Abstract
The science of spatial fisheries management, which combines ecology, oceanography, and economics, has matured significantly. As a result, there have been recent advances in exploiting spatially explicit data to develop spatially explicit management policies, such as networks of marine protected areas (MPAs). However, when data are sparse, spatially explicit policies become less viable, and we must instead rely on blunt policies such as total allowable catches or imprecisely configured networks of MPAs. Therefore, spatial information has the potential to change management approaches and thus has value. We develop a general framework within which to analyze the value of information for spatial fisheries management and apply that framework to several US Pacific coast fisheries. We find that improved spatial information can increase fishery value significantly (>10% in our simulations), and that it changes dramatically the efficient management approach—switching from diffuse effort everywhere to a strategy where fishing is spatially targeted, with some areas under intensive harvest and others closed to fishing. Using all available information, even when incomplete, is essential to management success and may as much as double fishery value relative to using (admittedly incorrect) assumptions commonly invoked.
Keywords: dispersal kernel, fisheries management, value of information
Spatially explicit policies are increasingly used for conservation and management of marine resources. Broad patterns of resource decline, increasing conflicts in coastal zones among competing users, and pressures from a complex set of human impacts are some of the reasons policy makers are turning to spatial management as an evolving paradigm for ocean policy (1, 2).
A substantial body of literature indicates that spatial management can improve marine resource management. For example, siting a network of marine protected areas (MPAs) in strategic spatial locations can simultaneously enhance both the yields and profits of a fishery and improve ecosystem service provision (3–7). Other prominent examples include ocean zoning for various activities, ecosystem-based management, and the use of spatial concessions or territorial user rights in fisheries (TURFs) management. Several studies have documented the benefits arising from more explicit spatial management (2, 8–12, 13), although concerns about whether spatial management approaches increase or maintain fisheries catches still remain (e.g., refs. 14 and 15).
Achieving benefits from spatial management policies requires spatial information. For example, designing effective MPA networks requires spatial information on habitat, species distributions, larval, juvenile, and adult movements and source–sink dynamics of larval production and recruitment (7, 16–18). These data are often scarce and costly to obtain. The questions of which sources of uncertainty may be reduced cost effectively, and what management approaches are more robust to remaining uncertainties are fundamentally important in the management of fisheries and natural resources in general (e.g., ref. 19).
In this paper we develop and implement a framework for calculating the value of information that facilitates spatial management of marine resources. Value of information studies have been conducted on El Niño forecasts for salmon management (20), weather forecasting for agriculture (21), and other applications. This work most closely parallels that of Polasky and Solow (22), who investigate the value of spatial information (e.g., from site surveys) for terrestrial reserve site selection. A key difference in our work is that information resolves a structural process underlying a bioeconomic model, not just the spatial distribution of sedentary organisms. We are primarily concerned with two questions. First, how does improved information lead to improved spatial management of a fishery? We focus on the effects of information on abundance of fish, profits from the fishery, and MPA implementation. Second, what is the value of this improved information? The framework we illustrate here provides a means to calculate the economic value of information. We apply this general framework to several representative fisheries from the Southern California Bight (SCB) and quantify the value of information under different alternative larval dispersal scenarios. In this region, perfect spatial information can improve the economic values of the fisheries significantly and these optimal solutions often require spatial closures, even from a pure fisheries profit perspective.
Modeling a Spatially Optimized Fishery
Our model starts with a single fish species residing in a heterogeneous patchy marine environment. The spatial domain of the species is carved into a finite set of patches (or sites), which are connected via larval dispersal. Habitat quality is heterogeneous, which determines the patch’s carrying capacity. In the absence of harvest, this metapopulation settles down to an equilibrium in which each patch contains a different biomass of the species.
Harvest is set by a fishery manager and may be spatially heterogeneous. Thus, the fishery manager has the ability to direct harvest spatially—e.g., by setting zonal total allowable catches and/or by establishing marine protected areas. This mimics the approaches taken by Costello and Polasky (3), Sanchirico and Wilen (23), and Sanchirico et al. (7), among others. Although this kind of spatially targeted management is not the most common, it is not without precedent. For example, in the Alaskan halibut fishery, which is managed by individual transferable quota, quotas are zone specific. In the Washington state geoduck fishery, allowable catches are area specific. In Baja California, Mexico, cooperatives centrally control the location and timing of lobster harvest. And in California, although harvest quotas are not spatial, a network of marine protected areas in the northern Channel Islands creates a spatial mosaic of areas open and closed to fishing.
Objective Function.
We allow for a multicriterion objective function that simultaneously accommodates profit (from harvest) and conservation (standing biomass) of the species in question. We adopt a discount rate of 0; we seek to maximize the steady state value of the objective function. There are N patches; the steady state harvest in patch i is Hi and the set of harvests is H ≡ {H1, H2, …, HN}. Let π(H) be steady state profit and B(H) be steady state biomass. If π and/or B are uncertain, then the objective is to maximize their expected values, where the expectation operator is given by E[·]. The maximand is
The coefficient α controls the relative weight of profit vs. conservation in the overall objective. When α = 0, we recover the standard objective function from economics (to maximize profit). When α = 1 conservation drives the objective. In what follows we will explore a full range of the weight, α. Finally, the coefficient β allows for a unit conversion of B to π. In practice, we select β so that maximized value when α = 0 and maximized value when α = 1 are equal. Formally, 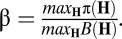
Management Under Uncertainty.
The focus of this analysis is on information that resolves uncertainty. Uncertainty can enter in numerous ways that affect the objective function (Eq. 1) and will thus have an impact on management. Although the framework we present is general and is agnostic about the source of uncertainty, it is instructive to provide a few examples. If habitat is unknown (or incompletely mapped spatially), then we will be uncertain about spatial production. This would introduce uncertainty into both π and B above. In contrast, uncertainty over harvest costs would induce uncertainty only over π, but conditional on spatial harvesting decisions, the biomass, B will be deterministic.
The modeling methods described in this paper extend both to problems with structural uncertainty and inherent stochasticity. When the manager is uncertain about a structural element of the system (e.g., uncertainty about the amount of sandy bottom in a patch), information can (at least in principle) resolve that uncertainty once and for all. When the system is inherently stochastic (e.g., upwelling varies interannually), information can inform the realization of the stochasticity in a particular period, but will not resolve the process once and for all. Without loss of generality, and to illustrate the concepts more concretely, we will work through a problem with structural uncertainty. In particular, the manager is uncertain about the larval dispersal kernel, D.The dispersal kernel, D is an N × N matrix where element Dij is the proportion of larvae originating in patch i that disperse to patch j. Accordingly, we assume D does not change over time; once it is learned, it is known once and for all. Although published models of fish populations almost unanimously assume a fixed D, an emerging literature suggests that D itself may vary from year to year (in which case, D itself would be inherently stochastic). The methods developed here apply equally well under that generalization; in Conclusions and Discussion we discuss its implications on our numerical results.
To completely characterize uncertainty over D we must, in principle, compile the set of all possible dispersal kernels (only one of which is the true dispersal kernel). Let {D1, …, DJ} be that set. Further, suppose we assign a probability weighting to each of those dispersal kernels: {p1, …, pJ}, where 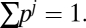 The question is how this uncertainty affects the fishery manager’s choice of harvest, H. It is well-known that for nonlinear problems, the optimal choice of H differs (sometimes quite substantially) from the optimal choice under a deterministic environment (24). In other words, selecting our “best guess” of the dispersal kernel, D, and proceeding with a deterministic analysis may lead us to arrive at the wrong policy. Loosely speaking, when we do not know which dispersal kernel is the correct one, we are forced to adopt a rather blunt policy that would perform well on average for any possible dispersal kernel. The objective is to maximize V(H) from Eq. 1, by choosing H, subject to the biological growth relationships, where D is a random variable. The important thing here is that a single spatial harvest pattern is being selected that performs well on average. Let the optimized spatial harvest pattern under uncertainty be given by HU, and the associated expected value be given by VU (where VU ≡ V(HU)).
The question is how this uncertainty affects the fishery manager’s choice of harvest, H. It is well-known that for nonlinear problems, the optimal choice of H differs (sometimes quite substantially) from the optimal choice under a deterministic environment (24). In other words, selecting our “best guess” of the dispersal kernel, D, and proceeding with a deterministic analysis may lead us to arrive at the wrong policy. Loosely speaking, when we do not know which dispersal kernel is the correct one, we are forced to adopt a rather blunt policy that would perform well on average for any possible dispersal kernel. The objective is to maximize V(H) from Eq. 1, by choosing H, subject to the biological growth relationships, where D is a random variable. The important thing here is that a single spatial harvest pattern is being selected that performs well on average. Let the optimized spatial harvest pattern under uncertainty be given by HU, and the associated expected value be given by VU (where VU ≡ V(HU)).
Management with Information.
Now suppose that we can obtain information about the uncertain variable(s). In our example this amounts to obtaining information about the larval dispersal kernel, D. Information on D allows us to make more precise management recommendations. In the extreme, we can acquire perfect information and know the “true” dispersal kernel. For that case, no uncertainty remains and we simply maximize Eq. 1 by choosing H subject to the biological growth equations. Before acquiring information, however, we do not know which dispersal kernel is the truth. If the dispersal kernel turns out to be Dj, then we maximize Eq. 1 using that information by choosing Hj, which yields a payoff V(Hj|Dj). Thus, we end up with one of J spatial harvest patterns: H1, …, HJ, with associated probabilities p1, …, pJ.
Calculating the Value of Information
We are interested in the ex ante expected value of information—a measure of the maximum willingness to pay for the information before the fishery manager knows what information will be revealed. This is the relevant statistic for determining whether to acquire information ahead of time. With information, the expected value of the objective is:
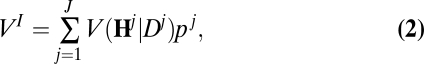 |
where the term pj weights each value by the associated probability of drawing that dispersal kernel. The ex ante value of information is simply the difference of the value of the system with information and the value of the system without information:
An important result is that VOI cannot be negative. If the fishery manager expected the information to be useless or faulty, he would simply disregarded it. Thus, he must expect the information to lead to better management decisions, so VOI ≥ 0. Its magnitude is an empirical question to which we turn in the following section.
Application to the Southern California Bight
We adopt a modified form of the biophysical model used to evaluate alternative MPA packages in California’s South Coast region as part of the Marine Life Protection Act process (25). The model is a stage structured spatial model representing populations of fish in the Southern California Bight (from Point Conception to the Mexican border including offshore islands). In this analysis, we divide the region into 135 patches of approximately equal size (∼10 km in diameter) and the amount of hard substrate in each patch is used as a measure of habitat availability. The model assumes Beverton-Holt form intracohort density dependence in juvenile survival, where the maximum number of survivors in a patch is linearly related to habitat availability. The model tracks fish in each patch in 1-year age classes (combining multiple cohorts spawning in the same year for simplicity), with adult fish undergoing von Bertalanffy growth and age-independent natural mortality. Fish are harvested in each patch at a rate dependent on the effort expended in the patch, and the unit of effort is the amount required to catch 1% of the fish in that patch in a year. The profit achieved in a given patch is the total harvest in that patch less the cost of the effort expended there. The costs are chosen so that patches can be profitably fished until the population is reduced to 10% of the maximum population occurring in any patch in the unfished system. Fish reproduce once a year, and the number of larvae produced by each fish is proportional to its weight. The larvae produced in each patch are then redistributed among all of the patches on the basis of the larval dispersal kernel described in the next section. Details on the model structure are given in ref. 25 and parameter values for the three species modeled here are reproduced in Tables S1 ‒ S3.
Empirical Larval Dispersal Estimates.
To calculate the value of information about larval dispersal, we require a set of J alternative potential dispersal kernels for the SCB. We generate a total of 10 dispersal kernels for use in this analysis. Kernels 1–8 are derived from an ocean circulation model, kernel 9 is from a Gaussian model of larval dispersal, and kernel 10 assumes a common larval pool. Our operating assumption is that one of kernels 1–8 is the “correct” dispersal kernel, but ex ante, the fishery manager does not know which one it is. Kernels 9–10 mimic kernels that are often assumed in the literature. We use these to illustrate how poorly management can perform under the incorrect assumption that larvae are dispersed according to a Gaussian kernel or a common larval pool.
Kernels 1–8 are derived from the output of a high-spatial resolution (1 km) circulation model (26) that advects surface-following larvae from source to destination locations given the plankton larval duration (PLD) and the spawning season for the species in question (27, 28). We generated output from this model for 7 years (1996–2002) so we used the dispersal kernel for each individual year and the average dispersal across all years as our eight alternatives. The 1-km SCB regional circulation model solutions well represent available oceanographic observations in the SCB (29). Ocean circulation model outputs are available for the period of 1996–2003, spanning the 1997–1998 El Niño and the 1999 La Niña events, providing larval dispersal estimates for seven spawning seasons and their arithmetic mean.
There remains a technical mismatch between the manner in which these kernels were derived (i.e., from an underlying model in which the kernel is stochastic, and varies from year to year) and our assumption that the kernel is fixed, but unknown. This reflects both the state of the literature (which typically assumes, either explicitly or implicitly, that the dispersal kernel is fixed), and our interest in deriving alternative dispersal kernels that were as realistic as possible. The methods developed here are equally applicable to an underlying model with a stochastic kernel (in which case, information would inform the kernel in a particular year, t). The implications of this change in assumption are discussed in Conclusions and Discussion.
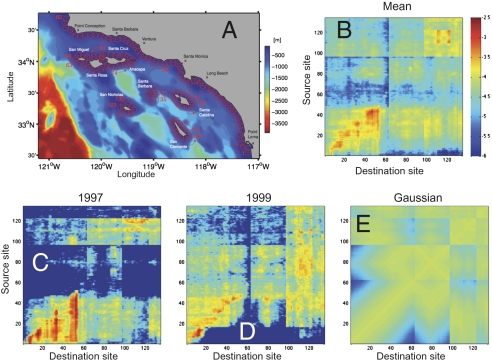
Larval dispersal kernels are calculated from millions of Lagrangian trajectories simulated within the 1-km model solution following procedures outlined in refs. 27 and 28. Potential larval connectivity is quantified as the probability that water parcels released at a source site are found within a destination site at the end of their PLD. This calculation of the site-to-destination probabilities defines potential larval connectivity (28). Potential larval dispersal kernels are calculated for three species: sheephead, kelp bass, and kelp rockfish. Sheephead is the focus of this manuscript and they typically spawn from July to October with a 40-day PLD (30, 31). Kelp bass spawn from May to September with a 30-day PLD (32, 33), and kelp rockfish spawn from March to June with a 60-day PLD (34, 35). Examples of potential dispersal kernels for sheephead from the 135 source sites in the Southern California Bight (Fig. 1A) are shown in Fig. 1 B–E (see Figs. S1 and S2 for dispersal kernels for kelp bass and kelp rockfish).
Fig. 1.
(A) Ocean circulation model domain and SCB site locations (after ref. 31). (B) Eight-year mean potential dispersal kernel for sheephead larvae in the SCB. Sheephead typically spawn from July to October and have a 40-day PLD (33, 34). Source sites are listed in the y axis and destination in the x axis and the strength of dispersal is shown in color (note the logarithmic scale and all dispersal kernels have the same color bar). (C) Potential dispersal kernel for sheephead larvae for the El Niño year of 1997. (D) Potential dispersal kernel for sheephead larvae for the La Niña year of 1999. (E) Dispersal estimates calculated as a function of geometric distance between sites using the Gaussian larval transport model (42). Site-to-site dispersal values are calculated assuming no mean flow and variable component of the flow of 10 km/day using the Gaussian dispersal formulae provided in Eq. 5 and table 1 of ref. 42.
The 8-year mean dispersal kernel for sheephead (Fig. 1B) shows several important factors characterizing potential dispersal in the SCB. First, the time mean of sheephead site-to-site dispersal varies by more than a thousand across the SCB. Second, the mean dispersal kernel is asymmetric (Fig. 1B), indicating that source–destination relationships are more complicated than simple distance assessments. Third, many strong regional associations are apparent. For example, sites 1–62 represent the mainland coast (Fig. 1A) and strong levels of mean larval dispersal are found within the mainland sites south of Ventura, indicating a large degree of regional self-settlement in the southern portion of the SCB (Fig. 1B). Mainland source sites are also fairly well connected to Northern Channel Island destinations (sites 63–96) and the Southern Channel Islands (sites 97–135) with the exception of source sites nearer the Mexican border (sites 1–5). By comparison Northern Channel Islands source sites (sites 63–96) are weakly connected with the other subregions and with themselves whereas sources from the Southern Channel Islands (sites 97–135) are only strongly connected with themselves.
Example potential dispersal kernels for 1997 and 1999 (Fig. 1 C and D, respectively) show expected changes in oceanographic conditions associated with the 1997–1998 El Niño and the 1999 La Niña events. Ocean circulation patterns in the California Current are largely poleward during El Niño events and equatorward during La Niña (e.g., 36, 37, 29) and is easily seen in Fig. 1 C and D. This representation illustrates the variability in larval dispersal due to interannual changes in ocean circulation and the inherent variability of eddy driven transport (e.g., 30, 38).
Dispersal kernel 9 derives from geometric distance between sites using the Gaussian larval transport model (39); see Fig. 1 legend. The Gaussian dispersal model (Fig. 1E) underestimates the scales of variability of dispersal and cannot explain any asymmetry in dispersal.
Finally, dispersal kernel 10 simply assumes a “common larval pool” for which any single site has the same probability for transporting larvae to any other site. All dispersal estimates are normalized by their absolute magnitude of dispersing larvae. This ensures that kernels differ only in their spatial patterns, not in the overall magnitude of larvae produced and dispersed throughout the system.
Experiments.
We ran a total of four experiments using the dispersal kernels introduced above. The “true” dispersal kernel is always one of dispersal kernels 1–8, each with probability 1/8. The experiments differ in the assumptions made by the fishery manager about what is the true dispersal kernel. For each experiment, we calculate the expected value, biomass, effort, and area in MPAs. We do so for a range of the weighting parameter, α.
Experiment 1: Choose spatial harvests, H, under uncertainty over the true dispersal kernel.
Experiment 2: Choose spatial harvests, H, with perfect information about the true dispersal kernel.
Experiment 3: Choose spatial harvests, H, under the incorrect assumption that the true dispersal kernel is Gaussian (dispersal kernel 9).
Experiment 4: Choose spatial harvests, H, under the incorrect assumption that the true dispersal kernel is common larval pool (dispersal kernel 10).
Results
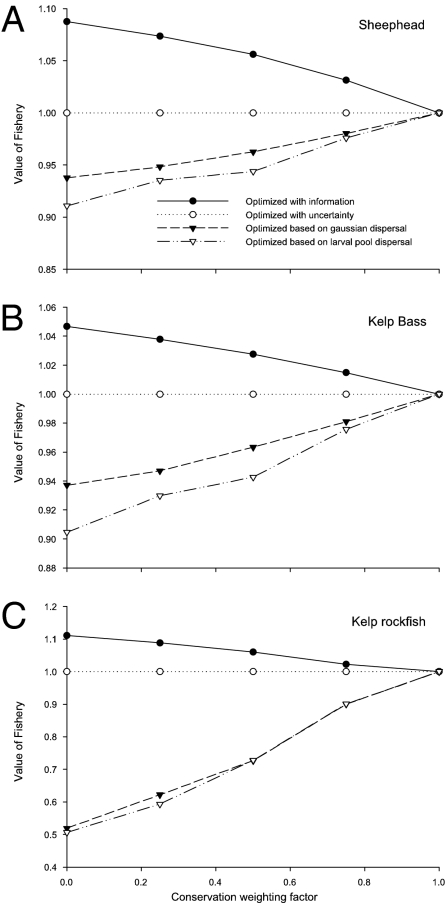
For each of three species, the value of the fishery, relative to optimal management under uncertainty, is shown in Fig. 2. Each of the four lines corresponds to a different experiment (see above), and the magnitude can be interpreted as the fractional value of information. For example with a conservation weighting factor of 0, the value of the fishery with perfect information on dispersal is 5–11% higher than the value of the same fishery under uncertainty over dispersal, depending on the species (Fig. 2). Here we will first characterize the value of information. We then explore the biological and management consequences of improved information.
Fig. 2.
The expected value of the (A) sheephead, (B) kelp bass, and (C) kelp rockfish fisheries optimized using different information about larval dispersal. Filled circles are with full information, open circles are with information about alternative possible dispersal kernels but uncertainty about which is correct, filled triangles are for the Gaussian larval transport model, and open triangles are for the larval-pool model. Results are given for a range of management goals, from purely profit maximizing (conservation weighting factor α = 0) to purely biomass maximizing (conservation weighting factor α = 1). All values are expressed relative to the value of the fishery optimized under uncertainty.
How Fishery Value Depends on Information.
The kind of information used to optimize management has a major impact on its expected value. As expected, the value of a fishery optimized with perfect information always has the highest value—as much as 11% more than the same fishery managed with uncertainty about the dispersal (Fig. 2). When perfect information is not available, the best one can do is to manage under uncertainty by using the available information on the types of dispersal patterns that might occur. When the fishing effort is optimally distributed on the basis of simpler (although incorrect) representations of dispersal, the value of the fishery can be significantly lower; for the case of kelp rockfish it is only half of the value of the fishery optimized under uncertainty (Fig. 2C). Of these simpler approximations, optimizing fishing using the distance-based Gaussian approximation yields only slightly higher values than optimizing on the basis of larval-pool dispersal.
The effect of information on the value of the fishery is largest when the system is managed solely for maximizing profit, but the qualitative patterns are maintained across a broad range of management goals. As the conservation weighting factor increases, the spread between the value of the fishery optimized with and without information narrows, and thus the value of information is reduced. In the extreme case in which the system is being managed solely to maximize biomass (i.e., when the conservation weighting factor is 1) the optimal solution is to cease fishing. In this situation the optimal fishing pattern does not depend on dispersal, so information has no value.
The effect of information is similar across the three species, although the magnitude of its value varies. For example, optimizing solely for profit with full information produces about 11% higher expected value than optimizing with uncertainty for kelp rockfish, 8% more for sheephead, and 5% more for kelp bass (Fig. 2 A–C). For these three species, the value of information is larger for species with longer PLDs, potentially reflecting greater potential to take advantage of source–sink dynamics in these longer dispersing species. Although there is considerable variation in the predicted values of information, the three species presented here represent a range of pelagic larval durations (30, 40, and 60 days) and spawning seasons (spring, summer, and fall), so these patterns may apply across a wide range of larval life histories and seasonal ocean circulation patterns. A much larger set of species would be necessary to draw any general conclusions, however.
How Fishing Effort and Biomass Depend on Information.
In the optimized fishing distributions, there is considerable variability in fishing effort from patch to patch. This variability arises both from heterogeneity in the habitat and from variability in larval dispersal. The more information about dispersal that is available, the more the distribution of fishing effort can be optimized to take advantage of source–sink dynamics.
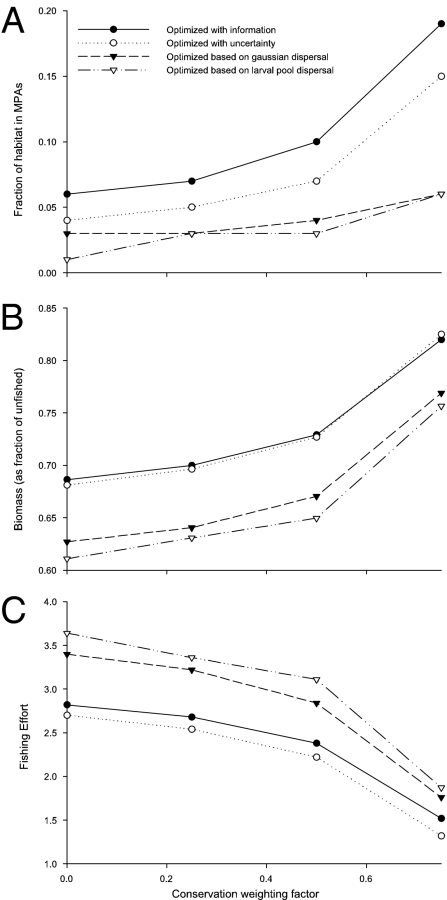
One striking feature of the optimized fishing distributions is that they include significant areas that are closed to fishing, even when the manager’s objective is to maximize fisheries profit. These marine reserves are defined here as patches where fish could be extracted profitably but which are left unfished in the optimal solution. A strong effect of information is found on the fraction of the habitat optimally dedicated to reserves. When the fishery is optimized with perfect information, substantially more habitat is in reserves than when the fishery is optimized under uncertainty (Fig. 3A). When the fishery is optimized on the basis of simpler approximations of dispersal, substantially less habitat is protected, with the larval pool approximation typically protecting the least amount of habitat. These results hold true for all three species and across a wide range of management goals (Figs. S3 and S4).
Fig. 3.
Characteristics of the sheephead fishery, which has been optimized using different information about larval dispersal. (A) The fraction of habitat in MPAs, (B) the equilibrium biomass, and (C) the total fishing effort. Filled circles are with full information, open circles are with information about alternative possible dispersal kernels but uncertainty about which is correct, filled triangles are for the Gaussian larval transport model, and open triangles are for the larval-pool model. Results are given for a range of management goals, from purely profit maximizing (conservation weighting factor α = 0) to mostly biomass maximizing (conservation weighting factor α = 0.75).
The overall level of fishing and the resulting biomass also depend on the availability of information. Overall fishing effort is substantially higher when using the simplified dispersal; optimized fishing on the basis of larval pool dispersal has the highest levels of fishing (Fig. 3C). Because of these high fishing rates, biomass levels are consistently lower in these cases and are the lowest for the larval pool case (Fig. 3B). Optimizations with perfect knowledge of the potential dispersal but uncertainty about which is correct tend to have the lowest levels of fishing effort, ∼20% lower than in the simplified cases and a few percentage points lower than the average fishing effort with full information. Biomass in these cases is correspondingly high, ∼10% higher than the simplified connectives, with biomass in the uncertainty case slightly higher than that with full information. These results are consistent for the range of management goals, which either focus on maximizing profit or on maximizing some mix of profit and conservation.
Discussion and Conclusions
We show that marine reserves can be economically profitable and that profit levels increase with the amount and quality of spatial information provided. This is because fishing effort can be allocated more efficiently to patches of varying productivity given dispersal information. Thus, spatial information informs better management and has substantial economic value, possibly >10% of the value of the fishery. Improved spatial information (even when incomplete) also tends to lead to lower overall fishing effort and correspondingly higher fish abundances than can occur—even in fishery being optimized for profits—without that information. Better information also leads to a greater heterogeneity in the spatial harvest and nearly doubles the number of reserves optimally designated, all while increasing overall profit.
These conclusions will depend on which parameters are uncertain and on the specific modeling choices made. For example, instead of postulating a fixed (but unknown) dispersal kernel, we could have allowed it to be stochastic. In that case, information would inform the value of Dt in period t. Because permanent reserves would tend to be less desirable under that condition (see ref. 3), we suspect that this change would dampen our result that information significantly increases the number of reserves designated. A related extension would assess the effect of information on structural changes to a truly stochastic kernel, e.g., if information resolved changes in the variance of weather arising from climate change.
Taken together, Figs. 2 and 3 paint an interesting picture of the determinants of increased value arising from improved information; notably that the value arising from increases in biomass are relatively small. When the conservation weighting factor (α) is low, the contribution from biomass is low because it receives little weight in the objective function. When the conservation weighting factor is large, the contribution from biomass is also low, but for a different reason. Here, the same policy is pursued regardless of the information, so the same biomass is achieved with, and without, information. We thus conclude that equilibrium biomass can play a direct role in the value of information, but only for intermediate values of the conservation weighting factor, α. Fishery profit plays a direct role, even for small values of α.
Our results contribute to a growing debate on how different objectives of marine management—maintaining populations and sustaining harvests—can be reconciled. Previous studies have shown that life-history characteristics of species, their mobility, and the behaviors and constraints to the fishing fleet are important determinants of whether both objectives can be met (4, 5, 40, 41). Here we propose a different aspect of this problem to be considered: the amount of information available on the dynamics of the biological systems and their interactions with the environment. Our results suggest that investment into acquiring spatial information, particularly on source–sink dynamics of exploited species, may be economically efficient and will benefit both the fisheries and the exploited populations.
Our modeling framework simplifies several features of real marine systems to produce general insights rather than addressing the specific characteristics of individual systems. Relaxing some of our assumptions may lead to different conclusions, although such differences are likely to be quantitative rather than qualitative. For example, this model assumes a sole owner of the fishery, thus no competition among fishermen. In contrast, under several management schemes, such as a quota system where fishing is regulated through a total allowable catch (TAC), fishing fleets compete for a common resource. Such competition would likely interfere with the efficient allocation of fishing effort among areas with varying (and known) productivity, especially in the case of overcapacity of the fishing fleets. This effect is likely to decrease the estimated value of information. Moreover, excessive competition among fishing fleets may be decreased, even under a TAC system, through private agreements among fishermen (e.g., via cooperatives) or by instituting spatial property rights. Whatever the management regime, the framework presented here can provide guidance about the value of information to improve spatial management.
We also found that optimally sited networks of marine reserves become considerably larger with improved information. Information on the source–sink dynamics allows the siting of no-take zones in the most efficient locations (typically the source areas). When this information is not available, reserves (albeit smaller networks) are still typically part of the optimal solution even though the increase in fishery performance is not as remarkable. This is consistent with other theoretical studies showing that the profit of a sole owner fishery under MPA implementation can be larger than optimized profit under traditional management (3). We also allowed for multiple objectives where conservation of unfished biomass (not just fishery profit) is the driving factor. As expected, we find that the fraction of area in reserves and the overall systemwide biomass increased as the weight on conservation increased.
We did not explicitly address the tradeoff between the value of information and the cost of acquiring it. When gathering information is costly, efficient information acquisition will depend on both the benefits and costs. In extreme cases, where costs are sufficiently high, it may be efficient to manage under uncertainty, without acquiring any additional information. On the other hand, there are likely to be additional benefits—beyond fisheries management—of improved understanding of larval dispersal dynamics.
Although we focused on the specific case of uncertainty over larval dispersal patterns, the framework developed here is sufficiently general to accommodate other mechanisms of dispersal and other sources of uncertainty and information. Biological, economic, and oceanographic variables may all be uncertain, and this approach could be used to evaluate which sources of uncertainty are most efficiently resolved by acquiring additional information.
Supplementary Material
Acknowledgments
We thank Satoshi Mitarai and James Watson for help compiling the larval dispersal kernels and National Center for Ecological Analysis and Synthesis working group participants for constructive feedback.The authors gratefully acknowledge the National Science Foundation’s Biocomplexity Program for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.D.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908057107/DCSupplemental.
References
- 1.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 2.Cudney-Bueno R, Lavín MF, Marinone SG, Raimondi PT, Shaw WW. Rapid effects of marine reserves via larval dispersal. PLoS One. 2009;4:e41–40. doi: 10.1371/journal.pone.0004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello CJ, Polasky S. Optimal harvesting of stochastic spatial resources. J Environ Econ Manage. 2008;56:1–18. [Google Scholar]
- 4.Gaylord B, Gaines SD, Siegel DA, Carr MH. Consequences of population structure and life history for fisheries yields using marine reserves. Ecol Appl. 2005;15:2180–2191. [Google Scholar]
- 5.Stefansson G, Rosenberg AA. Combining control measures for more effective management of fisheries under uncertainty: Quotas, effort limitation and protected areas. Philos Trans R Soc B. Biol Sci. 2005;360:133–146. doi: 10.1098/rstb.2004.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefansson G, Rosenberg AA. Designing marine protected areas for migrating fish stocks. J Fish Biol. 2006;69:66–78. [Google Scholar]
- 7.Sanchirico JN, Malvadkar U, Hastings A, Wilen JE. When are no-take zones an economically optimal fishery management strategy? Ecol Appl. 2006;16:1643–1659. doi: 10.1890/1051-0761(2006)016[1643:wanzae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.McClanahan TR, Mangi S. Spillover of exploitable fishes from a marine park and its effect on the adjacent fishery. Ecol Appl. 2000;10:1792–1805. [Google Scholar]
- 9.Gell FR, Roberts CM. The fishery effects of marine reserves and fishery closures. Trends Ecol Evol. 2003;18:448–455. [Google Scholar]
- 10.Micheli F, Halpern BS, Botsford LW, Warner RR. Trajectories and correlates of community change in no-take marine reserves. Ecol Appl. 2004;14:1709–1723. [Google Scholar]
- 11.Russ GR, Alcala AC, Maypa AP, Calumpong HP, White AT. Marine reserve benefits local fisheries. Ecol Appl. 2004;14:597–606. [Google Scholar]
- 12.Murawski SA, Wigley SE, Fogarty MJ, Rago PJ, Mountain DG. Effort distribution and catch patterns adjacent to temperate MPAs. ICES J Mar Sci. 2005;62:1150–1167. [Google Scholar]
- 13.Lester SE, et al. Biological effects within no-take marine reserves: A global synthesis. Mar Ecol Prog Ser. 2009;384:33–46. [Google Scholar]
- 14.Hilborn R, et al. When can marine reserves improve fisheries management? Ocean Coast Manage. 2004;47:197–205. [Google Scholar]
- 15.Walters CJ, Hilborn R, Parrish R. An equilibrium model for predicting the efficacy of marine protected areas in coastal environments. Can J Fish Aquat Sci. 2007;64:1009–1018. [Google Scholar]
- 16.Crowder LB, Lyman SJ, Figueira WF, Priddy J. Source-sink population dynamics and the problem of siting marine reserves. Bull Mar Sci. 2000;66:799–820. [Google Scholar]
- 17.Apostolaki P, Milner-Gulland EJ, McAllister MK, Kirkwood GP. Modelling the effects of establishing a marine reserve for mobile fish species. Can J Fish Aquat Sci. 2002;59:405–415. [Google Scholar]
- 18.Botsford LW, Micheli F, Hastings A. Principles for the design of marine reserves. Ecol Appl. 2003;13(sp1):25–31. [Google Scholar]
- 19.Botsford LW, Parma AM. Uncertainty in marine management. In: Norse E, Crowder L, editors. Marine Conservation Biology: The Science of Maintaining the Sea’s Diversity. Washington DC: Island Press; 2005. pp. 375–392. [Google Scholar]
- 20.Costello CJ, Adams RM, Polasky S. The value of El Nino forecasts in the management of salmon: a stochastic dynamic assessment. Am J Agric Econ. 1998;80:765–777. [Google Scholar]
- 21.Adams RM, et al. Value of improved long-range weather information. Contemp Econ Policy. 1995;13:10–19. [Google Scholar]
- 22.Polasky S, Solow AR. The value of information in reserve site selection. Biodivers Conserv. 2001;10:1051–1058. [Google Scholar]
- 23.Sanchirico JN, Wilen JE. Optimal spatial management of renewable resources: Matching policy scope to ecosystem scale. J Environ Econ Manage. 2005;50:23–46. [Google Scholar]
- 24.Laffont JJ. The Economics of Uncertainty and Information. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- 25.Science Advisory Team . Marine Life Protection Act. Draft Methods Used to Evaluate Marine Protected Area Proposals in the MLPA South Coast Study Region. Technical report, October. Sacramento, CA: California Department of Fish and Game; 2009. [Google Scholar]
- 26.Dong C, McWilliams JC. A numerical study of island wakes in the Southern California Bight. Cont Shelf Res. 2007;27:1233–1248. [Google Scholar]
- 27.Mitarai S, Siegel DA, Watson JR, Dong C, McWilliams JC. Quantifying connectivity in the coastal ocean with application to the Southern California Bight. J Geophys Res. 2009;114:C10026. [Google Scholar]
- 28.Watson JR, et al. Simulating larval connectivity in the Southern California Bight. Mar Ecol Prog Ser. 2009 [Google Scholar]
- 29.Dong C, Idica E, McWilliams JC. Circulation and multiple-scale variability in the Southern California Bight. Prog Oceanogr. 2009;82:168–190. [Google Scholar]
- 30.Cowen RK. Variation in the planktonic larval duration of the temperate wrasse Semicossyphus pulcher. Mar Ecol Prog Ser. 1991;69:9–15. [Google Scholar]
- 31.DeMartini EE, Barnett AM, Johnson TD, Ambrose RF. Growth and production estimates for biomass-dominant fishes on a southern California artificial reef. Bull Mar Sci. 1994;55:484–500. [Google Scholar]
- 32.Oda DL, Lavenberg RJ, Rounds JM. Reproductive biology of three California species of Paralabrax (Pisces: Serranidae) CCOFI Rep. 1993;34:122–132. [Google Scholar]
- 33.Cordes JF, Allen LG. Estimates of age, growth, and settlement from otoliths of young-of-the-year kelp bass (Paralabrax clathratus) Bull South Calif Acad Sci. 1997;96:43–60. [Google Scholar]
- 34.Love MS, Yoklavich M, Thorsteinson LK. The Rockfishes of the Northeast Pacific. Berkeley, CA: University of California Press; 2002. [Google Scholar]
- 35.Standish JD, Sheehy M, Warner RR. Use of otolith natal elemental signatures as natural tags to evaluate connectivity among open-coast fish populations. Mar Ecol Prog Ser. 2008;356:259–268. [Google Scholar]
- 36.Schwing FB, Moore CS, Ralston S, Sakuma KM. Record coastal upwelling in the California Current in 1999. Rep Calif Cooper Ocean Fish Investig. 2000;41:148–160. [Google Scholar]
- 37.Lynn RJ, Bograd SJ. Dynamic evolution of the 1997–1999 El Niño–La Niña cycle in the southern California Current system. Prog Oceanogr. 2002;54:59–75. [Google Scholar]
- 38.Siegel DA, et al. The stochastic nature of larval connectivity among nearshore marine populations. Proc Natl Acad Sci USA. 2008;105:8974–8979. doi: 10.1073/pnas.0802544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel DA, Kinlan BP, Gaylord B, Gaines SD. Lagrangian descriptions of marine larval dispersion. Mar Ecol Prog Ser. 2003;260:83–96. [Google Scholar]
- 40.Smith MD, Wilen JE. Economic impacts of marine reserves: The importance of spatial behavior. J Environ Econ Manage. 2003;46:183–206. [Google Scholar]
- 41.Hilborn R, Micheli F, De Leo GA. Integrating marine protected areas with catch regulation. Can J Fish Aquat Sci. 2006;63:642–649. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.