Abstract
Synaptic dysfunction and the loss of synapses are early pathological features of Alzheimer's disease (AD). Synapses are sites of high energy demand and extensive calcium fluctuations; accordingly, synaptic transmission requires high levels of ATP and constant calcium fluctuation. Thus, synaptic mitochondria are vital for maintenance of synaptic function and transmission through normal mitochondrial energy metabolism, distribution and trafficking, and through synaptic calcium modulation. To date, there has been no extensive analysis of alterations in synaptic mitochondria associated with amyloid pathology in an amyloid β (Aβ)-rich milieu. Here, we identified differences in mitochondrial properties and function of synaptic vs. nonsynaptic mitochondrial populations in the transgenic mouse brain, which overexpresses the human mutant form of amyloid precursor protein and Aβ. Compared with nonsynaptic mitochondria, synaptic mitochondria showed a greater degree of age-dependent accumulation of Aβ and mitochondrial alterations. The synaptic mitochondrial pool of Aβ was detected at an age as young as 4 mo, well before the onset of nonsynaptic mitochondrial and extensive extracellular Aβ accumulation. Aβ-insulted synaptic mitochondria revealed early deficits in mitochondrial function, as shown by increased mitochondrial permeability transition, decline in both respiratory function and activity of cytochrome c oxidase, and increased mitochondrial oxidative stress. Furthermore, a low concentration of Aβ (200 nM) significantly interfered with mitochondrial distribution and trafficking in axons. These results demonstrate that synaptic mitochondria, especially Aβ-rich synaptic mitochondria, are more susceptible to Aβ-induced damage, highlighting the central importance of synaptic mitochondrial dysfunction relevant to the development of synaptic degeneration in AD.
Keywords: synaptic AB, mitochondrial trafficking, mitochondrial oxidative stress, mitochondrial dysfunction, synaptic injury
Brain mitochondria are a mixture of synaptic and nonsynaptic mitochondria. Synaptic mitochondria differ from nonsynaptic mitochondria in size, motility, life span, and other properties. Synaptic mitochondria are energy warehouses that sustain the activity of neurons/synapses (1, 2). Synaptic mitochondria are synthesized in neuronal soma; they are then transported to nerve terminals (dendrites and axons), are distributed abundantly around synapses where mitochondria modulate calcium balance (3), and actively provide energy to fuel the synaptic function (4, 5). Synaptic mitochondria thus undergo constant activation to maintain synaptic function. Defects in synaptic mitochondria obviously compromise synaptic function (1, 6, 7), and synaptic mitochondria are vulnerable to accumulative damages. Multiple studies have shown that synaptic mitochondria undergo increased oxidation during aging (8, 9). In addition, synaptic mitochondria demonstrate higher levels of cyclophilin D (CypD), and are thereby more susceptible to calcium insult (10, 11). Given the importance of synaptic mitochondria in maintaining neuronal function and the relatively high risk for mitochondrial stress, it is important to delineate the role of synaptic mitochondrial dysfunction in synaptic degeneration relevant to neurodegenerative diseases, such as Alzheimer's disease (AD).
Mitochondrial dysfunction and synaptic damage are early pathological features of AD. Abnormalities of mitochondrial function, including decreased mitochondrial respiration, reactive oxygen species (ROS) generation, and hypometabolism, occur in the AD brain (12–16) and in brains of AD mouse models (17–22). Amyloid β (Aβ), a major component that accumulates in the brain, is an important pathogenic peptide that directly disturbs mitochondrial function, causing oxidative stress, decreased ATP, and increased Ca2+ influx (23–26). Furthermore, the interaction of mitochondrial Aβ with its binding proteins, such as amyloid-binding alcohol dehydrogenase (ABAD) (20, 25) and CypD (26, 27), exacerbates Aβ-induced mitochondria and neuronal stress. These studies suggest that in an Aβ-rich environment, overt mitochondrial dysfunction occurs and that mitochondria provide a direct site for Aβ-mediated cellular perturbation. However, it is not yet known whether Aβ accumulates predominantly in synaptic mitochondria and whether synaptic mitochondria enriched for Aβ are more vulnerable.
The goals of the present study are (i) to examine the accumulation of Aβ in synaptic mitochondria of amyloid precursor protein transgenic (APP Tg) mice overexpressing APP/Aβ in neurons, (ii) to analyze synaptic mitochondrial function and its correlation with mitochondrial Aβ levels and aging, and (iii) to assess the direct effect of Aβ on mitochondrial distribution and mitochondrial trafficking within axons. Results generated from the study will provide substantial evidence for the role of synaptic mitochondrial dysfunction in AD pathogenesis, especially as related to Aβ-induced synaptic mitochondrial injury. The Tg mutant form of APP (mAPP) mouse is a well-established mouse model for AD and has been used previously to study the role of mitochondrial Aβ in AD (19, 20, 25–28).
Results
Aβ Accumulation in Synaptic Mitochondria of Tg mAPP Mice.
To determine whether Aβ accumulates in synaptic mitochondria in an Aβ-rich environment, Tg mice overexpressing human APP/Aβ (Tg mAPP mice) and non-Tg littermate controls lacking human Aβ were applied to our studies. First, synaptic mitochondria were prepared from mice. The purity of synaptic mitochondria was ascertained by the enrichment of mitochondrial markers [cytochrome c oxidase (CcO); HSP60; and VDAC] and relative absence of endoplasmic reticulum (Calnexin) and lysosomal (LAMP-1) and neuronal (synaptophysin) markers (Fig. S1A) as well as by ultrastructural criteria, as previously described (19, 25, 26) (Fig. S1 B and C). Synaptic mitochondria isolated by this method were >95% homogeneous when visualized using EM (Fig. S1C).
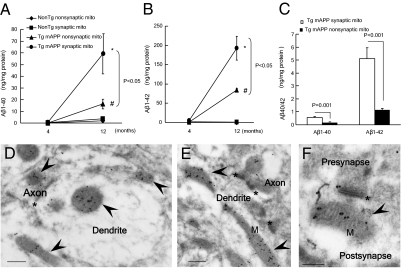
We then measured Aβ levels in synaptic and nonsynaptic mitochondria from Tg mAPP mice and non-Tg mice. To examine the age-dependent accumulation of mitochondrial Aβ and its association with mitochondrial abnormalities, mice were analyzed at the age of 4 mo (at the start of cerebral Aβ accumulation) and 12 mo (full cerebral Aβ deposit). Mitochondrial fractions were subjected to ELISAs to detect the two species of human Aβ, Aβ1-40 and Aβ1-42. As early as 4 mo of age, Tg mAPP synaptic mitochondria had Aβ1-40 and Aβ1-42 concentrations (ng/mg of mitochondrial fraction) of 0.569 ± 0.077 and 5.11 ± 0.828, respectively, as compared with nonsynaptic mitochondria at 0.134 ± 0.017 and 1.13 ± 0.116, respectively (Fig. 1C; P < 0.05). By 12 mo of age, Aβ1-40 and Aβ1-42 in synaptic mitochondria reached ∼58.9 ± 17.0 and 192.6 ± 31.1, respectively (Fig. 1 A and B), suggesting that Aβ accumulated progressively in synaptic mitochondria of Tg mAPP mice.
Fig. 1.
Increased accumulation of Aβ in synaptic mitochondria (mito) of Tg mAPP mice. (A and B) Age-related mitochondrial Aβ1-40 and Aβ1-42 accumulation in synaptic and nonsynaptic mitochondria from non-Tg and APP mice at 4 and 12 mo of age (n = 4–6 mice per group). *P < 0.05 vs. 4-mo-old Tg mAPP synaptic mitochondria; #P < 0.05 vs. 4-mo-old Tg mAPP nonsynaptic mitochondria. (C) Comparison of Aβ levels in synaptic mitochondria with those in nonsynaptic mitochondria from Tg mAPP mice at the age of 4 mo. (D–F) Immunogold EM images with a specific Aβ1-42 antibody, followed by a gold-conjugated antibody (18 nm), demonstrating the presence of intramitochondrial Aβ accumulation (black particles) in 12-mo-old Tg mAPP mice. Arrows denote mitochondria (M), and the asterisk denotes a synapse. (Scale bars = 200 nm.)
To confirm the presence of Aβ in synaptic mitochondria further, immunogold EM was performed in the intact brain tissues of Tg mAPP mice and non-Tg littermates using anti-Aβ antibody specific for human Aβ1-42, as previously described (19, 25, 26). Aβ immunogold particles were clearly localized in pre- and postsynaptic mitochondria of Tg mAPP mice (Fig. 1 D–F and Fig. S2A). No detectable Aβ immunogold particles were found in non-Tg synaptic mitochondria (Fig. S2B). Control experiments were performed to verify the specificity of this immunogold staining pattern. The specific Aβ staining pattern was lost when Aβ antibody was replaced by nonimmune IgG (Fig. S2C) or neutralized by its antigen (Aβ-42) (Fig. S2D).
Synaptic Mitochondrial Dysfunction in Tg mAPP Mice.
Impaired mitochondrial respiratory function in Tg mAPP synaptic mitochondria.
To determine if synaptic mitochondrial accumulation of Aβ was associated with abnormalities in mitochondrial function, we detected the known characteristic bioenergetic abnormalities of AD brain (12, 13, 32). First, we studied mitochondrial respiratory function by assessing the activity of CcO, a key mitochondrial respiration enzyme. CcO inactivation is a predominant brain mitochondrial deficit in human patients with AD and Tg mAPP mice (12, 19, 20, 26, 27). As shown in Table 1, decreased CcO activity (nmol/mg of mitochondrial protein per minute) in Tg mAPP synaptic mitochondria occurred as early as 4 mo of age in comparison to non-Tg synaptic mitochondria (∼20% decreased in Tg mAPP, 113.2 ± 2.19 vs. 147.8 ± 14.12; P < 0.05), although CcO activity was comparable in the non-Tg synaptic, nonsynaptic, and Tg mAPP nonsynaptic mitochondria. By 12 mo, CcO activity was progressively reduced in Tg mAPP synaptic mitochondria by 30% as compared with that in non-Tg synaptic mitochondria (89.67 ± 5.29 vs. 129.68 ± 6.65; P < 0.05). Similarly, Tg mAPP nonsynaptic mitochondria also demonstrated age-dependent reduction of CcO activity as compared with that in non-Tg nonsynaptic mitochondria; however, the impairment of nonsynaptic mitochondrial CcO activity was less than that of synaptic mitochondria in 12-mo-old Tg mAPP mice (13% reduction in nonsynaptic mitochondria vs. 30% reduction in synaptic mitochondria; Table 1). Notably, levels of CcO activity in synaptic mitochondria from WT APP-overexpressing (wtAPP) mice were comparable to those in non-Tg synaptic mitochondria (Fig. S3), even at the age of 12 mo.
Table 1.
CcO activity in synaptic and nonsynaptic mitochondria from Tg mAPP mice and non-Tg mice
| Age | Non-Tg nonsynaptic mitochondria | Non-Tg synaptic mitochondria | APP nonsynaptic mitochondria | APP synaptic mitochondria |
| 4 mo | 133.8 ± 11.88 | 147.8 ± 14.12 | 136.45 ± 10.28 | 113.18 ± 2.19* |
| 12 mo | 134.59 ± 4.84 | 129.68 ± 6.65 | 101.92 ± 5.85** | 89.67 ± 5.29*** |
CcO activity is measured in nmol/mg of mitochondrial fraction per minute (n = 7–15 mice per group).
*P < 0.05 vs. other mitochondrial fractions.
**P < 0.05 vs. 12-mo-old non-Tg mitochondrial fractions.
***P < 0.05 vs. other mitochondrial fractions.
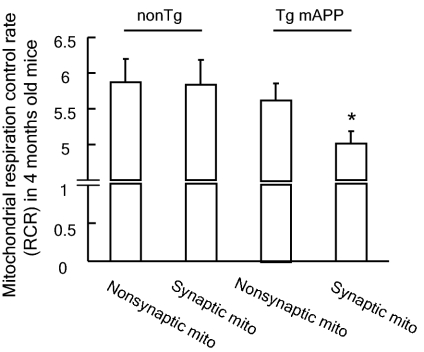
Decreased CcO activity is indicative of a defect in mitochondrial respiration. To evaluate whether early deficits in key respiratory enzyme (CcO) activity in Tg mAPP synaptic mitochondria impair respiratory function in Tg mice at a young age, we compared the mitochondrial respiration control ratio (RCR; ratio of oxygen consumption in state III/state IV respiration) of different mitochondrial fractions from mice aged 4 mo using glutamate/malate as the substrate. Indeed, Tg mAPP synaptic mitochondria showed a significant decline in the RCR when compared with Tg mAPP nonsynaptic mitochondria as well as with the non-Tg synaptic and nonsynaptic mitochondria (Fig. 2; P < 0.05). In contrast, the RCR of Tg mAPP nonsynaptic mitochondria was well preserved and, in fact, comparable to that of non-Tg nonsynaptic mitochondria (Fig. 2; P > 0.05). In an analysis of the state III and IV respiration of the different mitochondrial fractions, oxygen consumption of state III respiration was significantly lower in Tg mAPP synaptic mitochondria than in Tg mAPP nonsynaptic, non-Tg synaptic, and nonsynaptic mitochondria (Table S1; P < 0.05), whereas the oxygen consumption of state IV respiration of Tg mAPP synaptic mitochondria was preserved (Table S1; P > 0.05).
Fig. 2.
Mitochondrial RCRs of synaptic and nonsynaptic mitochondria from 4-mo-old non-Tg and Tg mAPP mice (n = 5–7 mice per group). *P < 0.05 vs. other mitochondrial fractions.
Increased mitochondrial permeability transition probability in Tg mAPP synaptic mitochondria.
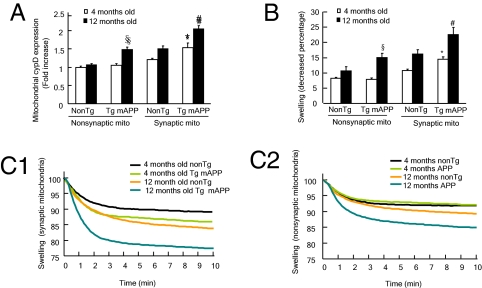
Mitochondrial permeability transition (mPT) is generally accepted as irreversible mitochondrial damage leading to mitochondrial calcium perturbation, mitochondrial swelling, and ROS production and release (15, 26, 27, 29). Increased mPT probability in brain mitochondria has been reported in Aβ milieus, as evidenced by increased expression and translocation of CypD and impaired mitochondrial calcium-handling capacity in aging Tg mAPP mice (26, 27). Given that CypD is a key component of the mitochondrial permeability transition pore (mPTP) and increased levels of CypD are associated with vulnerability of mPT (10, 11), we first examined the expression levels of CypD in synaptic and nonsynaptic mitochondria isolated from Tg mAPP and non-Tg mice at 4 and 12 mo of age by Western blotting. Consistent with previous results (26), CypD expression was elevated in both aged and Aβ-rich brain, but its level was significantly enhanced in Aβ-insulted mitochondria. We found that significant up-regulation of CypD in Tg mAPP synaptic mitochondria occurred as early as 4 mo of age (1.5-fold vs. nonsynaptic mitochondria from age-matched non-Tg mice; Fig. 3A; P < 0.05), whereas CypD in synaptic mitochondria was elevated 2-fold in 12-mo-old Tg mAPP mice compared with non-Tg nonsynaptic mitochondria. Although there was no significant difference in CypD levels between 4-mo-old non-Tg and Tg mAPP nonsynaptic mitochondria, nonsynaptic mitochondria from 12-mo-old Tg mAPP mice showed 1.5-fold higher CypD than nonsynaptic mitochondria from the age-matched non-Tg mice (Fig. 3A). Non-Tg synaptic mitochondria also demonstrated an age-dependent increase in CypD level (Fig. 3A). These results indicate that the levels of CypD in synaptic mitochondria are related to aging and are significantly enhanced in an Aβ-rich environment.
Fig. 3.
CypD expression level and Ca2+-induced mitochondrial swelling in Tg mAPP and non-Tg mice. (A) Age-dependent CypD expression in synaptic and nonsynaptic mitochondria from non-Tg and Tg mAPP mice at 4 and 12 mo of age. Data are expressed as fold increases compared with the CypD level in 4-mo-old non-Tg nonsynaptic mitochondria (n = 4–9 mice per group). (B) Mitochondrial swelling in synaptic (C1) and nonsynaptic (C2) mitochondria. Data are shown as percentages decreased from initial values (n = 5–9 mice per group). *P < 0.05 vs. 4-mo-old other mitochondrial fractions; #P < 0.05 vs. 4- and 12-mo-old non-Tg synaptic and Tg mAPP nonsynaptic mitochondria; §P < 0.05 vs. 4- and 12-mo-old non-Tg nonsynaptic mitochondria. Representative curves of the swelling in synaptic (C1) and nonsynaptic (C2) mitochondria are shown.
We then assessed whether Tg mAPP synaptic mitochondria are more vulnerable to calcium-induced mPTP formation. We compared mitochondrial swelling in response to calcium (500-nmol/mg of mitochondrial fraction) among the mitochondrial fractions from Tg mAPP and non-Tg mice. Both Tg mAPP and non-Tg synaptic mitochondria showed increased mitochondrial swelling compared with nonsynaptic mitochondria at 4 and 12 mo of age. At 4 mo of age, Tg mAPP synaptic mitochondria demonstrated the largest increase in mitochondrial swelling among all the mitochondrial fractions from age-matched mice (Fig. 3 B and C1; P < 0.05); the difference was more pronounced at 12 mo of age (Fig. 3 B and C1; P < 0.05). In addition, nonsynaptic mitochondria from Tg mAPP mice underwent more swelling than those from non-Tg mice at 12 mo of age (Fig. 3 B and C2; P < 0.05), although the mitochondrial swelling of Tg mAPP nonsynaptic mitochondria was comparable to that of the non-Tg nonsynaptic mitochondria in young mice.
Increased oxidative stress in Tg mAPP synaptic mitochondria.
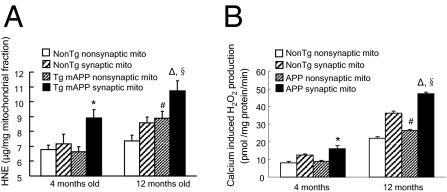
Mitochondria are principal sites of ROS generation, and oxidative stress is closely associated with impaired mitochondrial respiration and mPTP formation. Because Aβ is known to trigger oxidative stress, we next examined whether synaptic mitochondrial Aβ levels correlate with mitochondrial oxidative stress. We measured 4-hydroxynonenal (4-HNE) levels and calcium-induced hydrogen peroxide (H2O2) production in mitochondrial fractions from Tg mice. The 4-HNE levels were significantly increased in non-Tg synaptic mitochondria and Tg mAPP synaptic and nonsynaptic mitochondria in an age-dependent manner. Tg mAPP synaptic mitochondria showed significantly higher 4-HNE production than the other mitochondrial fractions at 4 mo of age, and the difference was still predominant at 12 mo of age (Fig. 4A), although there were no significant differences in 4-HNE levels in non-Tg synaptic, nonsynaptic, and Tg mAPP nonsynaptic mitochondria from young Tg mice.
Fig. 4.
Accumulation of mitochondrial oxidative stress in Tg mice. (A) 4-HNE levels in synaptic and nonsynaptic mitochondria from the indicated Tg mice (n = 4–10 mice per group). *P < 0.05 vs. other mitochondrial fractions from 4-mo-old mice; ΔP < 0.05 vs. other 12-mo-old mitochondrial fractions; #P < 0.05 vs. 4-mo-old Tg mAPP nonsynaptic mitochondria; §P < 0.05 vs. 12-mo-old Tg mAPP nonsynaptic mitochondria. (B) Calcium-induced (1 mM) H2O2 production in synaptic and nonsynaptic mitochondria from the indicated Tg mice at 4 and 12 mo of age (n = 4–10 mice per group). *P < 0.05 vs. other 4-mo-old mitochondrial fractions; ΔP < 0.05 vs. other 12-mo-old mitochondrial fractions; #P < 0.05 vs. 4-mo-old Tg mAPP nonsynaptic mitochondria; §P < 0.05 vs. 12-mo-old Tg mAPP nonsynaptic mitochondria.
Next, we compared calcium-induced H2O2 production in mitochondrial fractions from Tg mAPP mice and the age-matched non-Tg mice at 4 and 12 mo of age. Consistent with the 4-HNE results, H2O2 production was associated with age and also enhanced in Aβ-containing synaptic mitochondria. As shown in Fig. 4B, as early as 4 mo of age, Tg mAPP synaptic mitochondria generated a higher level of H2O2 in response to 1 mM calcium than non-Tg synaptic mitochondria or Tg mAPP nonsynaptic mitochondria (Fig. 4B; P < 0.05). These results suggest that synaptic mitochondrial oxidative stress positively correlates with the accumulation of Aβ in synaptic mitochondria. Notably, at 12 mo of age, non-Tg synaptic mitochondria demonstrated an apparent increase in H2O2 production compared with non-Tg nonsynaptic mitochondria and also with 4-mo-old non-Tg synaptic mitochondria, indicating that synaptic mitochondria are prone to the accumulation of oxidative stress during aging (Fig. 4B; P < 0.05).
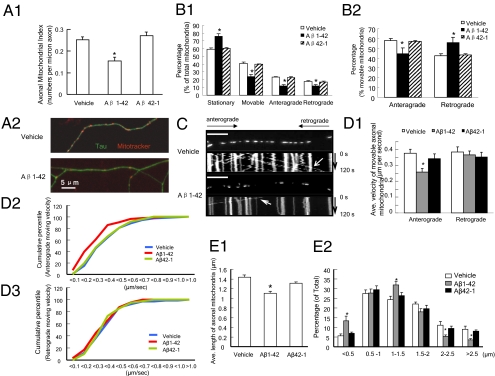
Decreased Axonal Mitochondrial Density and Impaired Mitochondrial Trafficking and Transport: Effect of Aβ.
To fulfill their synaptic roles, such as energy supply maintenance, calcium buffering, synaptic transmission, and vesicle release, mitochondria must transport from the soma to distal synapses via mitochondrial transport and constantly reconfigure to meet synaptic needs. These observations led us to analyze the direct effect of Aβ on axonal mitochondrial behavior. Because of the technical limitations for observing mitochondrial trafficking in vivo, we performed studies in a hippocampal neuronal culture model. We selected axonal processes for quantitative analysis of mitochondrial distribution and mobility because of their known morphologic characteristics (30) and significance in synaptic pathology of AD. With respect to the previously reported Aβ concentration around 60 nM in the hippocampi of Tg mAPP mice as young as 2 mo of age (28), non-Tg neurons were treated with low concentration of Aβ1-42 (200 nM) for 24 h to mimic the chronic low Aβ toxicity in young Tg mAPP mice.
First, we measured axonal mitochondrial density by counting Mitotracker red-positive particles in axonal processes. Aβ-treated hippocampal neurons exhibited significantly decreased (by ∼38%) axonal mitochondrial density in comparison to vehicle-treated neurons (0.155 ± 0.017 vs. 0.252 ± 0.013 per micrometer; Fig. 5 A1 and A2; P < 0.05). The control reversed Aβ peptide did not affect mitochondrial density (0.271 ± 0.017 vs. 0.252 ± 0.013 per micrometer; Fig. 5 A1; P < 0.05).
Fig. 5.
Effect of Aβ on mitochondrial distribution and trafficking in axons. (A) Axonal Mitochondrial Index (numbers per micron in axon) after Aβ1-42 (200 nM) treatment for 24 h on cultured hippocampal neurons. (A1) Data were collected from three independent experiments. *P < 0.05 vs. other groups. (A2) Representative images for vehicle- or Aβ-treated axonal mitochondrial distribution. Double-immunostaining with Mitotracker (red, mitochondrial marker) and Tau (green, axonal marker) was performed. (B) Axonal mitochondria trafficking in cultured non-Tg hippocampal neurons. Data were collected from 1,176, 546, and 563 mitochondria from vehicle, Aβ1-42, and Aβ42-1 groups, respectively, in four independent experiments. (B1) Percentages of stationary, moveable, anterograde-transported, and retrograde-transported mitochondria were compared with those of total mitochondria, respectively. (B2) Percentages of anterograde-transported and retrograde-transported mitochondria were compared with the total amount of movable mitochondria, respectively. *P < 0.05 vs. other groups. (C) Representative kymograph images of the vehicle and Aβ1-42–treated axonal mitochondrial movement. (Scale bars = 10 μm.) (D) Mitochondrial velocity. (D1) Average anterograde and retrograde transport velocity of movable mitochondria (μm/s) is shown. *P < 0.05 vs. other groups. Cumulative data for the anterograde transport velocity (D2) and retrograde transport velocity (D3) are shown. (E1) Average length of axonal mitochondria in micrometers. *P < 0.05 vs. other groups. (E2) Distribution of mitochondrial length.
Second, we monitored mitochondrial trafficking within axonal processes. Quantitatively, two motility characteristics were recorded for each mitochondrion observed: (i) whether it moved and (ii) the net direction of movement (anterograde or retrograde). Subsequently, the relative percentages of stationary, net-plus, and net-minus end-directed events were calculated. When compared with the total mitochondria in the studied axonal segment (the middle part of axons), Aβ-treated neurons showed a 30% increase in the percentage of stationary mitochondria (Fig. 5 B1; P < 0.05). The total of movable mitochondria, including both directions (anterograde and retrograde), was significantly reduced in Aβ-superimposed axons compared with those treated with vehicle or control-reversed Aβ peptide (Fig. 5 B1 and C). After Aβ treatment, the anterograde mobile mitochondria decreased by ∼23% (44.44 ± 5.66%) compared with those of vehicle-treated (57.65 ± 2.11%; P < 0.05) or reversed peptide-treated (56.82 ± 1.10%; P < 0.05) control neurons. In contrast, the percentage of retrograde mobile mitochondria increased in neurons treated with Aβ (Fig. 5 B2).
Third, we measured mitochondrial velocity as previously described (31). We compared the velocity of anterograde and retrograde movement. Interestingly, Aβ exerted a more deleterious effect on anterograde than retrograde mitochondrial movement velocity (Fig. 5 D1–D3). Aβ-treated axonal mitochondria demonstrated a substantial decline (by ∼32%) in anterograde transport velocity (0.258 ± 0.024 μm/s; P < 0.05) in comparison to those of vehicle (0.376 ± 0.038 μm/s) and reversed Aβ (0.343 ± 0.03 μm/s) groups (Fig. 5 D1), whereas the retrograde transport velocity was not significantly changed (Fig. 5 D1 and D3). Cumulative data displayed a left shift in anterograde transport velocity (Fig. 5 D2) but not in retrograde velocity (Fig. 5 D3) of Aβ-treated mitochondria.
Finally, we evaluated the changes in mitochondrial morphology by measuring average axonal mitochondrial length. There was a significantly decreased average mitochondrial length following Aβ treatment as compared with mitochondria treated with vehicle or reversed peptide (1.14 ± 0.035 μm in Aβ-treated neurons vs. 1.436 ± 0.044 μm in vehicle-treated neurons or 1.309 ± 0.026 μm in reversed Aβ-treated neurons; Fig. 5 E1; P < 0.05). In contrast, the percentage of mitochondria less than 0.5 μm in length after Aβ exposure was significantly higher than that of vehicle-treated or reversed peptide-treated control neurons (Fig. 5 E2). These data suggest increased mitochondrial fragmentation in neurons exposed to Aβ.
Discussion
Neuronal mitochondria play a crucial role in regulating synaptogenesis and supporting the release and recycling of neurotransmitters by means of their ATP provision and calcium modulation abilities (3–5, 33, 34). Given the critical role of neuronal degeneration and synaptic failure in AD pathogenesis, it is important to delineate mechanisms of neuronal mitochondrial perturbation contributing to synaptic stress, which will facilitate our understanding of AD pathology. Indeed, reports on synaptic mitochondrial structural and functional changes are quite limited, particularly regarding Aβ-induced synaptic mitochondrial alterations.
In this study, we demonstrated that Aβ, principally Aβ1-42, progressively accumulated in Tg mAPP synaptic mitochondria with age. Notably, accumulation of synaptic mitochondrial Aβ in Tg mAPP mice occurred as early as 4 mo of age, before extensive extracellular Aβ deposits were evidenced, a finding in agreement with previous reports showing that intracellular and mitochondrial accumulation of Aβ can occur before extracellular Aβ deposition (19, 35–37). Such results suggest that intracellular accumulation of Aβ may be a precipitating factor or early marker in Aβ-mediated neuronal dysfunction.
Accordingly, Aβ-rich synaptic mitochondria revealed detectable pathological and functional alterations as early as 4 mo of age, including decreased mitochondrial respiration, decreased key respiratory enzyme activity, elevated CypD and ABAD expression and oxidative stress, increased vulnerability to mPT, and compromised calcium handling capacity. These deleterious effects occur before the observed impairments in nonsynaptic mitochondria in Tg mAPP mice and in synaptic mitochondria in non-Tg mice. Although both Tg mAPP synaptic and nonsynaptic mitochondria underwent age-dependent exacerbation of mitochondrial dysfunction, Tg mAPP synaptic mitochondrial function worsened significantly more than nonsynaptic mitochondrial function, even at 12 mo of age. Additionally, in the presence of a low concentration of Aβ, axonal mitochondria displayed significantly suppressed mobility and increased fragmentation. These findings suggest that synaptic mitochondria are more susceptible to Aβ-induced injury and that synaptic mitochondrial stress is an early pathological alteration of AD.
Aβ is considered to be a causative factor underlying AD pathogenesis. Recent studies provide substantial evidence of progressive accumulation of mitochondrial Aβ, which is linked directly to mitochondrial toxicity (18–22, 25–27, 38). Previous studies found that Aβ was present inside synaptosomes and synaptosomal mitochondria on exposure of exogenous Aβ (38). Further, Aβ-superimposed synaptosomal mitochondria exhibited pronounced metabolic change and oxidative stress (39–40). Mitochondrial pooling of Aβ may be attributable to the cleavage of APP with the N terminus targeted to the mitochondrial membrane through γ-secretase that has been identified within mitochondria (41). Aβ is able to transport into mitochondria via the translocase of the outer membrane machinery (42). Moreover, the receptor for advanced glycation end products (RAGE) is involved in the transport of Aβ from the cell surface to the intracellular space, including mitochondrial localization. Blockade of RAGE significantly decreases mitochondrial uptake of Aβ, and thereby protects mitochondria from Aβ toxicity (43).
We observed that the progressive accumulation of Aβ in synaptic mitochondria is associated with many abnormalities in synaptic mitochondria. CypD is a key component of mPTP, and the level of CypD influences mPTP formation. Low expression of CypD in brain mitochondria increases resistance to mPT (44), whereas high levels of CypD in neuronal mitochondria result in greater vulnerability to mPT and require higher levels of cyclosporin A (an inhibitor of CypD) to inhibit mPTP opening (11). CypD is elevated during the aging of both human and mouse brains (26, 27). This could mean that CypD levels are an integral part of a mechanism by which aging mitochondria can become more vulnerable and susceptible to various damages. Moreover, levels of CypD in the Aβ-rich synaptic mitochondria isolated from Tg mAPP mice were significantly higher than in the synaptic mitochondria isolated from age-matched non-Tg controls, as shown in Fig. 3A, which would make Tg mAPP synaptic mitochondria more susceptible to injury attributable to increased mPTP, resulting in lowered calcium handling ability of brain mitochondria and enhanced mitochondrial oxidative stress. Recent studies provide substantial evidence that genetic deficiency of CypD protects cells from Ca2+- and oxidative stress-induced cell death (29, 45). Indeed, we have demonstrated that the abrogation of CypD protects against Aβ- or oxidative stress-induced mitochondrial and neuronal toxicity as well as cognitive decline (26, 27). Furthermore, high levels of ABAD have been found in AD-affected brain regions of AD brains and the AD mouse model (18, 25, 46–48). In the present study, we have observed that the ABAD expression level was significantly elevated in synaptic mitochondria from Tg mAPP mice as compared with those from non-Tg mice (Fig. S3). ABAD has been suggested as a contributing factor in the loss of synapses in the hippocampus, as observed in the animal model of AD-type pathology (47). These results suggest that increased expression of ABAD in Aβ-containing neuronal/synaptic mitochondria, could also contribute to synaptic damage through the interaction of ABAD with Aβ, leading to mitochondrial and neuronal stress (20, 25, 46, 49–52). Therefore, we propose that the accumulation of synaptic mitochondrial Aβ and up-regulation of Aβ-binding protein (CypD or ABAD) might be involved in facilitating and exacerbating mitochondrial and neuronal malfunction. The detailed mechanisms underlying synaptic mitochondrial perturbation in aging and Aβ-rich environments require further investigation.
In our published studies, wtAPP mice are known to produce much lower amounts of Aβ (28) and to have normal spatial learning and memory as compared with those mAPP/ABAD mice that had higher levels of Aβ and revealed significant impairments in spatial memory (51). Furthermore, CcO activity in wtAPP synaptic or nonsynaptic mitochondria was comparable to that in non-Tg synaptic or nonsynaptic mitochondria (Fig. S4) from 12-mo-old mice. These data suggest that overexpression of APP might not be a strong causative factor in synaptic mitochondrial dysfunction and impaired learning/spatial memory. Thus, the synaptic mitochondrial defects observed in Tg mAPP mice are more likely attributable to the effect of Aβ toxicity rather than the overexpression of human APP.
Normal synaptic mitochondrial mobility and dynamics are required for synaptic maintenance. The transport of mitochondria in axons and mitochondrial distribution at synapses are critical for neurotransmission, synaptic plasticity, and axonal outgrowth (1). Mutations in proteins that regulate mitochondrial dynamics compromise synaptic function and plasticity (1, 4), and defective mitochondrial trafficking and dynamics are implicated in neurodegenerative diseases, including AD (7, 25, 53, 54). Our findings clearly demonstrate that even at a low concentration, Aβ significantly impaired synaptic mitochondrial distribution and axonal mitochondrial mobility and increased axonal mitochondrial fragmentation. Interestingly, anterograde movements were more susceptible to Aβ insult, although both the retrograde and anterograde movements were impaired during these experiments. These findings are in agreement with those of recent studies indicating that Aβ causes rapid and severe impairment of mitochondrial transport (54) and alters mitochondrial dynamics (53). In view of the critical role of synaptic mitochondria in energy production, synaptic calcium buffering, and regulation of synaptic function, impaired movement to and sequestration of mitochondria at synapses by Aβ could be a mechanism of the pathogenesis at the distal synapses, together with early changes in synaptic mitochondrial function.
In summary, we have demonstrated early and progressive accumulation of Aβ in synaptic mitochondria and that such accumulation is associated with perturbation of synaptic mitochondrial function and oxidative stress. These findings indicate that central nervous system mitochondria respond heterogeneously to Aβ toxicity and that synaptic mitochondria are early victims in the AD milieu, highlighting the importance of synaptic mitochondria in regulation of synaptic strength and the impact of their perturbation on the pathogenesis of AD. Intervention in synaptic mitochondrial dysfunction may be a therapeutical target in defeating AD at an early stage.
Materials and Methods
Details on materials and methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported by the National Institute on Aging (Grants PO1AG17490 and AG037319) and the Alzheimer Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006586107/-/DCSupplemental.
References
- 1.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Guo X, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verstreken P, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 6.Kang JS, et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banaclocha MM, Hernández AI, Martínez N, Ferrándiz ML. N-acetylcysteine protects against age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1997;762:256–258. doi: 10.1016/s0006-8993(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 9.Martinez M, Hernández AI, Martínez N, Ferrándiz ML. Age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1996;731:246–248. doi: 10.1016/0006-8993(96)00708-1. [DOI] [PubMed] [Google Scholar]
- 10.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 11.Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan JP, et al. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer's disease. J Neurosci. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 15.Du H, Yan SS. Mitochondrial medicine for neurodegenerative diseases. Int J Biochem Cell Biol. 2010;42:560–572. doi: 10.1016/j.biocel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin MT, Beal MF. Alzheimer's APP mangles mitochondria. Nat Med. 2006;12:1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 17.Eckert A, et al. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5:157–159. doi: 10.1159/000113689. [DOI] [PubMed] [Google Scholar]
- 18.Yao J, et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspersen C, et al. Mitochondrial Abeta: A potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 20.Takuma K, et al. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 21.Shukkur EA, et al. Mitochondrial dysfunction and tau hyperphosphorylation in Ts1Cje, a mouse model for Down syndrome. Hum Mol Genet. 2006;15:2752–2762. doi: 10.1093/hmg/ddl211. [DOI] [PubMed] [Google Scholar]
- 22.Manczak M, et al. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: Implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield DA, Hensley K, Harris M, Mattson M, Carney J. beta-Amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: Implications to Alzheimer's disease. Biochem Biophys Res Commun. 1994;200:710–715. doi: 10.1006/bbrc.1994.1508. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso SM, Santana I, Swerdlow RH, Oliveira CR. Mitochondria dysfunction of Alzheimer's disease cybrids enhances Abeta toxicity. J Neurochem. 2004;89:1417–1426. doi: 10.1111/j.1471-4159.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- 25.Lustbader JW, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 26.Du H, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.003. 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mucke L, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 30.Banker GA, Cowan WM. Further observations on hippocampal neurons in dispersed cell culture. J Comp Neurol. 1979;187:469–493. doi: 10.1002/cne.901870302. [DOI] [PubMed] [Google Scholar]
- 31.Macaskill AF, et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosetti F, et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 33.Hollenbeck PJ. Mitochondria and neurotransmission: Evacuating the synapse. Neuron. 2005;47:331–333. doi: 10.1016/j.neuron.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 35.Wirths O, et al. Intraneuronal Abeta accumulation precedes plaque formation in beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci Lett. 2001;306:116–120. doi: 10.1016/s0304-3940(01)01876-6. [DOI] [PubMed] [Google Scholar]
- 36.Mori C, et al. Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid. 2002;9:88–102. [PubMed] [Google Scholar]
- 37.Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer's disease. J Mol Neurosci. 2001;17:137–145. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]
- 38.Crouch PJ, et al. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1-42. J Neurosci. 2005;25:672–679. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mungarro-Menchaca X, Ferrera P, Morán J, Arias C. beta-Amyloid peptide induces ultrastructural changes in synaptosomes and potentiates mitochondrial dysfunction in the presence of ryanodine. J Neurosci Res. 2002;68:89–96. doi: 10.1002/jnr.10193. [DOI] [PubMed] [Google Scholar]
- 40.Keller JN, et al. Amyloid beta-peptide effects on synaptosomes from apolipoprotein E-deficient mice. J Neurochem. 2000;74:1579–1586. doi: 10.1046/j.1471-4159.2000.0741579.x. [DOI] [PubMed] [Google Scholar]
- 41.Hansson CA, et al. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J Biol Chem. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- 42.Hansson Petersen CA, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci USA. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takuma K, et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci USA. 2009;106:20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eliseev RA, et al. Role of cyclophilin D in the resistance of brain mitochondria to the permeability transition. Neurobiol Aging. 2007;28:1532–1542. doi: 10.1016/j.neurobiolaging.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Schinzel AC, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan SD, et al. An intracellular protein that binds amyloid-beta peptide and mediates neurotoxicity in Alzheimer's disease. Nature. 1997;389:689–695. doi: 10.1038/39522. [DOI] [PubMed] [Google Scholar]
- 47.He XY, et al. Abundant type 10 17 beta-hydroxysteroid dehydrogenase in the hippocampus of mouse Alzheimer's disease model. Brain Res Mol Brain Res. 2002;99:46–53. doi: 10.1016/s0169-328x(02)00102-x. [DOI] [PubMed] [Google Scholar]
- 48.Chen JX, Yan S. Amyloid-beta-induced mitochondrial dysfunction. J Alzheimer's Disease. 2007;12:177–184. doi: 10.3233/jad-2007-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao J, et al. Interaction of amyloid binding alcohol dehydrogenase/Aβ mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mole Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Ren Y, et al. Endophilin I expression is increased in the brain of Alzheimer's disease patients. J Biol Chem. 2008;283:5685–5691. doi: 10.1074/jbc.M707932200. [DOI] [PubMed] [Google Scholar]
- 51.Yan SD, Chen X, Lustbader J, Arancio O, Wu H. ABAD: Mitochondrial target for amyloid-induced cellular perturbation relevant to Alzheimer disease In: In: Sun M.-K., editor. Research Progress in Alzheimer Disease and Dementia. Nova Science Publishers, Hauppauge, NY; 2006. [Google Scholar]
- 52.Oppermann UC, Salim S, Tjernberg LO, Terenius L, Jörnvall H. Binding of amyloid beta-peptide to mitochondrial hydroxyacyl-CoA dehydrogenase (ERAB): Regulation of an SDR enzyme activity with implications for apoptosis in Alzheimer's disease. FEBS Lett. 1999;451:238–242. doi: 10.1016/s0014-5793(99)00586-4. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.