Abstract
Marine protected areas (MPAs) that exclude fishing have been shown repeatedly to enhance the abundance, size, and diversity of species. These benefits, however, mean little to most marine species, because individual protected areas typically are small. To meet the larger-scale conservation challenges facing ocean ecosystems, several nations are expanding the benefits of individual protected areas by building networks of protected areas. Doing so successfully requires a detailed understanding of the ecological and physical characteristics of ocean ecosystems and the responses of humans to spatial closures. There has been enormous scientific interest in these topics, and frameworks for the design of MPA networks for meeting conservation and fishery management goals are emerging. Persistent in the literature is the perception of an inherent tradeoff between achieving conservation and fishery goals. Through a synthetic analysis across these conservation and bioeconomic studies, we construct guidelines for MPA network design that reduce or eliminate this tradeoff. We present size, spacing, location, and configuration guidelines for designing networks that simultaneously can enhance biological conservation and reduce fishery costs or even increase fishery yields and profits. Indeed, in some settings, a well-designed MPA network is critical to the optimal harvest strategy. When reserves benefit fisheries, the optimal area in reserves is moderately large (mode ≈30%). Assessing network design principals is limited currently by the absence of empirical data from large-scale networks. Emerging networks will soon rectify this constraint.
Keywords: biodiversity, fishery profit, ocean policy, sustainability
Global concern for ocean ecosystems has driven growing calls for marine protected areas (MPAs) (e.g., refs. 1, 2). Although the diversity of ocean threats is large (3), only a few of these threats can be mitigated by designating places for protection. Fishing is the human impact that has been the focus of most MPAs. In the extreme, a small subset of the MPAs, known as “marine reserves,” bans all forms of fishing. Only a tiny fraction of the ocean has been set aside as marine reserves, but the global number of reserves now exceeds 200 (4). Syntheses of reserve impacts both inside (5–7), and outside their borders (ref. 8 and Pelc et al., in this issue of PNAS) commonly show large benefits. Biomass typically triples relative to control areas outside the reserves, but the range of responses is enormous (5, 9). Hundreds of scientific studies have explored the details of these ecosystem responses, including the time course of change (10–13), the species that benefit (14, 15), the species that do not benefit (16–18), the cascading effects through foodwebs of excluding human fishing (12, 19, 20), and the challenges in separating true reserve impacts from confounding effects (9).
Our intent here, however, is not to evaluate the successes of marine reserves. Rather, we focus on a consistent failure of single reserves and an emerging solution—networks. For nearly all species in the sea, individual marine reserves provide only minor conservation benefits. The problem is that typically the reserve size is minute compared with the geographic extent of the species it is designed to protect (21–23). Large relative changes in biomass or density within a single small reserve provide relatively limited benefits to the species as a whole. If marine reserves and other MPAs are to provide significant conservation benefits to species, they must be scaled up. One means is to increase their size greatly (e.g., the Papahānaumokuākea National Monument, which covers nearly 360,000 km2). Given the potential economic and social costs of such large individual reserves in heavily populated coastal areas, this option is unlikely to be common. Alternatively, a number of nations (e.g., Australia, the United States; refs. 24, 25) have pursued an alternative approach to scaling up marine reserve benefits: networks of multiple MPAs. By aggregating the benefits of multiple MPAs, the network can have larger impacts. More importantly, a number of theoretical models suggest that networks can have emergent benefits that make the network more than the sum of its individual parts (26–28).
Marine reserve networks are inherently spatial management tools, and their design criteria (e.g., location, size, spacing, and configuration) theoretically correlate with their likelihood of effectiveness. The pace of scientific exploration of these issues has been brisk (5). Given the diversity of goals (e.g., conservation versus fisheries), processes included (e.g., patterns of movement, forms of density dependence), and assumptions made about fishery management outside the reserve, it is not surprising that model findings often have been at odds across studies (e.g., 14, 29–31). Here we synthesize this large body of research to evaluate how best to meet objectives in marine conservation and fishery management and to demonstrate how the perceived inherent tradeoffs between these two goals can be reduced or eliminated by network designs that provide simultaneous benefits to conservation and fishery prosperity. We address four categories of optimal marine reserve design: (i) location attributes of single reserves without regard to network factors, (ii) size and spacing of reserves in a network, (iii) location attributes of reserves driven by network benefits, and (iv) the proportion of a region to be placed in a network of reserves to achieve conservation and fishery goals. We conclude with a discussion of factors affecting optimal reserve design that need further investigation.
Locating Single Reserves
Single, isolated marine reserves constitute the majority of marine reserves worldwide (5). A multitude of environmental, ecological, and socioeconomic criteria have been established for the optimal siting of single reserves (e.g., 32, 33), especially for enhancing biological conservation. We highlight the criteria we consider especially important (Table 1). The fundamental factor for enhancing biological conservation and fishery prosperity is that an isolated reserve must be self-persistent and therefore must have a positive population growth (34). Although this concept is axiomatic, it is rarely followed. For example, in terrestrial landscapes convenient placement of reserves in de facto refuges can fail the persistence criterion when such areas represent extreme environmental conditions (e.g., high elevation) that are ecological sinks dependent on immigration from less extreme, unprotected areas nearby (35). Protecting de facto marine refuges (e.g., a remote reef that is inaccessible) can present similar problems of persistence if the refuge exhibits a net loss of biomass over time because there is more export of production (36, 37) than input of new recruits from neighboring fished areas. Further, placing reserves in de facto refuges may add little new protection, and therefore, by definition, has little effect on fishery prosperity. Even protecting de facto refuges of relatively high population density may be ineffective in meeting conservation goals, because these refuges can fail to persist amid a matrix of intensive fishing. Key to avoiding such pitfalls is consideration of spatial variation in historic regional fishing rates. Intense historical fishing can create remnant populations that are high in density (3, 38) but not self-persistent because of degraded local conditions (39), and protecting such an area in a reserve may require supplemental restoration to meet conservation objectives (23).
Table 1.
Patch (i.e., local species population) attribute and decision to protect it within a reserve, given conservation or fishery objective
| Place in reserve, given objective? |
||
| Patch attribute | Conservation | Fishery |
| Persistent | Y | Y |
| High population growth rate | Y | N |
| High carrying capacity | Y | Y |
| Larval source | Y | Y |
| Already heavily/overexploited | Y | Y |
| Costly to harvest | N | |
| —Fishery goal: minimize costs | Y | |
| —Fishery goal: maximize profits | N | |
| Has distinct habitat edge | Y | N |
Large conservation gains can come from protecting populations that are inherently persistent and have a high local carrying capacity but have been historically overfished. Economic benefits to a fishery also are expected if a persistent reserve area is a source of biomass to neighboring fished areas via adult spillover and/or larval export (27, 40, 41). As with conservation, the optimal reserve location for fisheries prosperity need not have high productivity; areas with high productive capacity can be more profitable as fished sites than as sources of spillover or export (40, 42). Protecting a source population may compensate for harvest lost from the reserve area, although this potential fishery benefit certainly depends on the life history and demography of the species and will not always be realized (18, 31, 43).
The location of a reserve in relation to coastal habitat also can strongly influence its effectiveness, sometimes in ways that differ for conservation versus economic perspectives. Matching a reserve's edge with a habitat boundary (e.g., reef edge) can enhance conservation benefits for mobile species by limiting spillover into nonprotected waters (44, 45). Conversely, placing a reserve edge in the midst of continuous habitat may enhance adult spillover and thus benefit fisheries (46). From a fishery perspective, the optimal geographic placement of a reserve also depends on whether the goal is to maximize benefits or minimize costs. Placeing a reserve edge near an access point (e.g., port) can maximize benefits by increasing the value of “fishing the line,” minimizing transportation costs to the reserve edge (41). Such areas near ports are often the first to be overexploited and thus also may be good candidate locations for conservation. Conversely, when spillover benefits are not expected, fisheries are better served by reserves placed further from port in order to minimize displacement of harvest effort, and thus minimally increase associated travel costs to fishing grounds on the far side of the reserve (47).
Why a Network?
One reserve may play an important role in enhancing or stabilizing adult marine populations locally (5), but if the reserve is too small, persistence may require input from the surrounding area. Modeling studies estimate that single reserves must be at least as large as the average dispersal distance for a species without contributions from elsewhere (48–50). Larval and adult movements typically are long enough to require that reserves be at least tens, and perhaps hundreds, of kilometers wide (20, 41). Few reserves are this large (3, 5, 7), and so by these criteria few single reserves are self-sustaining.
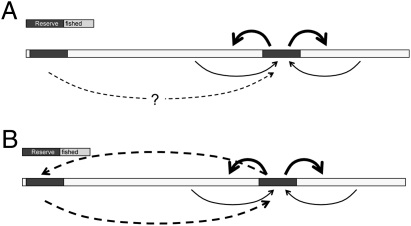
Reserves facing recruitment deficits can receive demographic bailouts from two sources: fished areas and other reserves. With high fishing pressure, the input of larvae from surrounding fished areas drops as the adult fished population declines (51), so the fate of populations within the reserve are linked directly to the health and trajectory of the outside fishery (52, 53). For reserves to help stabilize a fishery or reverse its declines (a fishing goal), or for the populations within reserves to be on a demographic trajectory separate from that of a declining fishery (a conservation goal), reserves must be individually large enough to mitigate the recruitment overfishing problem or must be able to receive sufficient larvae from nonfished sources, i.e., from other reserves (Fig. 1A). As a result, adding more reserves to a system may benefit the overall system and help meet both fishery and conservation goals.
Fig. 1.
Population connectivity among reserve and fished patches along a coast. (A) Larval export from a reserve (bold arrows) may benefit the fishery but may make a population in a single reserve non–self-sustainable. Sustainable reserves can be achieved through larval input from fished areas (thin arrows). For small reserves, this larval input dominates the population biology of the reserves. When populations outside the reserve decline, populations inside the reserve also decline unless they can receive larval input from other reserves (dashed arrow). As a result, unless there is significant input from nonfished areas, the demography of the reserve and the demography of fished areas will be tightly coupled, and the success of the reserve ultimately will be determined by the success of fisheries regulations outside the reserve. (B) If a second reserve with a large enough population is sufficiently close, its connections with the original reserve can help stabilize its populations (dashed, two-way arrows).
Hastings and Bostford (34) model the ability of a new reserve to rescue a population that is declining in a prior reserve, under the severe restriction that there is no input from fished areas. The new reserve can alter the fate of the original reserve only if the larvae it contributes to the original reserve are sufficient to reverse the population decline. Two reserve populations, each sufficiently large and sufficiently close, therefore can be more stable demographically than either one separately (Fig. 1B). Under these criteria, a second reserve would help achieve both fishery and management goals. As a result, one simplified rule of thumb for reserve spacing is that reserves should contribute sufficient larvae to and receive sufficient larvae from other reserves. Without this criterion, the fate of populations within non–self-sustaining reserves is tied primarily to the fate of the fished populations outside the reserves.
Two other considerations are generally applicable to establishing reserves in a network: habitat representation and replication (54). Reserve placement in all major marine habitats (i.e., representation) is a key network feature for meeting conservation and fishery goals, because marine species tend to segregate by habitat (e.g., depth, substrate, salinity, and other factors) and often use different habitats during different life stages (32). Placement of multiple reserves in each habitat (i.e., replication) promotes persistence not only through demographic coupling of reserves (discussed above) but also by providing insurance against catastrophes. When disasters (e.g., oil spills, tsunamis, ship groundings) strike the coast, reserves in the region that are smaller than the scale of the disturbance are at risk (55). Because disasters, human-caused and natural, are a common feature of most marine environments, replication of reserves for insurance purposes is critical over the long term (55, 56). The greater the frequency, size, and intensity of disturbance, the greater is the need for added replication to achieve any conservation or fisheries goal.
Size and Spacing of Reserves in a Network
Optimal size and spacing of marine reserves in a network is strongly influenced by the spatial scale of movement of the target species (21). The consequences of movement differ dramatically depending on when movement occurs during the life cycle. When adults leave the boundary of a reserve, they are at risk of becoming part of the fishery. When larvae leave a reserve, however, they can disperse without elevated risk because of their minute size and limited exposure to the fishery. Thus, if larvae disperse successfully to another reserve, where they can grow to adulthood under protection, they may be just as successful as if they never dispersed from their natal reserve. Therefore, scales of adult movement constrain the effectiveness of reserves of different sizes, and scales of larval movement provide a means of connecting the dynamics of multiple reserves in a network (21, 33, 53, 57–60).
Theoretical models of the impacts of reserves on population dynamics suggest that multiple reserves can have three kinds of effects on ecosystems. First, multiple reserves can have additive effects, e.g., the increase in population size from the entire network is simply the sum of the increases that would be observed from each reserve in isolation. Second, additional reserves may have declining effects if the marginal value of extra protection declines as more areas are protected. For example, the number of species protected within a reserve is a function of its placement, habitat heterogeneity, and area (61). Because species–area curves increase at a diminishing rate as area increases (62), doubling the area in reserves will not necessarily double the number of species protected. Similarly, if some locations are population sinks that contribute few offspring to future generations, protecting sink sites and shifting fishing effort to more productive sources of larval production could reduce conservation benefits. Third, multiple reserves may have a multiplicative effect on ecosystems if the reserve system as a whole has benefits that are more than the additive sum of benefits of individual reserves.
Achieving such multiplicative effects is the goal when designing reserve networks (63). Individual reserves can enhance population growth outside their borders when enhanced larval production from a reserve seeds larger populations in fished areas. However, a more powerful source of network benefits derives from the demographic coupling of populations in separate reserves. If each reserve can enhance the rate of population growth in the other reserve, then this population synergy potentially can result in increased numbers both within reserves and outside (26, 64). Demographic synergy can be achieved only when reserve populations are directly connected by larval dispersal with sufficient numbers of larvae moving between reserves to affect their demographics (Fig. 1B). As a result, a profitable network strategy is to space reserves close enough to allow substantial larval movement among them. Such placement is necessary but not sufficient to guarantee a multiplicative effect.
To benefit fisheries, the overwhelming majority of theoretical and empirical results indicate that reserve area should be divided into a network of closures whose size and spacing maximize the net effect of spillover of fishable adults and export of larvae to fished areas between the reserves (1, 26, 43, 49, 65, 66). Designs characterized by a greater number of smaller reserves increase the amount of reserve edge and decrease the average distance from fished areas to the reserve edge. Thus many, smaller reserves spaced closely can enhance fishery prosperity by maximizing larval export from reserves to the fished area, but only to the extent that the reserves can sustain themselves at the high biomass densities needed to produce the high larvae densities (53). Minimum reserve size is limited by the rate of adult spillover, which directly benefits the fishery but compromises the density of protected biomass within the reserve. From a conservation perspective, larger reserves are always preferred, because they enhance population persistence by increasing the protection of adults (i.e., limiting spillover) and the likelihood that larvae settle within their natal reserve (49, 53, 67). Consequently, there is a tradeoff of conservation benefits versus fishery benefits that is mediated by the interaction of the size, number, and spacing of reserves in a network.
Given the enormous variability in scales of adult movement among marine species, the choice of reserve size represents an inherent compromise that creates winning and losing species. Using reserves to protect highly mobile adult stocks (e.g., tuna and other pelagics) may be impractical in most coastal settings, because the requisite reserve size would be politically impractical. However, rapidly emerging data on scales of movement for a diverse range of species suggest that moderately sized reserves that are several to tens of kilometers in alongshore length and extend offshore to encompass depth-related movements should be suitable to contain adult movement for much of the diversity of nearshore species (21, 53, 68). Additionally, reserves with moderate spacing may enhance both fisheries and conservation benefits. Interreserve distances from tens of kilometers to about one hundred kilometers can enhance both conservation and fishery benefits, because they approach without exceeding the mean larval dispersal distances estimated for many fished coastal marine species (21, 36, 53, 69–74). In these cases, mean dispersal over multiple generations [not dispersal in any one year, which can be quite different (75)] is the most critical metric, because population build-up and persistence in a reserve depends on the average dispersal over multiple years that generates multiple age classes.
Overall, moderately sized reserves with moderate spacing are most likely to maintain their adult populations (and to be self-persistent) and to exhibit interreserve connectivity via larval dispersal (for promoting metapopulation and metacommunity persistence). Furthermore, a network of reserves maximizes larval export to adjacent fishing areas, potentially allowing high yields and profits.
Locating Reserves in a Network
Reserve location can be critical to network effectiveness. In a system using multiple reserves, some locations provide strategic benefits to the collective impact of the network. Although many of the criteria for siting a single reserve apply to configuring multiple reserves (Table 1), the net effect of an optimally designed network can exceed the sum of the individual reserve effects (63). For example, a reserve network might benefit from a reserve placed in a region that exports all its larvae, whereas a single reserve in such a place would be unsustainable. Furthermore, multiple configurations may be able to achieve similar outcomes (56), thereby offering considerable flexibility for stakeholders in choosing a reserve design.
Long-term population and biodiversity persistence depends on multigenerational dispersal pathways involving source and sink locations of variable vulnerability and resilience and may be achieved by reserve networks with a configuration different from that desired when the goal is to maximize current conservation (34, 53). For example, representative positioning of reserves across multiple habitat types—a simple mechanism commonly exploited for maximizing current biodiversity conservation (e.g., Australia's Great Barrier Reef and California Northern Channel Islands network reserve designs, refs. 25, 54, 76)—may fail to protect selectively particular (e.g., low-risk) areas that compromise conservation of current biodiversity levels but can enhance long-term persistence (56). Redundancy of habitat protection in reserves also promotes persistence, especially when reserves are configured to promote gene flow among them (e.g., via larval dispersal, ref. 77), although such a network has the potential to compromise current biodiversity conservation when the proportion of protection in a region is fixed.
Fishery yields and profits are expected to be maximized in a reserve network when reserves are configured to maximize larval export to fished areas (49). As with siting a single reserve, a simple rule of thumb is to protect the greatest sources or upstream patches and fish sinks or downstream patches (27, 63). However, in the case of multiple reserves, heterogeneity in larval dispersal can result in multiple, nearly equivalent, optimal reserve network configurations containing source patches of variable (not necessarily maximum) strength (28). Furthermore, as was the case for maximizing conservation, optimal reserve configuration for maximizing fishery prosperity may include reserves that serve as intermediary sites connecting other reserves in the network. Regardless of the particular network, the fundamental messages are that (i) larval connectivity drives optimal reserve configuration, (ii) fishery prosperity is maximized when the network maximizes overall—not individual reserve—larval export to fished areas, and (iii) the more spatially heterogeneous the connectivity pattern, the greater is the potential increase in fishery prosperity (28, 53, 78, 79). It also is important to note that the fisheries-based effectiveness of a reserve network, i.e., its ability to maximize larval export, depends not only on reserve configuration but also on optimal, heterogeneous regulation of harvest in the fished areas (28).
Proportion of Protection in a Region
It is a common perception that conservation and fishery perspectives promote dramatically different, irreconcilable estimates of the optimal percentage of a region to protect in reserves. For many species, however, this assumption may not be true. Although the two management objectives differ markedly in how they are measured, both rely on abundant and persistent populations, features promoted by reserves. For many species both objectives may be nearly maximized across a range of overlapping reserve percentages (30). Below we highlight this region of overlap that is critical for minimizing the tradeoffs involved in using reserves to achieve fishery and conservation goals.
In this synthesis we assume the use of marine reserves despite uncertainty about the profitability of reserves and our recognition that fishery profit is influenced by numerous, often interacting, factors characterizing fish population dynamics and fishery management, including reserve design (17, 29, 40, 80, 81). Table 2 presents a list of factors with predicted positive and negative individual effects on fishery profit as mediated by attributes of reserve design. Model evaluations of marine reserves typically focus on one or a few of these factors and invariably (and understandably) make a large number of simplifying demographic, ecological, environmental, and socioeconomic assumptions. The outcome is a collection of results, each linked to a specific set of assumptions, advocating for and against using reserves to benefit fisheries. This strong divergence of results can exacerbate efforts to determine an optimal reserve policy satisfying both conservation and fishery stakeholders.
Table 2.
Factors that affect fishery profitability of marine reserves, with brief descriptions of underlying mechanisms and reserve design implications to promote profitability
| Factor | Increases reserve profitability | Reduces reserve profitability | Reserve design attributes |
|---|---|---|---|
| Adult age/stage structure | Older/larger fish in reserves produce more offspring, resulting in greater larval export to fished areas (65). Reserves increase biomass contribution to harvested populations by buffering against evolution for reduced size at maturity (98). | Density dependent stock-recruitment dynamics enable reserves to increase biomass yield only for overexploited populations (17). Reserves generally reduce yields of species for whom biomass, reproduction or mortality vary with age (18). | Scale reserve network to maximize net export of larvae to fished areas (e.g., 49). |
| Adult movement | Reserves may increase yield if properly sized relative to adult movement (33). For both dispersal and migration, reserves can increase long-term yield and recovery probabilities (99). Reserves protecting nursery or spawning grounds can increased yields of mobile fishery species (100). | Adult spillover from reserves increases catch only under a limited set of conditions (41, 60, 101). Reserves may be ineffective if not configured to account for nondiffusive movement behavior of adult fish (102). | Scale reserve larger than adult home range size to promote population build-up (68, 103). Place boundaries at continuous habitat to allow spillover (46). Use additional conservation measure for pelagics. |
| Density-dependent recruitment | Reserves may increase fishery profits under mixed intra/intercohort density dependence (43). | Reserves increase yield only under intercohort density dependence (104). | Place reserves in source locations and fish larval sinks (e.g., 28, 40). |
| Density-dependent growth | Crowding in fished areas from larval input from reserves decreases individual growth and can reduce biomass yield (105). | ||
| Species interactions | Indirect negative effects of protection through competition or predation may reduce population sizes with reserves (20). | ||
| Spatial heterogeneity | Reserves can increase profits under ecological and/or economic spatial heterogeneity (40). | Protect area with low growth rate but high carrying capacity that is net biomass exporter (40). | |
| Habitat degradation | High fishing pressure between reserves may reduce yield through habitat disturbance (e.g., via trawling; 106). | Protect sensitive habitat within reserves. Limit damaging harvest practices elsewhere (107). | |
| Environmental stochasticity/ management uncertainty | Reserves can increase yield by buffering against population instability/management uncertainty (95). Reserves can increase profits of stochastic spatial resource (28). | Redundancy in habitat coverage by reserves can provide spatial buffering against shocks to system (56). | |
| Fishermen behavior | Reallocation of spatial fishing effort can reduce positive benefits of reserves (47). | Place reserves beneficial to fishery near port and detrimental reserves far from port (47, 108). | |
| Enforcement costs | Low monitoring and enforcement costs may provide reserves with a comparative cost advantage (109). | Poaching can reduce or eliminate the positive effects of fish dispersal on yields with reserves (110). | Make reserve design permanent and simple (88, 111). |
| Economic discount rate | Increasing the discount rate reduces the incentive to preserve high densities (in reserves) for generating future profits (40). |
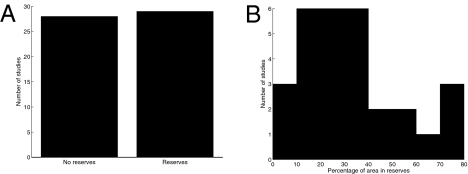
A survey of 33 modeling exercises with 57 case studies explicitly testing the impact of marine reserves on expected fisheries yields and/or profits reveals that reserves have the potential to enhance fishery prosperity for a range of species and ecological settings: Approximately half of the studies suggest higher fishery yields/profits are obtained when marine reserves are part of the management strategy (Fig. 2A). Moreover, when marine reserves enhance fishery prosperity, peak benefits are projected to occur when reserves constitute as much as half the total habitat (Fig. 2B). These projected benefits with moderately large portions of area in reserves support the conclusion that reserve networks can provide simultaneous conservation and fisheries benefits for some species and locations. Placing a still larger proportion of the area in reserves would provide even greater conservation benefits, but the revenue lost because of the displacement of fishing effort from protected areas typically would outweigh the gains generated by the reserves (e.g., via larval export, ref. 43). Furthermore, for many species, reserves may not increase long-term profits compared with those attainable under optimal nonreserve management or may increase profits only under limited conditions (18). Importantly, our synthesis focuses on optimal management; when a fishery is being overexploited (i.e., management is not optimal) or when environmental or management uncertainty is considered, reserves benefit fisheries across an even greater set of species (17, 82, 83).
Fig. 2.
Synthesis of 33 peer-reviewed scientific publications representing 57 case studies that explicitly examined how much area should be protected from fishing to maximize long-term fishery yield and/or profit. (Papers were obtained via an ISI Web of Science search on July 14, 2009 using keywords “marine reserve(s)” OR “marine protected area(s)” AND “yield” OR “profit.” We recovered 131 papers and considered all appropriate ones.) (A) Number of studies concluding that fishery yields/profits were maximized via management without versus with the use of marine reserves. (B) Frequency distribution of the percentage of fishing grounds recommended to be included in marine reserves in the studies that found reserves to be a part of yield/profit-maximizing management. Literature included in the survey is available on request from the authors.
Within their boundaries, reserves almost invariably increase biomass and biodiversity (10), with greater absolute increases (especially in biomass) expected for larger protected areas (5, 6). Given the economic constraints suggested by the fishery perspective, what proportion of protected area to fished area is sufficient to support conservation goals? Answering this question requires determining at what reserve proportions do conservation benefits begin to saturate and whether this point is low enough not to compromise fishery prosperity significantly. Without replenishment of larvae from fished areas, Botsford et al. (50) estimate persistence within reserves (i.e., self-replenishment) requires that the reserve be larger than the mean larval dispersal distance of the fished species; if the reserve area is configured in a network of smaller reserves, network-wide replenishment can be achieved if reserves collectively constitute one-third to one-half of the region. Although these estimates are based on the extreme assumption that overexploitation has reduced local fished stocks to negligible levels in fished areas, overexploitation does exist in many parts of the world (38, 84, 85), and increased local fishing pressure in response to displacement from reserves is an expected feature of optimal management for maximizing fishery prosperity (9, 43). A similar range of reserve coverage may be required to achieve marine biodiversity conservation goals as well (64, 86, 87). Consequently, in the face of poor fisheries management, a conservative estimate of the minimum proportion of a region to be placed in reserves lies at approximately one-third, a value comfortably within the maximum proportion estimated to maximize fishery prosperity for a number of species (Fig. 2B). A comparable figure has been recommended by scientists involved in empirical case studies (88), and this range of protection is consistent with estimates derived from analyses of minimum reserve sizes and maximum reserve spacing as described above. For example, under California's Marine Life Protection Act, preferred alongshore size (20 km) and maximum spacing (50 km) guidelines derived from patterns of adult and larval dispersal would yield a fractional area in reserves of 29%. Such target proportions for reserves would decrease if fishing rates outside reserves decrease or if reserves are placed strategically to maximize conservation benefits. Intriguingly, these estimates derived from seeking simultaneous benefits for conservation and fisheries prosperity exceed the global targets driven solely by conservation interests (e.g., Convention for Biological Diversity targets of 10% MPAs in 2010 and 20% MPAs in 2020).
Larval Export in Marine Reserve Models
Nearly all the results used to characterize optimal reserve design assume export of dispersing larvae beyond reserve boundaries despite limited knowledge of the spatial details of this key process. There is growing empirical evidence for larval export and its potential benefits to conservation and fisheries, but the results are species-specific and difficult to quantify accurately (ref. 21 and Pelc et al., in this issue of PNAS). Nonetheless, simple messages (e.g., that larval dispersal is spatially and temporally variable and correlated with oceanographic conditions, refs. 89–91) have guided us in generating increasingly complex depictions of larval dispersal for use in marine reserve models (37, 92, 93). Recent efforts have demonstrated that larval connectivity is inherently an intermittent and heterogeneous process on annual time scales (93). These findings alter our perceptions of species persistence and what it means for a patch to be a larval source or sink (75, 94). Although stochastic larval dispersal patterns can resemble simple functional forms (e.g., Gaussian) when averaged over many years (37), and average larval dispersal distances of 50–150 km from stochastic models are similar to those based on simpler models (37, 69), the stochastic nature of larval connectivity creates an unavoidable uncertainty in the assessment of fish recruitment and fishery yields. This finding begs the question of whether results from past marine reserve models based on simpler assumptions of larval dispersal still hold. Over long time scales, it is reassuring that results may change little. For example, reserves provide a buffer against ecological uncertainty (95), and a sound network design of reserves is expected to promote metapopulation resilience to stochastic larval recruitment dynamics (83, 94). Over short-term time scales, however, expectations of larval recruitment and marine reserve effects will change from those based on simpler models. Matching these expectations with empirical observations remains one of the greatest challenges in future investigations of the effects of marine reserve networks. Emerging tools in tracking dispersal (96) may help us move rapidly from theory to empirical analysis.
Outstanding Issues in Reserve Network Design
Although conceptual advances in reserve network design have been rapid, several challenges remain. First, marine ecosystems include enormous biological diversity with a broad range of ecologies and life histories. Although some simple rules of thumb are emerging that may provide benefits for many species in a diversity of settings, no design ever can provide benefits for all. Broadening the range of species that benefit is a clear goal that may entail designs focused specifically on reducing the variability in benefits among species (e.g., ref. 36). In addition, if species benefit differentially, how will emerging MPA networks alter interactions between species that benefit in different ways and to different extents (12, 19, 20)? Second, MPA networks are being designed on the basis of conditions, both biological and physical, that exist today. However, these features of the ecosystem often are strongly coupled to climate variability. Marine reserves are fixed in place, but the species they are designed to benefit may shift dramatically in their geographic distributions over coming decades in the face of climate change. Species already have shown poleward range shifts and shifts to deeper habitats. Moreover, larval connectivity depends partially on how long larvae take to develop, and this time scale is tightly coupled to temperature (97). Will network designs focused on persistence or profitability today promote persistence and profitability in an altered seascape? Third, as we have discussed, nearly all current work on network designs to meet fisheries goals focus on yield and profitability. There are, however, many other fishery management goals that might be benefited by networks of marine reserves. These goals include estimating ecosystem-wide effects of fishing to inform ecosystem-based fisheries management, spatially explicit stock assessments, and disentangling effects of fishing from climate change and other impacts. Moreover, MPA networks have other goals, such as preserving natural and cultural heritage, education, and research in “pristine” settings, and these goals may require different design decisions (32, 33). Finally, the efficacy of the design elements we have discussed remains largely untested, because networks of MPAs on appropriate geographical scales have been implemented only recently. Can we empirically assess whether the theoretical expectations for network effectiveness (e.g., those listed in Table 2) in the competing arenas of conservation and fisheries management hold true in real ecosystems? In particular, information about the actual effects of reserve networks on socioeconomic processes is lacking; all the predictions listed in Table 2 are generated using theoretical models whose results need to be validated empirically. With the maturing of existing networks and rapid increase in implementation of new networks, scientists and managers are poised to challenge ecological theory with data from real world networks.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gell FR, Roberts CM. Benefits beyond boundaries: The fishery effects of marine reserves. Trends Ecol Evol. 2003;18:448–455. [Google Scholar]
- 2.Norse EA, et al. Marine reserves: The best option for our oceans? Front Ecol Environ. 2003;1:495–502. [Google Scholar]
- 3.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 4.Spalding MD, Fish L, Wood LJ. Toward representative protection of the world's coasts and oceans—progress, gaps, and opportunities. Conservation Letters. 2008;1:217–226. [Google Scholar]
- 5.Lester SE, et al. Biological effects within no-take marine reserves: A global synthesis. Mar Ecol Prog Ser. 2009;384:33–46. [Google Scholar]
- 6.Halpern BS. The impact of marine reserves: Do reserves work and does reserve size matter? Ecol Appl. 2003;13:S117–S137. [Google Scholar]
- 7.Lester SE, Halpern BS. Biological responses in marine no-take reserves versus partially protected areas. Mar Ecol Prog Ser. 2008;367:49–56. [Google Scholar]
- 8.Halpern BS, Lester S, Kellner J. Spillover from marine reserves and the replenishment of fish stocks. Environmental Conservation. in press. [Google Scholar]
- 9.Halpern BS, Gaines SD, Warner RR. Confounding effects of the export of production and the displacement of fishing effort from marine reserves. Ecol Appl. 2004;14:1248–1256. [Google Scholar]
- 10.Halpern BS, Warner RR. Marine reserves have rapid and lasting effects. Ecol Lett. 2002;5:361–366. [Google Scholar]
- 11.Russ GR, et al. Rapid increase in fish numbers follows creation of world's largest marine reserve network. Curr Biol. 2008;18:R514–R515. doi: 10.1016/j.cub.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Micheli F, Halpern BS, Botsford LW, Warner RR. Trajectories and correlates of community change in no-take marine reserves. Ecol Appl. 2004;14:1709–1723. [Google Scholar]
- 13.Claudet J, et al. Marine reserves: Size and age do matter. Ecol Lett. 2008;11:481–489. doi: 10.1111/j.1461-0248.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 14.White C, Kendall BE. A reassessment of equivalence in yield from marine reserves and tra-ditional fisheries management. Oikos. 2007;116:2039–2043. [Google Scholar]
- 15.Russ GR, Alcala AC, Maypa AP, Calumpong HP, White AT. Marine reserve benefits local fisheries. Ecol Appl. 2004;14:597–606. [Google Scholar]
- 16.Hilborn R, et al. When can marine reserves improve fisheries management? Ocean Coast Manage. 2004;47:197–205. [Google Scholar]
- 17.Hart DR. When do marine reserves increase fishery yield? Can J Fish Aquat Sci. 2006;63:1445–1449. [Google Scholar]
- 18.Kaplan DM. Fish life histories and marine protected areas: An odd couple? Mar Ecol Prog Ser. 2009;377:213–225. [Google Scholar]
- 19.Baskett ML. Prey size refugia and trophic cascades in marine reserves. Mar Ecol Prog Ser. 2006;328:285–293. [Google Scholar]
- 20.Baskett ML, Micheli F, Levin SA. Designing marine reserves for interacting species: Insights from theory. Biol Conserv. 2007;137:163–179. [Google Scholar]
- 21.Palumbi SR. Marine reserves and ocean neighborhoods: The spatial scale of marine populations and their management. Annu Rev Environ Resour. 2004;29:31–68. [Google Scholar]
- 22.Roberts CM, Halpern BS, Palumbi SR, Warner RR. Designing marine reserve networks: Why small, isolated protected areas are not enough. Conservation Biology in Practice. 2001;2:11–17. [Google Scholar]
- 23.Allison GW, Lubchenco J, Carr MH. Marine reserves are necessary but not sufficient for marine conservation. Ecol Appl. 1998;8:S79–S92. [Google Scholar]
- 24.Avasthi A. Ecosystem management—California tries to connect its scattered marine reserves. Science. 2005;308:487–488. doi: 10.1126/science.308.5721.487. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes L, et al. Establishing representative no-take areas in the Great Barrier Reef: Large-scale implementation of theory on marine protected areas. Conserv Biol. 2005;19:1733–1744. [Google Scholar]
- 26.Neubert MG. Marine reserves and optimal harvesting. Ecol Lett. 2003;6:843–849. [Google Scholar]
- 27.Crowder LB, Lyman SJ, Figueira WF, Priddy J. Source-sink population dynamics and the problem of siting marine reserves. Bull Mar Sci. 2000;66:799–820. [Google Scholar]
- 28.Costello C, Polasky S. Optimal harvesting of stochastic spatial resources. J Environ Econ Manage. 2008;56:1–18. [Google Scholar]
- 29.Hastings A, Botsford LW. Equivalence in yield from marine reserves and traditional fisheries management. Science. 1999;284:1537–1538. doi: 10.1126/science.284.5419.1537. [DOI] [PubMed] [Google Scholar]
- 30.White C, Kendall BE, Gaines S, Siegel DA, Costello C. Marine reserve effects on fishery profit. Ecol Lett. 2008;11:370–379. doi: 10.1111/j.1461-0248.2007.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart DR, Sissenwine MP. Marine reserve effects on fishery profits: A comment on White et al. (2008) Ecol Lett. 2009;12:E9–E11. doi: 10.1111/j.1461-0248.2008.01272.x. [DOI] [PubMed] [Google Scholar]
- 32.Roberts CM, et al. Ecological criteria for evaluating candidate sites for marine reserves. Ecol Appl. 2003;13:S199–S214. [Google Scholar]
- 33.Botsford LW, Micheli F, Hastings A. Principles for the design of marine reserves. Ecol Appl. 2003;13:S25–S31. [Google Scholar]
- 34.Hastings A, Botsford LW. Persistence of spatial populations depends on returning home. Proc Natl Acad Sci USA. 2006;103:6067–6072. doi: 10.1073/pnas.0506651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen AJ, Rotella JJ. Biophysical factors, land use, and species viability in and around nature reserves. Conserv Biol. 2002;16:1112–1122. [Google Scholar]
- 36.Kinlan BP, Gaines SD. Propagule dispersal in marine and terrestrial environments: A community perspective. Ecology. 2003;84:2007–2020. [Google Scholar]
- 37.Siegel DA, Kinlan BP, Gaylord B, Gaines SD. Lagrangian descriptions of marine larval dispersion. Mar Ecol Prog Ser. 2003;260:83–96. [Google Scholar]
- 38.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 39.Trimble AC, Ruesink JL, Dumbauld BR. Factors preventing the recovery of a historically overexploited shellfish species, Ostrea Lurida Carpenter 1864. J Shellfish Res. 2009;28:97–106. [Google Scholar]
- 40.Sanchirico JN, Malvadkar U, Hastings A, Wilen JE. When are no-take zones an economically optimal fishery management strategy? Ecol Appl. 2006;16:1643–1659. doi: 10.1890/1051-0761(2006)016[1643:wanzae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Kellner JB, Tetreault I, Gaines SD, Nisbet RM. Fishing the line near marine reserves in single and multispecies fisheries. Ecol Appl. 2007;17:1039–1054. doi: 10.1890/05-1845. [DOI] [PubMed] [Google Scholar]
- 42.Schnier KE. Revisiting biological “hot spots” and marine reserve formation. Ecol Econ. 2005;54:111–113. [Google Scholar]
- 43.White C. Density dependence and the economic efficacy of marine reserves. Theoretical Ecology. 2009;2:127–138. [Google Scholar]
- 44.Lowe CG, Topping DT, Cartamil DP, Papastamatiou YP. Movement patterns, home range, and habitat utilization of adult kelp bass Paralabrax clathratus in a temperate no-take marine reserve. Mar Ecol Prog Ser. 2003;256:205–216. [Google Scholar]
- 45.Popple ID, Hunte W. Movement patterns of Cephalopholis cruentata in a marine reserve in St Lucia, WI, obtained from ultrasonic telemetry. J Fish Biol. 2005;67:981–992. [Google Scholar]
- 46.Starr RM, O'Connell V, Ralston S. Movements of lingcod (Ophiodon elongatus) in southeast Alaska: Potential for increased conservation and yield from marine reserves. Can J Fish Aquat Sci. 2004;61:1083–1094. [Google Scholar]
- 47.Smith MD, Wilen JE. Economic impacts of marine reserves: The importance of spatial behavior. J Environ Econ Manage. 2003;46:183–206. [Google Scholar]
- 48.Lockwood DR, Hastings A, Botsford LW. The effects of dispersal patterns on marine reserves: Does the tail wag the dog? Theor Popul Biol. 2002;61:297–309. doi: 10.1006/tpbi.2002.1572. [DOI] [PubMed] [Google Scholar]
- 49.Hastings A, Botsford LW. Comparing designs of marine reserves for fisheries and for biodiversity. Ecol Appl. 2003;13:S65–S70. [Google Scholar]
- 50.Botsford LW, Hastings A, Gaines SD. Dependence of sustainability on the configuration of marine reserves and larval dispersal distance. Ecol Lett. 2001;4:144–150. [Google Scholar]
- 51.Kaplan DM, Botsford LW, Jorgensen S. Dispersal per recruit: An efficient method for assessing sustainability in marine reserve networks. Ecol Appl. 2006;16:2248–2263. doi: 10.1890/1051-0761(2006)016[2248:dpraem]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Gerber LR, Kareiva PM, Bascompte J. The influence of life history attributes and fishing pressure on the efficacy of marine reserves. Biol Conserv. 2002;106:11–18. [Google Scholar]
- 53.Botsford LW, et al. Connectivity, sustainability, and yield: Bridging the gap between conventional fisheries management and marine protected areas. Rev Fish Biol Fish. 2009;19:69–95. [Google Scholar]
- 54.Leslie H, Ruckelshaus M, Ball IR, Andelman S, Possingham HP. Using siting algorithms in the design of marine reserve networks. Ecol Appl. 2003;13:S185–S198. [Google Scholar]
- 55.Allison GW, Gaines SD, Lubchenco J, Possingham HP. Ensuring persistence of marine reserves: Catastrophes require adopting an insurance factor. Ecol Appl. 2003;13:S8–S24. [Google Scholar]
- 56.Game ET, Watts ME, Wooldridge S, Possingham HP. Planning for persistence in marine reserves: A question of catastrophic importance. Ecol Appl. 2008;18:670–680. doi: 10.1890/07-1027.1. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan DM, Botsford LW. Effects of variability in spacing of coastal marine reserves on fisheries yield and sustainability. Can J Fish Aquat Sci. 2005;62:905–912. [Google Scholar]
- 58.Shanks AL, Grantham BA, Carr MH. Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl. 2003;13:S159–S169. [Google Scholar]
- 59.Gerber LR, Heppell SS, Ballantyne F, Sala E. The role of dispersal and demography in determining the efficacy of marine reserves. Can J Fish Aquat Sci. 2005;62:863–871. [Google Scholar]
- 60.Moffitt EA, Botsford LW, Kaplan DM, O'Farrell MR. Marine reserve networks for species that move within a home range. Ecol Appl. 2009;19:1835–1847. doi: 10.1890/08-1101.1. [DOI] [PubMed] [Google Scholar]
- 61.Carr MH, et al. Comparing marine and terrestrial ecosystems: Implications for the design of coastal marine reserves. Ecological Applications. 2003;13:S90–S107. [Google Scholar]
- 62.MacArthur RH, Wilson EO. Equilibrium-theory of insular zoogeography. Evolution. 1963;17:373. [Google Scholar]
- 63.Gaines SD, Gaylord B, Largier JL. Avoiding current oversights in marine reserve design. Ecol Appl. 2003;13:S32–S46. [Google Scholar]
- 64.Sala E, et al. A general model for designing networks of marine reserves. Science. 2002;298:1991–1993. doi: 10.1126/science.1075284. [DOI] [PubMed] [Google Scholar]
- 65.Gaylord B, Gaines SD, Siegel DA, Carr MH. Marine reserves exploit population structure and life history in potentially improving fisheries yields. Ecol Appl. 2005;15:2180–2191. [Google Scholar]
- 66.Roberts CM, Bohnsack JA, Gell F, Hawkins JP, Goodridge R. Effects of marine reserves on adjacent fisheries. Science. 2001;294:1920–1923. doi: 10.1126/science.294.5548.1920. [DOI] [PubMed] [Google Scholar]
- 67.Stockhausen WT, Lipcius RN. Single large or several small marine reserves for the Caribbean spiny lobster? Mar Freshw Res. 2001;52:1605–1614. [Google Scholar]
- 68.Kramer DL, Chapman MR. Implications of fish home range size and relocation for marine reserve function. Environ Biol Fishes. 1999;55:65–79. [Google Scholar]
- 69.Petersen CH, Drake PT, Edwards CA, Ralston SA. numerical study of inferred rockfish larval dispersal along the central California coast. Fisheries Oceanography. 19(1):21–41. [Google Scholar]
- 70.Cowen RK, Paris CB, Srinivasan A. Scaling of connectivity in marine populations. Science. 2006;311:522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- 71.Palumbi SR. Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl. 2003;13:S146–S158. [Google Scholar]
- 72.Barber PH, Moosa MK, Palumbi SR. Rapid recovery of genetic populations on Krakatau: Diversity of stomatopod temporal and spatial scales of marine larval dispersal. Proc R Soc Lond B Biol Sci. 2002;269:1591–1597. doi: 10.1098/rspb.2002.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Treml EA, Halpin PN, Urban DL, Pratson LF. Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landscape Ecol. 2008;23:19–36. [Google Scholar]
- 74.Halpern BS, Warner RR. Matching marine reserve design to reserve objectives. Proc R Soc Lond B Biol Sci. 2003;270:1871–1878. doi: 10.1098/rspb.2003.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watson J R, et al. Simulating larval dispersal in the Southern California Bight. Mar Ecol-Prog Ser. 2010;401:31–48. [Google Scholar]
- 76.Davis GE. Science and society: Marine reserve design for the California Channel Islands. Conserv Biol. 2005;19:1745–1751. [Google Scholar]
- 77.Bode M, Bode L, Armsworth PR. Larval dispersal reveals regional sources and sinks in the Great Barrier Reef. Mar Ecol Prog Ser. 2006;308:17–25. [Google Scholar]
- 78.Kaplan DM. Alongshore advection and marine reserves: Consequences for modeling and management. Mar Ecol Prog Ser. 2006;309:11–24. [Google Scholar]
- 79.Sanchirico JN, Wilen JE. The impacts of marine reserves on limited-entry fisheries. Nat Resour Model. 2002;15:291–310. [Google Scholar]
- 80.Botsford LW. Potential contributions of marine reserves to sustainable fisheries: Recent modeling results. Bull Mar Sci. 2005;76:245–259. [Google Scholar]
- 81.Rowley RJ. Marine reserves in fisheries management. Aquatic Conservation of Marine and Freshwater Ecosystems. 1994;4:233–254. [Google Scholar]
- 82.Stefansson G, Rosenberg AA. Combining control measures for more effective management of fisheries under uncertainty: Quotas, effort limitation and protected areas. Philos Trans R Soc Lond B Biol Sci. 2005;360:133–146. doi: 10.1098/rstb.2004.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grafton RQ, Kompas T, Lindenmayer D. Marine reserves with ecological uncertainty. Bull Math Biol. 2005;67:957–971. doi: 10.1016/j.bulm.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 85.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 86.Gladstone W. Requirements for marine protected areas to conserve the biodiversity of rocky reeffishes. Aquatic Conservation: Marine and Freshwater Ecosystems. 2007;17:71–87. [Google Scholar]
- 87.Beger M, Jones GP, Munday PL. Conservation of coral reef biodiversity: A comparison of reserve selection procedures for corals and fishes. Biol Conserv. 2003;111:53–62. [Google Scholar]
- 88.Airame S, et al. Applying ecological criteria to marine reserve design: A case study from the California Channel Islands. Ecol Appl. 2003;13:S170–S184. [Google Scholar]
- 89.Cudney-Bueno R, Lavin MF, Marinone S, Raimondi PT, Shaw WW. Rapid effects of marine reserves via larval dispersal. PLoS One. 2009;4:e4140. doi: 10.1371/journal.pone.0004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wing SR, Botsford LW, Morgan LE, Diehl JM, Lundquist CJ. Inter-annual variability in larval supply to populations of three invertebrate taxa in the northern California Current. Estuar Coast Shelf Sci. 2003;57:859–872. [Google Scholar]
- 91.Morgan LE, Wing SR, Botsford LW, Lundquist CJ, Diehl JM. Spatial variability in red sea urchin (Strongylocentrotus franciscanus) recruitment in northern California. Fish Oceanogr. 2000;9:83–98. [Google Scholar]
- 92.Paris CB, Cherubin LM, Cowen RK. Surfing, spinning, or diving from reef to reef: Effects on population connectivity. Mar Ecol Prog Ser. 2007;347:285–300. [Google Scholar]
- 93.Siegel DA, et al. The stochastic nature of larval connectivity among nearshore marine populations. Proc Natl Acad Sci USA. 2008;105:8974–8979. doi: 10.1073/pnas.0802544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mitarai S, Siegel DA, Winters KB. A numerical study of stochastic larval settlement in the California Current system. J Mar Syst. 2008;69:295–309. [Google Scholar]
- 95.Armsworth PR, Roughgarden JE. The economic value of ecological stability. Proc Natl Acad Sci USA. 2003;100:7147–7151. doi: 10.1073/pnas.0832226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Planes S, et al. Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci USA. 2009;106:5693–5697. doi: 10.1073/pnas.0808007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Connor MI, et al. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA. 2007;104:1266–1271. doi: 10.1073/pnas.0603422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baskett ML, Levin SA, Gaines SD, Dushoff J. Marine reserve design and the evolution of size at maturation in harvested fish. Ecol Appl. 2005;15:882–901. [Google Scholar]
- 99.Stefansson G, Rosenberg AA. Designing marine protected areas for migrating fish stocks. J Fish Biol. 2006;69:66–78. [Google Scholar]
- 100.Apostolaki P, Milner-Gulland EJ, McAllister MK, Kirkwood GP. Modelling the effects of estab-lishing a marine reserve for mobile fish species. Can J Fish Aquat Sci. 2002;59:405–415. [Google Scholar]
- 101.Le Quesne WJF, Codling EA. Managing mobile species with MPAs: The effects of mobility, larval dispersal, and fishing mortality on closure size. ICES J Mar Sci. 2009;66:122–131. [Google Scholar]
- 102.Codling EA. Individual-based movement behaviour in a simple marine reserve-fishery system: Why predictive models should be handled with care. Hydrobiologia. 2008;606:55–61. [Google Scholar]
- 103.Kellner JB, Nisbet RM, Gaines SD. Spillover from marine reserves related to mechanisms of population regulation. Theoretical Ecology. 2008;1:117–127. [Google Scholar]
- 104.Ralston S, O'Farrell MR. Spatial variation in fishing intensity and its effect on yield. Can J Fish Aquat Sci. 2008;65:588–599. [Google Scholar]
- 105.Gårdmark A, Jonzén N, Mangel M. Density-dependent body growth reduces the potential of marine reserves to enhance yields. J Appl Ecol. 2006;43:61–69. [Google Scholar]
- 106.Hiddink JG, et al. Cumulative impacts of seabed trawl disturbance on benthic biomass, production, and species richness in different habitats. Can J Fish Aquat Sci. 2006;63:721–736. [Google Scholar]
- 107.Armstrong CW, Skonhoft A. Marine reserves: A bio-economic model with asymmetric density dependent migration. Ecol Econ. 2006;57:466–476. [Google Scholar]
- 108.Sanchirico JN. Designing a cost-effective marine reserve network: A bioeconomic metapopulation analysis. Mar Resour Econ. 2004;19:41–65. [Google Scholar]
- 109.Rudd MA, Tupper MH, Folmer H, van Kooten GC. Policy analysis for tropical marine reserves: Challenges and directions. Fish Fish. 2003;4:65–85. [Google Scholar]
- 110.Byers JE, Noonburg EG. Poaching, enforcement, and the efficacy of marine reserves. Ecol Appl. 2007;17:1851–1856. doi: 10.1890/07-0067.1. [DOI] [PubMed] [Google Scholar]
- 111.Roberts CM, Polunin NVC. Marine reserves—simple solutions to managing complex fisheries. Ambio. 1993;22:363–368. [Google Scholar]