Abstract
The present experiment investigated whether pigeons can show associative symmetry on a two-alternative matching-to-sample procedure. The procedure consisted of a within-subject sequence of training and testing with reinforcement, and it provided (a) exemplars of symmetrical responding, and (b) all prerequisite discriminations among test samples and comparisons. After pigeons had learned two arbitrary-matching tasks (A–B and C–D), they were given a reinforced symmetry test for half of the baseline relations (B1–A1 and D1–C1). To control for the effects of reinforcement during testing, two novel, nonsymmetrical responses were concurrently reinforced using the other baseline stimuli (D2–A2 and B2–C2). Pigeons matched at chance on both types of relations, thus indicating no evidence for symmetry. These symmetrical and nonsymmetrical relations were then directly trained in order to provide exemplars of symmetry and all prerequisite discriminations for a second test. The symmetrical test relations were now B2–A2 and D2–C2 and the nonsymmetrical relations were D1–A1 and B1–C1. On this test, 1 pigeon showed clear evidence of symmetry, 2 pigeons showed weak evidence, and 1 pigeon showed no evidence. The previous training of all prerequisite discriminations among stimuli, and the within-subject control for testing with reinforcement seem to have set favorable conditions for the emergence of symmetry in nonhumans. However, the variability across subjects shows that methodological variables still remain to be controlled.
Keywords: associative symmetry, stimulus equivalence, exemplar training, two-alternative matching-to-sample, reinforced tests, key peck, pigeons
A subject taught to relate perceptually dissimilar stimuli in a matching-to-sample (MTS) task can establish new conditional discriminations among them without any additional training. For example, following reinforcement for choosing B1 and B2 comparisons given A1 and A2 samples, respectively (A–B baseline training), a subject can continue to match the same pair of stimuli even when their respective functional roles are reversed (B–A symmetry). This kind of emergent performance, also named backward association (Gray, 1966; Hogan & Zentall, 1977) or associative symmetry (Frank & Wasserman, 2005; Urcuioli, 2008), reveals that related samples and comparisons become mutually substitutable. Furthermore, emergent symmetry provides behavioral evidence for equivalence-class formation when it co-occurs with two other emergent performances, that is, matching each stimulus to itself (A–A and B–B reflexivity), and matching two unpaired stimuli that had been related to a third, common stimulus (A–C transitivity, following additional B–C training) (Sidman, Rauzin, Lazar, Cunninghan, Tailby, & Carrigan, 1982; Sidman & Tailby, 1982).
Emergent conditional discriminations, such as those defining stimulus equivalence, have been largely demonstrated in verbally-able humans with both typical and atypical development (e.g., Devany, Hayes, & Nelson, 1986; Lazar, Davis-Lang, & Sanchez, 1984; Sidman & Cresson, 1973; Sidman & Tailby, 1982; Spradlin, Cotter, & Baxley, 1973). However, such a demonstration has proved to be much more elusive with nonverbal subjects (e.g., Devany et al., 1986), especially nonhuman animals (e.g., D'Amato, Salmon, Loukas, & Tomie, 1985; Hogan & Zentall, 1977; Lionello-DeNolf & Urcuioli, 2002; Lipkens, Kop, & Matthijs, 1988; Sidman et al., 1982; Tomonaga, Matsuzawa, Fujita, & Yamamoto, 1991; Yamamoto & Asano, 1995).
Despite this difficulty, a few instances of emergent performance have been reported in nonhuman animals. Generalized identity matching has been demonstrated in monkeys (Barros, Galvão, & McIlvane, 2002; Oden, Thompson, & Premack, 1988), pigeons (Zentall, Edwards, Moore, & Hogan, 1981; Zentall & Hogan, 1976), sea lions (Kastak & Schusterman, 1994), dolphins (Herman & Gordon, 1974; Herman, Hovancik, Gory, & Bradshaw, 1989), and rats (Peña, Pitts, & Galizio, 2006). Transitivity has been documented in monkeys (D'Amato et al., 1985), in a chimpanzee (Yamamoto & Asano, 1995), and in a sea lion (Schusterman & Kastak, 1993). In addition, somewhat weaker evidence was found in one pigeon (Kuno, Kitadate, & Iwamoto, 1994). On the other hand, attempts to obtain symmetry with nonhuman animals have been mostly unsuccessful (e.g., Barros, Galvão, & Fontes, 1996; D'Amato et al., 1985; Dugdale & Lowe, 2000; Sidman et al., 1982, with primates; and also Gray, 1966; Hogan & Zentall, 1977; Holmes, 1979; Lionello-DeNolf & Urcuioli, 2002; Lipkens et al., 1988; Richards, 1988; Rodewald, 1974, with pigeons), although some exceptions exist (e.g., Frank & Wasserman, 2005, and Urcuioli, 2008, with pigeons; Schusterman & Kastak, 1993, with a sea lion; Yamamoto & Asano, 1995, with a chimpanzee). This set of results suggests that emergent symmetry may be a critical relation that separates the performance of humans from that of nonhumans in studies on stimulus equivalence (Yamamoto & Asano, 1995).
The discrepancy concerning results of verbal and nonverbal subjects has led some researchers to argue that emergent performances, especially symmetry, may depend on behavioral prerequisites presumably acquired through exposure to training on language competencies (Devany et al., 1986; Dugdale & Lowe, 1990; Hayes, 1989; Hayes, Barnes-Homes, & Roche, 2001, Horne & Lowe, 1996). On the other hand, it has also been argued that equivalence could be the very foundation of language development (Mackay & Sidman, 1984; Schusterman & Kastak, 1993; Sidman et al., 1982), perhaps even a basic, irreducible behavioral process that humans share with animals (Sidman, 1990; 1994; 2000). If this is the case, then the difficulty in demonstrating equivalence-class formation in nonverbal subjects would stem from methodological shortcomings, such as inadequate training, inadequate testing or both (Dube, McIlvane, Callahan, & Stoddard, 1993; Lionello-DeNolf, 2009).
Although the role of language on equivalence-class formation remains controversial, experimental results have shown that providing a history of reinforced symmetrical responding (multiple-exemplar training) — an experience incidentally embedded in linguistic training but not restricted to it — seems to encourage generalized symmetrical responding (as suggested by Sidman et al., 1982). Such has been the case for both nonhuman animals (Schusterman & Kastak, 1993; Yamamoto & Asano, 1995) and young children who initially fail to show symmetry (Barnes-Holmes, Barnes-Holmes, Roche, & Smeets, 2001).
Moreover, to ensure that the emergent performance has not been directly trained, untaught conditional discriminations are usually tested in extinction. In a typical testing procedure, after subjects have learned the baseline relations, the probability of reinforcement on baseline trials is reduced to prepare the subjects for the forthcoming unreinforced probe trials (e.g., Kuno et al., 1994, Sidman et al., 1982). In this case, the absence of reinforcement on test trials may account for the failure to obtain symmetry in some nonverbal subjects. Compared to the initial training, extinction during testing may change considerably the sources of stimulus control (Dube & McIlvane, 1996; Galvão, Calcagno, & Sidman 1992; Kuno et al., 1994; Schusterman & Kastak, 1993; Sidman, 1994; Sidman et al., 1982), allow subjects to discriminate that there is no reinforcement during probe trials (Brino, Galvão, & Barros, 2009; Galvão et al., 2005) and thereby prevent the emergence of conditional relations. Furthermore, extinction can have multiple and complex effects, such as resurgent behaviors (Epstein, 1983, 1985; Wilson & Hayes, 1996), aggression (Azrin, Hutchinson, & Hake, 1966), urination and defecation (Keller & Schoenfeld, 1950; Skinner, 1938), and increases in behavioral variability (Antonitis, 1951; Lerman & Iwata, 1996) that may also interfere with the emergence of conditional relations. This may be especially true given that unreinforced test trials reproduce the same consequences the subjects experience during baseline training following incorrect responses.
Another important issue to consider when testing for symmetry is that accurate performance on MTS requires successive discriminations among samples and simultaneous discriminations among comparisons. Therefore, new discriminations are required during the symmetry testing because stimuli that previously were comparisons (requiring a simultaneous discrimination) become samples (requiring a successive discrimination) and vice-versa (Saunders & Green, 1999). Typical baseline training procedures do not guarantee that the requisite discriminations are in place prior to testing.
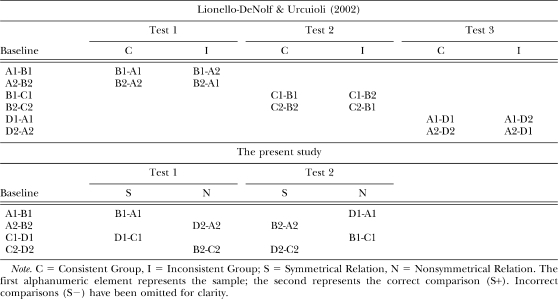
Lionello-DeNolf and Urcuioli (2002) conducted two experiments, Experiments 3 and 4, that resolved this methodological issue to some extent. Pigeons were given exemplar training on symmetrical relations and were tested for symmetry under differential reinforcement conditions. In addition to the target A–B matching task, two additional matching tasks (B–C and D–A) were trained in order to give the pigeons experience with A and B stimuli as both samples and comparisons. In this way, successive discriminations between the B stimuli and simultaneous discriminations between the A stimuli, both required for accurate symmetry-test performance, were directly trained. Because test-trial performances were reinforced, pigeons were divided into two test groups, consistent and inconsistent. For the consistent group, reinforced responses were the symmetrical versions of the baseline relations. For the inconsistent group, reinforced responses were the opposite of the symmetrical version of the baseline relations. The inconsistent group controlled for the potential effects of the direct training of the tested (symmetry) relations. Table 1 (top panel) summarizes Lionello-DeNolf and Urcuioli's procedure.
Table 1.
Summary of the Procedure for Experiments 3 and 4 of Lionello-DeNolf and Urcuioli (2002), and for the Present Experiment.
For this experimental design, evidence for symmetry is indicated by first-test-session accuracy well above chance for the consistent group and well below chance for the inconsistent group, and by faster acquisition of the B–A relations by the consistent group. Because no difference was found between matching performances by the consistent and inconsistent groups (i.e., no evidence for symmetry), pigeons were retrained on the additional B–C baseline matching task and tested for C–B symmetry until high accuracy was obtained. Afterwards, the D–A baseline matching task was retrained and A–D symmetry was tested. Although this sequence of training and testing had provided a history of reinforced symmetrical responding (or multiple exemplar training), no evidence of symmetry was found on either the second or third symmetry tests: First-session accuracies were at or close to chance for both the consistent and inconsistent groups, and both groups learned the tasks at the same rate.
Lionello-DeNolf and Urcuioli (2002) reasoned that the relatively limited history of reinforced symmetrical responding (only two sets: B–A and C–B) might have been insufficient to produce generalized symmetrical responding. Nevertheless, another variable may have contributed to the pigeons' failure to show symmetry. Although pigeons experienced the A and B stimuli as both samples and comparisons via the trained B–C and D–A relations, the same was not true for the C and D stimuli. In other words, before the second (C–B) and third (A–D) symmetry tests, pigeons had never learned to discriminate among the C stimuli successively and the D stimuli simultaneously.
The present study was designed to examine whether pigeons show emergent associative symmetry after experiencing a relatively short history of reinforced symmetrical responding in a procedure that provides all prerequisite successive and simultaneous discriminations for the critical test. In a two-alternative MTS procedure, pigeons were initially trained on two independent arbitrary-matching tasks (A–B and C–D) involving Set 1 and Set 2 stimuli. Then, a first reinforced symmetry test was conducted with the Set 1 stimuli (B1–A1 and D1–C1). To show that accurate matching on symmetrical relations was not a result of rapid symmetry learning due to reinforcement, responses on two novel, nonsymmetrical relations were reinforced by repairing stimuli from Set 2 (B2–C2 and D2–A2). Evidence for symmetry is indicated by (1) response accuracies above chance on symmetrical trials and near chance on nonsymmetrical trials on the first test session, and (2) faster acquisition of the symmetrical relations than the nonsymmetrical relations across repeated test sessions.
Notice, however, that at the onset of the first symmetry test, the pigeons will not have been exposed to all the prerequisite discriminations between the stimuli. Therefore, if such exposure is necessary, one should expect no evidence for associative symmetry during the first test. In contrast, by the end of the first symmetry test, all prerequisite discriminations will have been trained to high accuracy levels. That is, the successive discriminations among B and D stimuli and the simultaneous discriminations among A and C stimuli will have been directly trained. Hence, after a baseline retraining, a second symmetry test was given using the Set 2 relations (B2–A2 and D2–C2). For this test, the nonsymmetrical relations were formed from the Set 1 stimuli (D1–A1 and B1–C1). If exposure to all prerequisite discriminations is necessary, evidence for associative symmetry should be seen in this second test: response accuracies should be above chance on symmetrical trials and near chance on nonsymmetrical trials.
Table 1 (bottom panel) summarizes the present procedure and allows for comparisons with the procedure used by Lionello-DeNolf and Urcuioli (2002). One advantage of using novel (nonsymmetrical) relations rather than inconsistent relations to control for reinforcement procedures during testing is that both symmetrical and nonsymmetrical responses can be concurrently reinforced for the same subject. By contrast, the consistent/inconsistent control method necessitates a group design; if both consistent and inconsistent responses are reinforced within subjects, there is a risk of all the stimuli merging into a single, larger class such that neither relation would be acquired. A within-subject control for the use of reinforcement procedures during testing is critical given the variability often observed on symmetry tests because it should improve experimental control and highlight possible evidence for symmetry in individual subjects that might be obscured when group designs are used.
METHOD
Subjects
The subjects were 4 pigeons (P1, P2, P3 and P4) that had been exposed to a previous experiment on timing in which they learned to match different sample durations to hue comparisons (e.g., 1-s signal to yellow key and 4-s signal to blue key). Each pigeon was individually housed in a stainless-steel cage and had free access to water and grit when not in the experimental chamber. A 13/11-h light/dark cycle was in effect in the pigeon colony with lights on at 8:00 am. Throughout the experiment, the pigeons were maintained at 80% of their free-feeding body weights by restricting feeding to the experimental sessions.
Apparatus
Two custom-built operant conditioning chambers constructed of brushed aluminum, and measuring 37 × 45 × 44 cm were used. The front wall of each chamber was equipped with a 15-in touchscreen LCD monitor (Elo Touch© 1515L) that required approximately 100 g to be activated. A pair of speakers located behind each monitor emitted a “click” every time the touchscreen was activated. An opaque Plexiglas plate, measuring 30 × 6 cm, covered the lower portion of the touchscreen making available to the subject only an area of 30 × 17 cm.
Visual stimuli were displayed individually or in pairs, on three possible screen locations (left, center and right). The three locations were 6 cm apart, center-to-center, and were horizontally aligned in a row 24 cm above the floor. Each location corresponded to an area of 5 cm2 into which pecks with a force greater than 100 g activated the screen. Pecking outside the location or inside an empty location had no consequences. The background of the screen remained black across the experiment. Nine visual stimuli were presented in different conditions. A 4 × 4-cm white square was used only in the preliminary training. Eight black irregular forms (designed by Detanico & Lain, 2001) superimposed on a 4 × 4-cm white square were used both in the preliminary training and in all subsequent experimental phases. These stimuli were randomly divided into two four-stimulus sets (Figure 1). Stimuli comprising Set 1 and Set 2 were counterbalanced across subjects.
Fig 1.
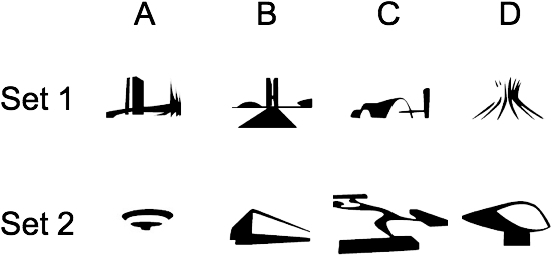
The eight stimuli used in all experimental conditions.
A food hopper was accessible through a 6 × 5.5-cm opening centered on the rear wall of the chamber, 8 cm above the floor. When activated, the food hopper was illuminated with a 7.5-W white light and allowed access to mixed grain. A 7.5-W white houselight located 16.5 cm above the hopper opening provided general illumination. A fan mounted on the sidewall of the chamber circulated air and provided masking noise. A digital interface (Keithley/KPCI-PDISO8) connected each chamber to a computer. All experimental events were controlled and recorded by software developed in Visual Basic.
Procedure
Preliminary training
After the subjects learned to eat from the food hopper, they were trained by the method of successive approximations to peck the white square displayed at the center location of the screen. Next, a 96-trial session was conducted in which the white square appeared equally often in each of the three locations. In subsequent 96-trial sessions, the eight irregular forms (Figure 1) appeared randomly and equally often in each of the three locations. A single peck on the stimulus (i.e., fixed-ratio 1 or FR 1) turned it off and immediately produced 3-s access to the food. Sessions were conducted until response latencies to each stimulus at each location averaged 2 s or less.
Over the course of 6 sessions, the number of pecks required to the center location, which later would serve as the sample location in the MTS tasks, was gradually increased to 10. Each FR training session was composed of 96 trials in which the eight irregular forms (Figure 1) appeared equally often and randomly at the center location. Food was contingent upon three pecks (FR 3) during the first session, five pecks (FR 5) during the second session, seven pecks (FR 7) during the third session, and ten pecks (FR 10) during the remaining three sessions. In the last FR 10 session, the time to complete the response requirement averaged 10 s or less for all pigeons. During all FR sessions, as well as during all subsequent experimental conditions, the reinforcement duration remained constant within a particular session, but varied between 2 and 4 s across sessions to maintain each pigeon as close as possible to its 80% free-feeding body weight. Successive stimulus presentations were separated by a 10-s intertrial interval (ITI) initiated immediately after reinforcement. The houselight was on during the initial 9 s of the ITI, but it was turned off for the last 1 s and remained off until the onset of the next ITI.
Matching-to-sample training
A 0-s delay arbitrary MTS procedure was used in the subsequent 96-trial sessions. Each trial began with the onset of the sample stimulus in the center location. Pecking the sample 10 times (FR 10) turned it off and immediately produced the two comparisons in the left and right locations. A single peck (FR 1) to the comparison arbitrarily set as the correct stimulus (S+) turned both stimuli off, activated the food hopper and then the 10-s ITI. Pecking the comparison arbitrarily set as the incorrect stimulus (S−) turned off both stimuli and initiated the ITI.
Baseline training
Subjects were trained to match the A1 and A2 samples to the B1 and B2 comparisons, respectively, and to match the C1 and C2 samples to the D1 and D2 comparisons, respectively (see Table 1, lower portion). The four possible trial types were equally likely throughout each 96-trial session. For each sample–comparison relation, the S+ and S- comparisons appeared equally often on the left and right locations. The eight possible configurations (four trial types × two locations) were randomized in blocks of eight trials to ensure a uniform distribution of each trial type within a session. The only constraints on trial order were that a particular sample could not appear more than twice in succession, and that a particular location could not contain an S+ on more than three consecutive trials.
During baseline training, incorrect choices resulted in that trial being repeated after the ITI (correction procedure) for a maximum of three more trials. If the pigeon made four consecutive errors, then on the next trial only the S+ was presented. Correction trials were not included in data analysis.
Because 1 pigeon (P3) showed a strong location bias during the first 20 sessions, an additional correction procedure was used for this subject. First, the probability of choosing the preferred location was calculated over the last five sessions. Then, in the subsequent session, correct comparisons (S+) were presented in the opposite location with the same probability. For example, if the right location was chosen with probability of .7 during the last five sessions, then, in the next session, the probability of presenting the S+ in the left location was adjusted to .7. This correction procedure was used until the location bias became less than .6, and it was reintroduced every time it crossed .7. This correction procedure was used in a total of 12 sessions.
Pigeons P2 and P4 did not achieve accuracy levels greater than 75% correct after 50 sessions, and consequently a 15-s timeout was introduced after each incorrect choice. During the timeout period, both the houselight and the screen were turned off.
Each pigeon received one training session daily until it matched correctly on 90% or more of the 96 programmed trials for two consecutive sessions, and with no more than four incorrect responses on a particular relation. After reaching the accuracy criteria, each subject was overtrained for 20 sessions without any correction procedure.
Symmetry Test 1
Pigeons were tested for symmetry with samples from the baseline being used as comparisons, and vice-versa. Test performances were differentially reinforced over the course of 96-trial sessions. On half of the trials, reinforced sample–comparison relations were the symmetrical versions of half of the baseline relations (see Table 1, lower portion). That is, pecking the A1 comparison was reinforced after the B1 sample, and pecking the C1 comparison was reinforced after the D1 sample.
To control for the use of reinforcement on symmetry test trials, reinforcement was also provided for correct choices on two novel, nonsymmetrical relations. These relations were formed from the remaining baseline relations. That is, pecking the A2 comparison was reinforced after the D2 sample, and pecking the C2 comparison was reinforced after the B2 sample (see Table 1, lower portion).
Symmetrical and nonsymmetrical responses were concurrently reinforced for each subject. All procedural details remained identical to baseline, except that incorrect choices were not followed by either the correction procedure or the timeout. As in baseline, testing sessions were conducted until overall accuracy was at least 90% correct for two consecutive sessions with no more than four incorrect choices on any particular relation.
Baseline retraining
After completing Symmetry Test 1, all pigeons were retrained on the original baseline relations. All procedural details were identical to those employed during the initial training except that the correction procedure and the timeout were not used.
Symmetry Test 2
Each pigeon was given a new symmetry test in which different stimuli were used for the symmetrical and nonsymmetrical tested relations (see Table 1, lower portion). The symmetrical relations were composed of stimuli from Set 2 (B2–A2 and D2–C2), whereas the nonsymmetrical relations were composed of stimuli from Set 1 (D1–A1 and B1–C1). All other procedural details were identical to those described for Symmetry Test 1.
RESULTS
Baseline training
Pigeons P1, P2, P3 and P4 required a total of 23, 79, 55 and 62 sessions, respectively, to acquire the overall baseline relations to a 90% or better accuracy and with no more than four errors in each particular relation. Pigeons required an average of 50 sessions to learn Set 1 relations (range: 20 to 79) and 49 sessions to learn Set 2 relations (range: 23 to 62).
Figure 2 shows matching accuracy for each pigeon over five-session blocks of the baseline training. All pigeons performed at chance level on both Set 1- and Set 2-relation trials during the first block. Accuracies on Set1 and Set 2 relations improved across blocks and did not differ from one another.
Fig 2.
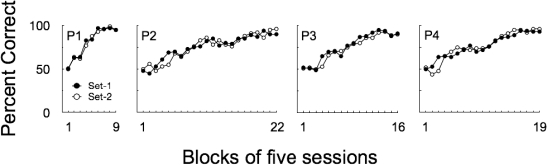
Matching accuracy for individual pigeons over five-session blocks of Baseline Training. Filled circles represent accuracy on Set 1 trained relations and open circles represent accuracy on Set 2 trained relations. The last four 5-session blocks of each subject refer to the overtraining sessions. The number of sessions in the last block preceding the overtraining was six for P1 and P3, three for P2 and two for P4.
The foregoing results provided an important experimental control for the reinforced symmetry test because the symmetrical and the nonsymmetrical relations were drawn from different sets of stimuli: When the symmetrical relations were formed with Set 1 stimuli, the nonsymmetrical relations were formed with Set 2 stimuli, and vice-versa. To the extent that there was no difference between the acquisitions of Set 1 and Set 2 relations, accuracies significantly above chance on symmetrical trials and near chance on nonsymmetrical trials would suggest associative symmetry rather than unknown confounds, such as intrinsic stimulus properties.
Accuracies on baseline trials remained high for all pigeons during the overtraining sessions. On the last two overtraining sessions, the individual matching accuracies averaged 94%, 93%, 91% and 95% for Subjects P1, P2, P3 and P4, respectively.
Symmetry Test 1
Figure 3 shows accuracy on both the symmetrical and nonsymmetrical test trials during the first symmetry test session. For 2 pigeons, P1 and P2, the percentage of correct choices on symmetrical test trials (51% and 54%, respectively) did not differ from chance. On nonsymmetrical trials, percentage of correct responses was below chance (38% and 40% for P1 and P2, respectively). For the remaining two pigeons, P3 and P4, the opposite was the case. Accuracy was at chance on the nonsymmetrical relations (56% and 54% for P3 and P4, respectively) but below chance on the symmetrical relations (35% and 31%, for P3 and P4, respectively).
Fig 3.
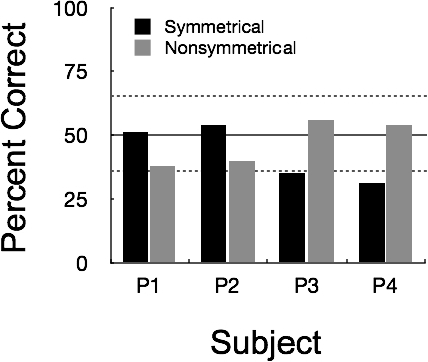
Matching accuracy for individual pigeons during the first 96-trial session of Symmetry Test 1. Black bars represent accuracy on symmetrical trials with the Set 1 stimuli and gray bars represent accuracy on nonsymmetrical trials with the Set 2 stimuli. Dashed lines show the 95% confidence interval associated with random responding (normal approximation to binomial).
Figure 4 shows individual matching accuracy averaged across five-session blocks of the first symmetry test for both the symmetrical and nonsymmetrical test trials. In the first block, P1 matched above chance on the symmetrical relations (63% correct), and below chance on the nonsymmetrical relations (40% correct). In contrast, matching accuracies on both symmetrical and nonsymmetrical relations were close to chance for Pigeons P2, P3 and P4. Accuracy on both symmetrical and nonsymmetrical relations improved gradually over 30 sessions for P1 and P4, and over 50 and 60 sessions for P2 and P3, respectively. In the first symmetry test, there was no difference between matching accuracies on symmetrical- and nonsymmetrical-relation trials throughout test sessions. Furthermore, the symmetrical relations were not acquired faster than the nonsymmetrical relations.
Fig 4.
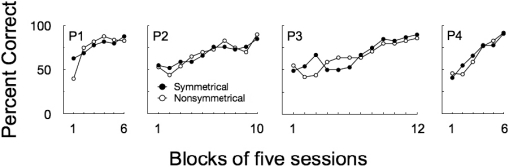
Matching accuracy for individual pigeons across five-session blocks in the Symmetry Test 1. Filled circles represent symmetrical test trials and open circles represent nonsymmetrical test trials. The number of sessions in the last block was three for P3 and two for P4.
Baseline retraining
On the baseline retraining, P1, P2, P3 and P4 required 4, 10, 13 and 14 sessions, respectively, to meet the accuracy criteria. Matching accuracy on the last two baseline retraining sessions averaged 94.5% for Set 1 relations (range: 90% to 99%) and 95.3% for Set 2 relations (range: 91% to 100%).
Symmetry Test 2
Figure 5 shows percentage of correct responses on the 96-trial session of the second symmetry test for both the symmetrical and nonsymmetrical test trials. All pigeons performed above chance on the symmetrical relations (range: 69% to 77%). In contrast, on the nonsymmetrical relations, P4 and P1 matched at chance (52% and 60%, respectively), while P2 and P3 matched well below chance (21% and 35%).
Fig 5.
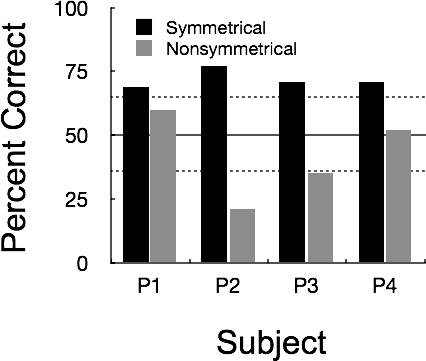
Matching accuracy for individual pigeons during the 96-trial session of Symmetry Test 2. Black bars represent accuracy on symmetrical trials with the Set 1 stimuli and gray bars represent accuracy on nonsymmetrical trials with the Set 2 stimuli. Dashed lines show the 95% confidence interval associated with random responding (normal approximation to binomial).
The fact that P2 and P3 responded above chance on the symmetrical relations but well below chance on the nonsymmetrical relations suggests that both pigeons might be performing simple rather than conditional discriminations. In other words, regardless of the sample, pigeons would prefer A2 over A1 and C2 over C1 (i.e., a bias for the S+'s of the symmetrical relations).
Figure 6 shows the probability of choosing the S+'s of the symmetrical relations as a function of the sample during the second symmetry test. The top panel shows the probability of choosing A2, given a choice between A1 and A2, as a function of B2 (symmetrical sample) or D1 (nonsymmetrical sample). The bottom panel shows the probability of choosing C2, given a choice between C1 and C2, as a function of D2 (symmetrical sample) or B1 (nonsymmetrical sample).
Fig 6.
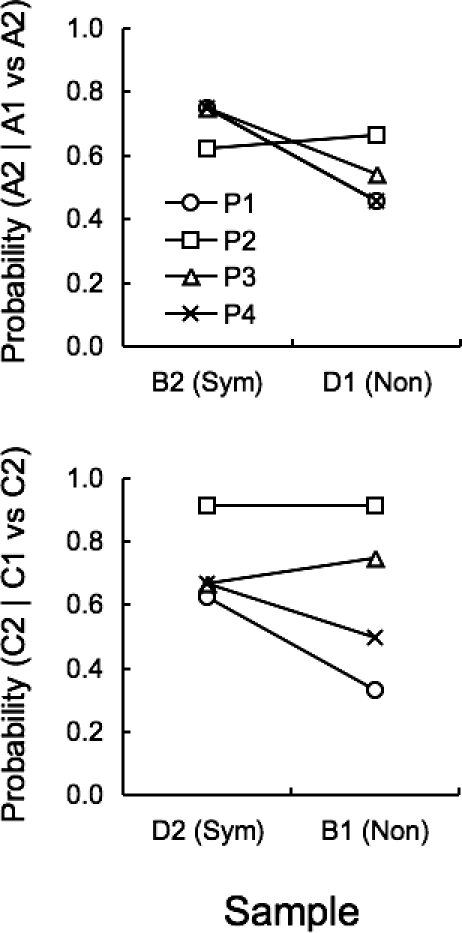
Probability of choosing A2, given a choice between A1 and A2 (top panel), and probability of choosing C2, given a choice between C1 and C2 (bottom panel), as a function of sample, during the 96-trial session of the Symmetry Test 2. Each curve corresponds to an individual pigeon.
For P1, P3 and P4, the probability of choosing A2 (top panel) was considerably higher for the sample B2 (.75 for P1, P3, and P4) than for the sample D1 (.46, .54 and .46, for P1, P3 and P4, respectively), providing clear evidence of conditional discrimination performances. On the other hand, P2 chose the A2 comparison with comparable probability when the sample was either B2 or D1 (.63 and .67, respectively), thus indicating no conditional control by the sample.
On the remaining test trials (bottom panel), when the sample was D2, the probability of choosing C2 was .63 for P1 and .67 for P4, whereas the probability of choosing B1 was .5 for P1 and .33 for P4, indicating conditional control of responding in both cases. However, P3 chose the C2 comparison with slightly higher probability when the sample was B1 than when it was D2 (.75 and .67, respectively) and P2 chose the C2 comparison with probability of .92 independently of the sample.
To summarize, choices by 2 pigeons, P1 and P4, indicated conditional control by the samples concerning both pairs of comparisons. For a 3rd pigeon, P3, choices indicated conditional control by the samples involving one pair of comparisons but not the other. Finally, the choices by P2 did not indicate any conditional control by the samples.
DISCUSSION
In a novel procedure that provides a within-subject control for testing symmetry with reinforcement, pigeons may show symmetrical responding following a sequence of training and testing in which exemplars of symmetry are provided and the prerequisite discriminations among test samples and comparisons are directly trained.
After pigeons had learned two arbitrary-matching tasks (A–B and C–D), they were given a reinforced symmetry test for half of the baseline relations (B1–A1 and D1–C1). To control for the effects of reinforcement on test trials, they were also given a reinforced test on two nonsymmetrical relations using familiar stimuli from the remaining baseline relations (D2–A2 and B2–C2). At the time of this first symmetry test, pigeons had no previous training on successive discriminations involving B and D stimuli, nor on simultaneous discriminations involving A and C stimuli. In this test, pigeons matched at chance on both symmetrical and nonsymmetrical trials, thus providing no evidence of symmetrical responding.
Nonetheless, over the course of the first symmetry test, as both symmetrical and nonsymmetrical relations were trained to high accuracy levels, subjects were provided with the missing successive and simultaneous discriminations that were necessary in the second symmetry test. In this second test, evidence of symmetry was finally observed: 1 pigeon showed clear evidence of symmetry (P4), 2 pigeons showed weak evidence (P1 and P3), and 1 pigeon showed no evidence (P2). Performances of P2 and P3 would be better described as simple rather than conditional discriminations. In both cases, the pigeons showed a systematic preference for choosing the correct comparison for the symmetrical relations on both symmetrical and nonsymmetrical trials. Such a stimulus preference may be part of the effects of the emergence of symmetry, something the present data do not allow us to ascertain.
The clear demonstration of symmetry for one pigeon (P4), and the weak evidence for another (P1), are not as robust as the evidence normally obtained with verbally able humans (e.g., Lazar et al., 1984; Sidman & Cresson, 1973; Sidman & Tailby, 1982; Spradlin, et al., 1973). However, such a finding is relevant given the absence of symmetry reported in most studies with nonhuman subjects (e.g., Barros et al., 1996; D'Amato et al., 1985; Dugdale & Lowe, 2000; Gray, 1966; Hogan & Zentall, 1977; Holmes, 1979; Lionello-DeNolf & Urcuioli, 2002; Lipkens et al., 1988; Richards, 1988; Rodewald, 1974; Sidman et al., 1982).
Especially interesting is the discrepancy between our results and those reported by Lionello-DeNolf and Urcuioli (2002). In both studies, pigeons were given symmetry-exemplar training and were tested for emergent symmetrical responding under differential reinforcement conditions (two variables deemed important for demonstration of symmetry in nonhuman animals). Nevertheless, unlike our pigeons, Lionello-DeNolf and Urcuioli's subjects had not learned all prerequisite discriminations among the stimuli used in the critical symmetry tests (i.e., second and third tests). Possibly, symmetry may have not emerged in their experiment simply because subjects lacked the prerequisite discriminative repertoire.
In the present experiment, all prerequisite discriminations seemed to be provided by training the symmetrical and nonsymmetrical relations to high accuracy levels over the course of the first symmetry test. The strategy of using novel, nonsymmetrical relations to control for reinforcement during the symmetry test (rather than reinforcing inconsistent responses) allowed symmetry to be repeatedly tested using the same baseline stimuli. Because the stimuli that first integrated the symmetrical relations were later used to form nonsymmetrical relations, and vice-versa, the first reinforced symmetry test provided all prerequisite discriminations for the subsequent test, in addition to familiarizing the pigeons with each stimulus in its respective test location.
As we ran the MTS task, samples and comparisons were presented on different locations, that is, samples always appeared in the center key and comparisons in the side keys. From the baseline training to the first symmetry testing, stimuli were presented on a novel location and their roles were reversed. Such a variation from baseline to testing could have precluded symmetry if the spatial attributes of the stimuli had become part of their defining properties (cf. Lionello-DeNolf & Urcuioli, 2002). In this case, the relations presented in the first symmetry test would not have been exactly the symmetrical versions of the baseline relations because presenting a stimulus on a novel location would have created a functionally different stimulus (cf. Iversen, 1997; Iversen, Sidman, & Carrigan, 1986; Lionello & Urcuioli, 1998; Lionello-DeNolf & Urcuioli, 2000; Sidman, 1992).
The present procedure did not explicitly control for the role of stimulus location on responding. While developing this novel procedure, we decided to focus on training the symmetry exemplars and the requisite discriminations, and to expose the pigeons to all stimuli as samples and comparisons in all possible locations. However, experiencing the stimuli as both samples and comparisons may not be enough to reduce or eliminate the role of stimulus location as a controlling variable (see Sidman et al., 1982). Lionello and Urcuioli (1998) also pointed out that training a conditional relation using multiple stimulus locations (i.e., randomly presenting the sample on any of the three keys and the comparisons on the remaining two keys) does not necessarily reduce the control exerted by location when another relation is tested. Therefore, a promising direction for future investigations would be to combine the present procedure with one in which stimuli appear in multiple locations throughout training and testing.
Our results are consistent with those of Schusterman and Kastak (1993) with a sea lion, and also with those of Yamamoto and Asano (1995) with a chimpanzee, which show emergent symmetrical responding after symmetry exemplar training. Notably, both the sea lion and the chimpanzee were also trained on identity-matching tasks, which provided them with experience with each stimulus as both sample and comparison. As in the present study, the sea lion and the chimpanzee learned before testing all the successive and simultaneous discriminations necessary for accurate performance on symmetry, and were also familiarized with each stimulus in its respective testing location.
In the present study, 1 pigeon (P4) showed emergent symmetrical responding after a very limited amount of exemplar training. Specifically, only two symmetrical relations were trained before symmetry emerged, whereas Schusterman and Kastak's (1993) sea lion was trained on six symmetrical relations before showing symmetry. Yamamoto and Asano (1995) reported only transitory evidence of emergent symmetry after training their chimpanzee on six symmetrical relations. Moreover, they found no evidence of symmetry in a subsequent test with new stimuli —a performance that might have undergone extinction due to the absence of reinforcement on test trials.
The limited amount of exemplar training in our study might lead to the conclusion that symmetrical responding already existed in the pigeons' repertoire. In that sense, the exemplar training provided over the course of the first symmetry test would have served only to establish the prerequisite discriminations required for the subsequent emergence of symmetry, as well as to set the context for such emergence. Although this hypothesis needs to be systematically checked (see suggestions below), it can be partially supported by two studies that succeeded in showing associative symmetry in pigeons in the absence of explicit exemplar training.
In one study, Frank and Wasserman (2005, Experiment 1) reported the strongest evidence to date for emergent symmetry in pigeons. They used a successive MTS procedure in which samples and comparisons appeared successively in a single location in order to prevent spatial dimensions from gaining control over responding. To control for temporal cues, two identity-matching tasks (A–A and B–B) were concurrently trained with the target arbitrary-matching task (A–B) so that during training each stimulus appeared as both the first (sample) and the second (comparison) stimulus. Half of the trials consisted of “positive” sample–comparison relations during which pecks to the comparison stimulus were reinforced (e.g., A1–B1 and A2–B2). The remaining half of the trials consisted of “negative” sample–comparison relations during which pecks to the comparison were extinguished (e.g., A1–B2 and A2–B1). Pigeons were trained until a discrimination ratio of 0.8 was reached. Afterwards, symmetry-testing sessions were conducted in which arbitrary and identity-matching trials were intermixed with symmetry testing trials (B–A) that involved the inverse of the arbitrary training relations. Even with no reinforcement on symmetry testing trials, pigeons pecked much more on these trials (B1–A1 and B2–A2) than on trials that were inconsistent with symmetry (B1–A2 and B2–A1), thus providing evidence of emergent symmetrical responding. Urcuioli (2008, Experiment 3) reported similar results, although less robust, that replicated the procedure also with pigeons.
To assess the role of multiple exemplar training on the emergence of symmetry, Lionello-DeNolf and Urcuioli's (2002) procedure may be combined with the present one so that additional baseline relations would be trained and a third and a fourth symmetry test conducted. Would we find more robust symmetry effects in a third or a fourth symmetry test compared to those obtained in the second test? On the one hand, by the time the subjects are exposed to the third and the fourth tests, they will have had a longer history of symmetry exemplars, which may play a positive role in both tests. On the other hand, not all simple discriminations will have been directly trained until the third symmetry test is completed. So, evidence for symmetry should be seen only in the fourth test. However, it would be interesting to investigate whether some evidence for symmetry would also start to appear in the third test, possibly due to the increased number of symmetry exemplars.
Acknowledgments
This research was supported by Doctoral Grant (CNPq 142544/2005-1) and Doctoral Sandwich Grant (CAPES 4457-07-2) to Saulo M. Velasco, Doctoral Grant (FAPESP 60678-4/05) and Doctoral Sandwich Grant (CAPES 0103/08-0) to Edson M. Huziwara, Researcher Grant (CNPq 302640/2007-0) and Research Support (CNPq 471953/2004-0) to Gerson Y. Tomanari. Armando Machado was funded by the Portuguese Foundation for Science and Technology (FCT).
The data was collected at the Animal Learning and Behavior Laboratory of the University of Minho in Portugal, and was presented at the 36th Annual Meeting of the Brazilian Society of Psychology (SBP), Goiânia, Brazil, Oct 2009. The authors express their gratitude to Andersen M. Picorone for the development of the software used to collect data, as well as to Cristiano V. dos Santos, Marcelo F. Benvenuti, Miriam G. Mijares, Olavo F. Galvão and Paula Debert for comments on earlier versions of the manuscript.
REFERENCES
- Antonitis J.J. Response variability in the white rat during conditioning, extinction, and reconditioning. Journal of Experimental Psychology. 1951;42:273–281. doi: 10.1037/h0060407. [DOI] [PubMed] [Google Scholar]
- Azrin N.H, Hutchinson R.R, Hake D.F. Extinction-induced aggression. Journal of the Experimental Analysis of Behavior. 1966;9:191–204. doi: 10.1901/jeab.1966.9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Holmes Y, Barnes-Holmes D, Roche B, Smeets P.M. Exemplar training and a derived transformation of function in accordance with symmetry: II. The Psychological Record. 2001;51:589–603. [Google Scholar]
- Barros R.S, Galvão O.F, Fontes J.C. Um teste de simetria após treino de discriminações condicionais de posição com macaco Ateles paniscus paniscus [A symmetry test after training position conditional discriminations with macacus Ateles paniscus paniscus] Acta Comportamentalia. 1996;4:181–204. [Google Scholar]
- Barros R.S, Galvão O.F, McIlvane W.J. Generalized identity matching-to-sample in Cebus apella. The Psychological Record. 2002;52:441–460. doi: 10.1007/s40732-014-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brino A.L.F, Galvão O.F, Barros R.S. Successive identity matching to sample tests without reinforcement in Cebus apella. Ciência & Cognição. 2009;14:2–11. [Google Scholar]
- D'Amato M.R, Salmon D.P, Loukas E, Tomie A. Symmetry and transitivity of conditional relations in monkeys (Cebus apella) and pigeons (Columbia livia) Journal of the Experimental Analysis of Behavior. 1985;44:35–47. doi: 10.1901/jeab.1985.44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detanico A, Lain R. (Designers) Utopia [Digital typography] 2001. Retrieved from http://www.detanicolain.com.br/utopia.
- Devany J.M, Hayes S.C, Nelson R.O. Equivalence class formation in language-able and language-disabled children. Journal of the Experimental Analysis of Behavior. 1986;46:243–257. doi: 10.1901/jeab.1986.46-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube W.V, McIlvane W.J. Some implications of a stimulus control topography analysis for emergent stimulus class. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. Amsterdam: Elsevier; 1996. pp. 197–218. (Eds.), [Google Scholar]
- Dube W.V, McIlvane W.J, Callahan T.D, Stoddard L.T. The search for stimulus equivalence in nonverbal organisms. The Psychological Record. 1993;43:761–778. [Google Scholar]
- Dugdale N, Lowe C.F. Naming and stimulus equivalence. In: Blackman D.E, Lejeune H, editors. Behaviour analysis in theory and practice: Contributions and controversies. Hillsdale, NJ: Erlbaum; 1990. pp. 115–138. (Eds.), [Google Scholar]
- Dugdale N, Lowe C.F. Testing for symmetry in conditional discriminations of language-trained chimpanzees. Journal of the Experimental Analysis of Behavior. 2000;73:5–22. doi: 10.1901/jeab.2000.73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R. Resurgence of previously reinforced behavior during extinction. Behaviour Analysis Letters. 1983;3:391–397. [Google Scholar]
- Epstein R. Extinction-induced resurgence: Preliminary investigations and possible applications. The Psychological Record. 1985;35:143–153. [Google Scholar]
- Frank A.J, Wasserman E.A. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão O.F, Calcagno S, Sidman M. Testing for emergent performances in extinction. Experimental Analysis of Human Behavior Bulletin. 1992;10:18–20. [Google Scholar]
- Galvão O.F, Barros R.S, Santos J.R, Brino A.L.F, Brandão S, Lavratti C.M, Dube W.V, McIlvane W.J. Extent and limits of the matching concept in Cebus apella: A matter of experimental control. The Psychological Record. 2005;55:219–232. [Google Scholar]
- Gray L. Backward association in pigeons. Psychonomic Science. 1966;4:333–334. [Google Scholar]
- Hayes S.C. Nonhumans have not yet shown stimulus equivalence. Journal of the Experimental Analysis of Behavior. 1989;51:385–392. doi: 10.1901/jeab.1989.51-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S.C, Barnes-Holmes D, Roche B. Relational frame theory: A post-Skinnerian account of language and cognition. New York: Plenum; 2001. [DOI] [PubMed] [Google Scholar]
- Herman L.M, Gordon J.A. Auditory delayed matching in bottlenose dolphin. Journal of the Experimental Analysis of Behavior. 1974;21:19–26. doi: 10.1901/jeab.1974.21-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L.M, Hovancik P.R, Gory J.D, Bradshaw G.L. Generalization of visual matching by a bottlenose dolphin (Tursiops truncatus): Evidence of invariance of cognitive performance with visual and auditory materials. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:124–136. [Google Scholar]
- Hogan D.E, Zentall T.R. Backward associations in the pigeon. American Journal of Psychology. 1977;90:3–15. [Google Scholar]
- Holmes P.W. Transfer of matching performance in pigeons. Journal of the Experimental Analysis of Behavior. 1979;31:103–114. doi: 10.1901/jeab.1979.31-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne P.J, Lowe C.F. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65:185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen I.H. Matching-to-sample performance in rats: A case of mistaken identity. Journal of the Experimental Analysis of Behavior. 1997;68:27–45. doi: 10.1901/jeab.1997.68-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen I.H, Sidman M, Carrigan P. Stimulus definition in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1986;45:297–304. doi: 10.1901/jeab.1986.45-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastak D, Schusterman R.J. Transfer of visual identity matching-to-sample in two California sea lions (Zalophus californianus) Animal Learning & Behaviour. 1994;22:427–435. [Google Scholar]
- Keller F.S, Schoenfeld W.N. Principles of psychology: A systematic text in the science of behavior. New York: Appleton-Century-Crofts; 1950. [Google Scholar]
- Kuno H, Kitadate T, Iwamoto T. Formation of transitivity in conditional matching to sample by pigeons. Journal of the Experimental Analysis of Behavior. 1994;62:399–408. doi: 10.1901/jeab.1994.62-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar R, Davis-Lang D, Sanchez L. The formation of visual stimulus equivalence in children. Journal of the Experimental Analysis of Behavior. 1984;41:251–266. doi: 10.1901/jeab.1984.41-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman D.C, Iwata B.A. Developing a technology for the use of operant extinction in clinical settings: An examination of basic and applied research. Journal of Applied Behavior Analysis. 1996;29:345–382. doi: 10.1901/jaba.1996.29-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello K.M, Urcuioli P.J. Control by sample location in pigeons' matching to sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M. The search for symmetry: 25 years in review. Learning & Behavior. 2009;37:188–203. doi: 10.3758/LB.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M, Urcuioli P.J. Transfer of pigeons' matching to sample to novel sample locations. Journal of the Experimental Analysis of Behavior. 2000;73:141–161. doi: 10.1901/jeab.2000.73-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M, Urcuioli P.J. Stimulus control topographies and test of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens R, Kop P.F.M, Matthijs W. A test for symmetry and transitivity in the conditional discrimination performances of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay H.A, Sidman M. Teaching new behavior via equivalence relations. In: Brooks P.H, Sperber R, McCauley C, editors. Learning and cognition in the mentally retarded. Hillsdale, NJ: Erlbaum; 1984. pp. 493–513. (Eds.), [Google Scholar]
- Oden D.L, Thompson R.K, Premack D. Spontaneous transfer of matching in infant chimpanzees. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:140–145. [PubMed] [Google Scholar]
- Peña T, Pitts R.C, Galizio M. Identity matching-to-sample with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior. 2006;85:203–221. doi: 10.1901/jeab.2006.111-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R.W. The question of bidirectional associations in pigeons' learning of conditional discrimination tasks. Bulletin of the Psychonomic Society. 1988;26:577–579. [Google Scholar]
- Rodewald H.K. Symbolic matching-to-sample by pigeons. Psychological Reports. 1974;34:987–990. [Google Scholar]
- Saunders R.R, Green G. A discrimination analysis of training-structure effects on stimulus equivalence outcomes. Journal of the Experimental Analysis of Behavior. 1999;72:117–137. doi: 10.1901/jeab.1999.72-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman R.J, Kastak D. A California see lion (Zalophus californianus) is capable of forming equivalence relations. The Psychological Record. 1993;43:823–839. [Google Scholar]
- Sidman M. Equivalence relations: Where do they come from. In: Blackman D.E, Lejeune H, editors. Behaviour analysis in theory and practice: Contributions and controversies. Hillsdale, NJ: Erlbaum; 1990. pp. 93–114. (Eds.), [Google Scholar]
- Sidman M. Adventitious control by the location of comparison stimuli in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1992;58:173–182. doi: 10.1901/jeab.1992.58-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations and behavior: A research story. Boston: Authors Cooperative; 1994. [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Cresson O., Jr Reading and crossmodal transfer of stimulus equivalence in severe retardation. American Journal of Mental Deficiency. 1973;77:515–523. [PubMed] [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discrimination of rhesus monkeys, baboons and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching to sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner B.F. The behavior of organisms: An experimental analysis. New York: Appleton-Century-Crofts; 1938. [Google Scholar]
- Spradlin J.E, Cotter V.W, Baxley N. Establishing a conditional discrimination without direct training: A study of transfer with retarded adolescents. American Journal of Mental Deficiency. 1973;77:556–566. [PubMed] [Google Scholar]
- Tomonaga M, Matsuzawa T, Fujita K, Yamamoto J. Emergence of symmetry in a visual conditional discrimination by chimpanzees (Pan troglodytes) Psychological Reports. 1991;68:51–60. doi: 10.2466/pr0.1991.68.1.51. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J. Associative symmetry, antisymmetry, and a theory of pigeons' equivalence-class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K.G, Hayes S.C. Resurgence of derived stimulus relations. Journal of the Experimental Analysis of Behavior. 1996;66:267–281. doi: 10.1901/jeab.1996.66-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Asano T. Stimulus equivalence in a chimpanzee (Pan troglodytes) The Psychological Record. 1995;45:3–21. [Google Scholar]
- Zentall T.R, Edwards C.A, Moore B.S, Hogan D.E. Identity: The basis for both matching and oddity learning in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:70–86. [PubMed] [Google Scholar]
- Zentall T.R, Hogan D.E. Pigeons can learn identity or difference, or both. Science. 1976;191:408–409. doi: 10.1126/science.191.4225.408. [DOI] [PubMed] [Google Scholar]