) and nonreinforced reflexivity probe trials (filled circles) averaged over the subsequent 10 consecutive test sessions for each Group Identity pigeon. Matching = trials on which the comparison physically matched the preceding sample. Non-matching = trials on which the comparison did not physically match the preceding sample. Note that the ordinate for IREF3 and IREF5 differs from that for the other 3 pigeons.
Fig 6.
Comparison pecks/sec (± 1 SEM) on form-oddity baseline trials (open circles) and non-reinforced reflexivity probe trials (filled circles) averaged over the subsequent 10 consecutive test sessions for each Group Oddity pigeon. Matching = trials on which the comparison physically matched the preceding sample. Non-matching = trials on which the comparison did not physically match the preceding sample. Note that the ordinate for pigeons OREF1 and OREF5 differs from that for pigeons OREF2 and OREF6.
The test results for Group Oddity were more varied. Pigeons OREF1 and OREF6 responded at higher rates on nonmatching than on matching probes, although the difference was statistically significant only for OREF1, Fs(1, 78) = 4.00 and 0.99, respectively. By contrast, the opposite pattern was exhibited by pigeons OREF2 and OREF5: They responded at higher rates on matching than on nonmatching probes, although the difference was significant only for OREF2, Fs(1, 78) = 4.33 and 2.30, respectively.
As before, the differences in matching versus nonmatching response rates on the hue–hue (A–A) probe trials for these 10 tests were considerably smaller than the corresponding differences on the form–form (B–B) baseline trials: Group Identity, all Fs(1, 9) ≥ 57.60; Group Oddity, all Fs(1, 9) ≥ 101.19.
DISCUSSION
This experiment showed that after successive matching training on A–B and B–A symbolic relations and B–B identity relations, most Group Identity pigeons responded appreciably more on novel A–A probe trials when an A comparison matched its preceding A sample than when it did not. This emergent A–A effect is predicted by, and provides additional support for, Urcuioli's (2008) theory of pigeons' equivalence-class formation which posits that this group's baseline training should generate 4-member stimulus classes containing the matching A sample and A comparison stimuli (see Figure 1).
The results from Group Oddity, whose baseline training involved B–B oddity relations, showed that the effect observed in Group Identity was not attributable just to A–B and B–A symbolic training which might otherwise be viewed as sufficient for the A–A effect via transitivity or some other mechanism (e.g., Zentall, Clement, & Weaver, 2003). If so, Group Oddity should have also exhibited higher comparison-response rates on matching than on nonmatching A–A probes. Clearly, they did not (with the possible exception of pigeon OREF2). Thus, explicit B–B identity training appears to be crucial for emergent (A–A) differential responding in Group Identity.
That said, Urcuioli's (2008) theory clearly states that Group Oddity's baseline training will generate four-member stimulus classes that should yield higher comparison-response rates on nonmatching A–A probes. Stated more specifically, the prediction was that these pigeons would respond more in testing to a green comparison after a red sample, and vice versa (see Figure 2). This result clearly did not materialize (with the possible exception of pigeon OREF1) and, thus, is inconsistent with the theory. The reason for the inconsistent finding is at present unclear.
One issue to be addressed is whether the apparent reflexivity effect in Group Identity is just another example of acquired equivalence (Urcuioli, 1996, 2006). After all, the A and B samples occasioned responding to the same reinforced comparisons in training (viz., to the B comparisons of the A–B and B–B relations). If such many-to-one relations (Urcuioli et al., 1989) were learned first, acquisition of the remaining B–A relations would be like “reassignment training” (Wasserman et al., 1992)—learning new (A) responses to one set (B) of already functionally equivalent samples. If so, those new responses should then occur to the other set (A) of functionally equivalent samples, yielding the observed A–A differential response patterns.
Unfortunately, because all baseline relations were trained concurrently, it is difficult in many cases to determine if A–B (hue–form) and B–B (form–form) matching were mostly acquired prior to B–A (form–hue) matching (cf. Urcuioli, Zentall, & DeMarse, 1995). Pigeon IREF3, however, provided at least one clear example of this acquisition profile. This pigeon later responded more on matching than on nonmatching A–A probes in testing, although the numerical difference in its probe-trial rates was among the smallest observed. By contrast, the acquisition profiles of IREF1 and IREF4 were not conducive to acquired equivalence, yet they exhibited the largest differences in probe-trial responding. It would seem, then, that acquired equivalence does not offer a compelling explanatory alternative to the results.
Another issue concerns the smaller difference in matching versus nonmatching comparison-response rates on the A–A probes than on the corresponding B–B baseline trials in Group Identity (see Figures 3 and 5). Complete interchangeability of stimuli in the hypothesized 4-member stimulus classes (see Figure 1) should yield differences of comparable magnitude across probe and baseline trials. But such an ideal result seems rather unlikely given the pigeons' very limited preexperimental histories and repertoires (compared to humans in studies of equivalence) and the fact that their experimental histories involved lengthy periods of baseline differential reinforcement on B–B matching (which continued during the test itself) followed by limited but consistently nonreinforced exposure to the A–A probes.
If Group Identity's results truly represent reflexivity, these data provide the first demonstration of this phenomenon in any animal. This claim might seem curious because pigeons and other animals exhibit generalized identity matching (e.g., Dube, Iennaco, & McIlvane, 1993; Kastak & Schusterman, 1994; Katz, Wright, & Bodily, 2007; Oden, Thompson, & Premack, 1988; Peña, Pitts, & Galizio, 2006; Wright, Cook, Rivera, Sands, & Delius, 1988), which is often regarded as an index of reflexivity (e.g., Saunders, Wachter, & Spradlin, 1988; Sidman, Kirk, & Willson-Morris, 1985; Zentall & Urcuioli, 1993). Generalized identity matching occurs when explicit identity training (e.g., on B–B matching) yields the ability to match other stimuli to themselves (i.e., A–A matching).
However, some (e.g., Saunders & Green, 1992) have argued that it is inappropriate to equate generalized identity matching with reflexivity. It is essential in the construct of equivalence that the same relation exists between all members of the class. In generalized identity matching, the functional relation is of the form “A is (physically) identical to A”. But A can be in the same equivalence class as other stimuli (e.g., B) without being physically identical to them. Stated otherwise, the required relation in equivalence is not identity per se but, rather, a relation that broadly captures the interchangeability of class members.
An equally important consideration is that the origins of generalized identity matching lie, by definition, in a history of reinforced responding to physically identical stimuli. By contrast, reflexivity can purportedly result solely from a history of reinforced responding to nonidentical stimuli (i.e., after training on purely symbolic relations like A–B and B–C). This, alone, argues against equating the two phenomena. Interestingly, Saunders and Green (1992, p. 236) note that “…there is no way to determine whether performance on reflexivity tests shows a general relation of equivalence…or some specific…relation that is a product of the stimulus control inherent in match-to-sample trials involving identical stimuli.” We would modify that statement by adding “with human subjects” after “reflexivity tests” given that developmentally normal and disabled children and adults (1) typically demonstrate generalized identity matching (Dube et al., 1993), and (2) have extensive identity-relevant experiences and repertoires. Research with nonhuman animals like the pigeon, then, has better potential for disentangling reflexivity from generalized identity given the greater control researchers have over the preexperimental histories of their animal subjects.
Did the present experiment fulfill this potential? By itself, it did not because one of the baseline tasks for Group Identity was identity matching with stimuli different from those appearing on the reflexivity test trials. Consequently, this group's results could be interpreted as another example of generalized identity matching (i.e., train B–B matching, observe A–A matching). Besides, not only was identity matching explicitly trained, the other two trained relations (A–B and B–A symbolic matching) insured that pigeons were familiar with both the A samples and the A comparisons prior to their A–A reflexivity test.
But there are reasons to question this account. First, if successive matching training for Group Identity was sufficient to produce generalized identity, why wasn't successive matching training for Group Oddity sufficient to produce generalized oddity? One rejoinder is to say that pigeons have a predisposition toward identity (cf. Zentall, Edwards, Moore, & Hogan, 1981; cf. OREF2's test results in Figure 6) which is bolstered by explicit identity training and is counteracted by explicit oddity training. An appeal to an identity predisposition, however, is contradicted by other findings showing no difference in the rates at which two-alternative identity or oddity are learned (Carter & Werner, 1978) and even evidence of an oddity bias (Berryman, Cumming, Cohen, & Johnson, 1965; Wilson, Mackintosh, & Boakes, 1985). Moreover, although the present experiment found an overall numerical difference in favor of form–form identity acquisition (cf. Table 1), there was no significant acquisition difference between form–form identity and form–form oddity.
Second, generalized identity matching in pigeons after identity training with only two stimuli would be at odds with most pigeon data in the relational concept literature (although see Wright, 1997). Generalized identity and generalized same/different performances are far more likely to be observed after explicit training with many exemplars or stimuli (Katz & Wright, 2006; Wright et al., 1988). Nevertheless, there are data indicating that same/different training with only a small number of stimuli will transfer to novel stimuli in modified versions of the go/no-go tasks of the sort used here (Cook, Kelly, & Katz, 2003).
A much better way to clarify the role, if any, of identity training in successive matching for obtaining the emergent results observed here is to train A–B, B–A, and C–C baseline relations prior to A–A testing. This training accomplishes many of the same things as the A–B, B–A, and B–B training for Group Identity. For example, it provides reinforced identity training, insures familiarity with the A samples and A comparisons prior to testing, and guarantees discrimination of each of the two A samples from one another and of each of the two A comparisons from one another prior to testing (Saunders & Green, 1999). The difference lies solely in the nature of the identity baseline relations (C–C vs. B–B). If our results reflect generalized identity matching, this difference should be inconsequential: Pigeons should respond more on matching A–A trials than on nonmatching A–A trials irrespective of whether training involves C–C or B–B successive matching.
In contrast, Urcuioli's (2008) theory predicts different test outcomes as a function of the identity task used in training. The theory views B–B training as indispensable for the A–A emergent effect because that training promotes the merger of otherwise separate stimulus classes (see Figure 1), thus yielding a larger class containing the elements of the reflexive (A–A) relation. By contrast, class merger cannot occur with C–C training because there would be no common elements whatsoever across the two-member classes arising from concurrent A–B, B–A and C–C training. The results from such a future experimental manipulation will not only be theoretically important but will also be important in advancing our understanding of the processes underlying emergent behavior.
Acknowledgments
This research represents the senior undergraduate honors thesis of Mary M. Sweeney, who is now in the Department of Psychology, Utah State University, Logan, UT. Some of these results were presented at the 50th Annual Convention of the Psychonomic Society, Boston, November 2009 and at the 33rd Annual Meeting of the Society for the Quantitative Analysis of Behavior, San Antonio, May 2010. Preparation of this manuscript was supported in part by NICHD Grant R01 HD061322. The authors thank Timothy Burnight, Nicole Coulardot, and Cody Neal for their assistance in conducting this research.
Footnotes
The location at which stimuli appear is also likely to be a component of the functional matching stimuli, as Lionello and Urcuioli (1998) have shown. However, the location at which the samples and comparisons appear in successive matching is the same (e.g., on the center key of a three-key display) so location is inconsequential for present considerations.
The data for these analyses were the response rates on the matching and nonmatching probe trials (four each per test session) over all eight test sessions for each pigeon.
REFERENCES
- Astley S.L, Wasserman E.A. Superordinate category formation in pigeons: Association with a common delay or probability of reinforcement makes perceptually dissimilar stimuli functionally equivalent. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:415–432. [PubMed] [Google Scholar]
- Berryman R, Cumming W.W, Cohen L.R, Johnson D.F. Acquisition and transfer of simultaneous oddity. Psychological Reports. 1965;17:767–775. doi: 10.2466/pr0.1965.17.3.767. [DOI] [PubMed] [Google Scholar]
- Bovet D, Vauclair J. Functional categorization of objects and of their pictures in baboons (Papio anubis) Learning and Motivation. 1998;29:309–322. [Google Scholar]
- Carter D.E, Werner T.J. Complex learning and information processing by pigeons: A critical analysis. Journal of the Experimental Analysis of Behavior. 1978;29:565–601. doi: 10.1901/jeab.1978.29-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R.G, Kelly D.M, Katz J.S. Successive two-item same–different discriminations and concept learning by pigeons. Behavioural Processes. 2003;62:125–144. doi: 10.1016/s0376-6357(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Cullinan V.A, Barnes D, Smeets P.M. A precursor to the relational evaluation procedure: Analyzing stimulus equivalence. The Psychological Record. 1998;48:121–145. [Google Scholar]
- D'Amato M.R, Salmon D.P, Loukas E, Tomie A. Symmetry and transitivity in the conditional relations in monkeys (Cebus apella) and pigeons (Columba livia) Journal of the Experimental Analysis of Behavior. 1985;44:35–47. doi: 10.1901/jeab.1985.44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater A.R, Joseph P. Common coding in symbolic matching tasks with humans: Training with common consequence or antecedent. Quarterly Journal of Experimental Psychology. 2000;53B:255–273. doi: 10.1080/027249900411182. [DOI] [PubMed] [Google Scholar]
- Devany J.M, Hayes S.C, Nelson R.O. Equivalence class formation in language-able and language-disabled children. Journal of the Experimental Analysis of Behavior. 1986;46:243–257. doi: 10.1901/jeab.1986.46-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougher M.J, Markham M.R. Stimulus classes and the untrained acquisition of stimulus functions. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. New York, NY: Elsevier; 1996. pp. 137–152. (Eds.), [Google Scholar]
- Dube W.V, Iennaco F.M, McIlvane W.J. Generalized identity matching to sample of two-dimensional forms in individuals with intellectual disabilities. Research in Developmental Disabilities. 1993;14:457–477. doi: 10.1016/0891-4222(93)90038-l. [DOI] [PubMed] [Google Scholar]
- Dugdale N, Lowe C.F. Naming and stimulus equivalence. In: Blackman D.E, Lejeune H, editors. Behaviour analysis in theory and practice: Contributions and controversies. Hillsdale, NJ: Erlbaum; 1990. pp. 115–138. (Eds.), [Google Scholar]
- Dugdale N, Lowe C.F. Testing for symmetry in the conditional discriminations of language-trained chimpanzees. Journal of the Experimental Analysis of Behavior. 2000;73:5–22. doi: 10.1901/jeab.2000.73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A.J. An examination of the temporal and spatial stimulus control in emergent symmetry in pigeons. 2007. (Doctoral dissertation). Available from Dissertations & Theses: Full Text. (Publication No. AAT 3271660).
- Frank A.J, Wasserman E.A. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G, Mitchell C, Graham S, Lavis Y. Acquired equivalence and distinctiveness in human discrimination learning: Evidence for associative mediation. Journal of Experimental Psychology: General. 2003;132:266–276. doi: 10.1037/0096-3445.132.2.266. [DOI] [PubMed] [Google Scholar]
- Hayes S.C. Nonhumans have not yet shown stimulus equivalence. Journal of the Experimental Analysis of Behavior. 1989;51:385–392. doi: 10.1901/jeab.1989.51-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey R.C, Hall G. The acquired equivalence and distinctiveness of cues. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:338–346. [PubMed] [Google Scholar]
- Horne P.J, Hughes J.C, Lowe C.F. Naming and categorization in young children: IV: Listener behavior training and transfer of function. Journal of the Experimental Analysis of Behavior. 2006;85:247–273. doi: 10.1901/jeab.2006.125-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne P.J, Lowe C.F. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65:185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne P.J, Lowe C.F. Toward a theory of verbal behavior. Journal of the Experimental Analysis of Behavior. 1997;68:271–296. doi: 10.1901/jeab.1997.68-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C.L. The problem of stimulus equivalence in behavior theory. Psychological Review. 1939;46:9–30. [Google Scholar]
- Kastak D, Schusterman R.J. Transfer of visual identity matching-to-sample in two California sea lions (Zalophus californianus) Animal Learning & Behavior. 1994;22:427–435. [Google Scholar]
- Katz J.S, Wright A.A. Mechanisms of same/different abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:80–86. doi: 10.1037/0097-7403.32.1.80. [DOI] [PubMed] [Google Scholar]
- Katz J.S, Wright A.A, Bodily K.D. Issues in the comparative cognition of abstract-concept learning. Comparative Cognition & Behavior Reviews. 2007;2:79–92. doi: 10.3819/ccbr.2008.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello K.M, Urcuioli P.J. Control by sample location in pigeons' matching to sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M. The search for symmetry: 25 years in review. Learning & Behavior. 2009;37:188–203. doi: 10.3758/LB.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M, Urcuioli P.J. Stimulus control topographies and tests of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens R, Kop P.F.M, Matthijs W. A test of symmetry and transitivity in the conditional discrimination performances of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvane W.J. Stimulus control analysis and nonverbal instructional methods for people with intellectual disabilities. International Review of Research in Mental Retardation. 1992;16:55–109. [Google Scholar]
- Oden D.L, Thompson R.K.R, Premack D. Spontaneous transfer of matching by infant chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:140–145. [PubMed] [Google Scholar]
- Peña T, Pitts R.C, Galizio M. Identity matching-to-sample with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior. 2006;85:203–221. doi: 10.1901/jeab.2006.111-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger R. The number of non-zero, post hoc contrasts from ANOVA and error-rate: I. British Journal of Mathematical and Statistical Psychology. 1975;28((1)):71–78. [Google Scholar]
- Saunders R.R, Wachter J, Spradlin J.E. Establishing auditory stimulus control over an eight-member equivalence class via conditional discrimination procedures. Journal of the Experimental Analysis of Behavior. 1988;49:95–115. doi: 10.1901/jeab.1988.49-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K.J, Williams D.C, Spradlin J.E. Derived stimulus control: Are there differences among procedures and processes. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. New York, NY: Elsevier; 1996. pp. 93–109. (Eds.), [Google Scholar]
- Saunders R.R, Green G. The nonequivalence of behavioral and mathematical equivalence. Journal of the Experimental Analysis of Behavior. 1992;57:227–241. doi: 10.1901/jeab.1992.57-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R.R, Green G. A discrimination analysis of training-structure effects on stimulus equivalence outcomes. Journal of the Experimental Analysis of Behavior. 1999;72:117–137. doi: 10.1901/jeab.1999.72-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman R.J, Kastak D. A California sea lion (Zalophus Californianus) is capable of forming equivalence relations. Psychological Record. 1993;43:823–839. [Google Scholar]
- Sidman M. Equivalence relations: Some basic considerations. In: Hayes S.C, Hayes L.J, editors. Understanding verbal relations. Reno, NV: Context Press; 1992. pp. 15–27. (Eds.), [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Kirk B, Willson-Morris M. Six-member stimulus classes generated by conditional-discrimination procedures. Journal of the Experimental Analysis of Behavior. 1985;43:21–42. doi: 10.1901/jeab.1985.43-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching-to-sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradlin J.E, Cotter V.W, Baxley N. Establishing a conditional discrimination without direct training: A study of transfer with retarded adolescents. American Journal of Mental Deficiency. 1973;77:556–566. [PubMed] [Google Scholar]
- Spradlin J.E, Saunders R.R. The development of stimulus classes using match-to-sample procedures: Sample classification versus comparison classification. Analysis and Intervention in Developmental Disabilities. 1986;6:41–58. [Google Scholar]
- Urcuioli P.J. Acquired equivalences and mediated generalization in pigeon's matching-to-sample. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. Amsterdam: Elsevier; 1996. pp. 55–70. (Eds.), [Google Scholar]
- Urcuioli P.J. Responses and acquired equivalence classes. In: Wasserman E.A, Zentall T.R, editors. Comparative cognition: Experimental explorations of animal intelligence. New York, NY: Oxford University Press; 2006. pp. 405–421. (Eds.), [Google Scholar]
- Urcuioli P.J. Associative symmetry, “anti-symmetry”, and a theory of pigeons' equivalence-class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, Lionello-DeNolf K.M. Some tests of the anticipatory mediated generalization model of acquired sample equivalence in pigeons' many-to-one matching. Animal Learning & Behavior. 2001;29:265–280. [Google Scholar]
- Urcuioli P.J, Lionello-DeNolf K.M. The role of common reinforced comparison responses in acquired sample equivalence. Behavioural Processes. 2005;69:207–222. doi: 10.1016/j.beproc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J, Zentall T.R, DeMarse T. Transfer to derived sample-comparison relations by pigeons following many-to-one versus one-to-many matching with identical training relations. Quarterly Journal of Experimental Psychology. 1995;48B:158–178. [Google Scholar]
- Urcuioli P.J, Zentall T.R, Jackson-Smith P, Steirn J.N. Evidence for common coding in many-to-one matching: Retention, intertrial interference, and transfer. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:264–273. [Google Scholar]
- von Fersen L, Delius J.D. Acquired equivalences between auditory stimuli in dolphins (Tursiops truncates) Animal Cognition. 2000;3:79–83. [Google Scholar]
- Wasserman E.A. Successive matching-to-sample in the pigeon: Variation on a theme by Konorski. Behavior Research Methods & Instrumentation. 1976;8:278–282. [Google Scholar]
- Wasserman E.A, DeVolder C.L, Coppage D.J. Nonsimilarity-based conceptualization in pigeons. Psychological Science. 1992;3:374–379. [Google Scholar]
- Wilson B, Mackintosh N.J, Boakes R.A. Matching and oddity learning in the pigeon: Transfer effects and the absence of relational learning. The Quarterly Journal of Experimental Psychology. 1985;37B:295–311. [Google Scholar]
- Wright A.A. Concept learning and learning strategies. Psychological Science. 1997;8:119–123. [Google Scholar]
- Wright A.A, Cook R.G, Rivera J.J, Sands S.F, Delius J.D. Concept learning by pigeons: Matching-to-sample with trial-unique video picture stimuli. Animal Learning & Behavior. 1988;16:436–444. [Google Scholar]
- Yamamoto J, Asano T. Stimulus equivalence in a chimpanzee (Pan troglodytes) The Psychological Record. 1995;45:3–21. [Google Scholar]
- Zentall T.R. An analysis of stimulus class formation in animals. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. New York, NY: Elsevier; 1996. pp. 15–34. (Eds.), [Google Scholar]
- Zentall T.R. Symbolic representation in animals: Emergent stimulus relations in conditional discrimination learning. Animal Learning & Behavior. 1998;26:363–377. [Google Scholar]
- Zentall T.R, Clement T.S, Weaver J.E. Symmetry training in pigeons can produce functional equivalences. Psychonomic Bulletin & Review. 2003;10:387–391. doi: 10.3758/bf03196496. [DOI] [PubMed] [Google Scholar]
- Zentall T.R, Edwards C.A, Moore B.S, Hogan D.E. Identity: The basis for both matching and oddity learning in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:70–86. [PubMed] [Google Scholar]
- Zentall T.R, Urcuioli P.J. Emergent relations in the formation of stimulus classes by pigeons. The Psychological Record. 1993;43:795–810. [Google Scholar]
- Zentall T.R, Wasserman E.A, Lazareva O.F, Thompson R.K.R, Rattermann M.J. Concept learning in animals. Comparative Cognition & Behavior Reviews. 2008;3:13–45. [Google Scholar]