Abstract
To study adaptive evolution in defined environments, we performed evolution experiments with Saccharomyces cerevisiae (yeast) in nitrogen-limited chemostat cultures. We used DNA microarrays to identify copy-number variation associated with adaptation and observed frequent amplifications and deletions at the GAP1 locus. GAP1 encodes the general amino acid permease, which transports amino acids across the plasma membrane. We identified a self-propagating extrachromosomal circular DNA molecule that results from intrachromosomal recombination between long terminal repeats (LTRs) flanking GAP1. Extrachromosomal DNA circles (GAP1circle) contain GAP1, the replication origin ARS1116, and a single hybrid LTR derived from recombination between the two flanking LTRs. Formation of the GAP1circle is associated with deletion of chromosomal GAP1 (gap1Δ) and production of a single hybrid LTR at the GAP1 chromosomal locus. The GAP1circle is selected following prolonged culturing in l-glutamine–limited chemostats in a manner analogous to the selection of oncogenes present on double minutes in human cancers. Clones carrying only the gap1Δ allele were selected under various non-amino acid nitrogen limitations including ammonium, urea, and allantoin limitation. Previous studies have shown that the rate of intrachromosomal recombination between tandem repeats is stimulated by transcription of the intervening sequence. The high level of GAP1 expression in nitrogen-limited chemostats suggests that the frequency of GAP1circle and gap1Δ generation may be increased under nitrogen-limiting conditions. We propose that this genomic architecture facilitates evolvability of S. cerevisiae populations exposed to variation in levels and sources of environmental nitrogen.
Keywords: adaptive evolution, genome plasticity, intrachromosomal recombination, double minute, retrotransposon
Adaptive evolution is the process by which a population becomes better suited to its environment through mutation and selection. The pace at which a population adapts to a novel environment is determined by the rate at which beneficial mutations arise in the population. In both experimental and natural populations, mutations underlying adaptive evolution include nucleotide changes (1, 2), transposition events (3, 4), and copy-number variants including gene amplifications and deletions (5, 6). Although the relative importance of these different classes of genomic variation for adaptive evolution is not known, their spontaneous rates differ dramatically. In the single-celled eukaryote Saccharomyces cerevisiae (budding yeast) the rate of point mutation is 10−9–10−10 per base pair/generation (7), whereas rates of retrotransposition are estimated to be on the order of 10−8 events/generation (8). Gene deletion events are estimated to occur at a very high rate of around 10−5 events/generation (9). Conversely, gene amplification events, which are much more difficult to assay accurately, have been estimated to occur at rates as low as 10−10 events/generation (10).
Repetitive elements often result in higher rates of deletion, translocation, and duplication. In the yeast genome, repetitive DNA at the ribosomal DNA (rDNA) (11) and telomeric (12) loci facilitates the formation of extrachromosomal circular DNA. Besides rDNA and telomeric repetitive elements, eukaryotic genomes carry a large number of dispersed repeats, including paralogues, tRNA genes, and transposons. Transposon sequences facilitate a higher rate of recombination than other repetitive elements in the genome (13), and their ability to transpose and rearrange is a driving force in the evolution of eukaryotic genomes (14). In yeast, long terminal repeat (LTR)-containing retrotransposons (Ty1–Ty5) are the dominant class of transposable elements. Ty retrotransposons are typified by two ORFs flanked by tandem LTRs. Ty elements are purged from the genome via intrachromosomal recombination, leaving a scar of a solo LTR and producing an extrachromosomal circle as an intermediate in the excision process (15). As a result of this process, the S. cerevisiae genome contains ≈300 solo LTRs dispersed throughout the genome. Given sufficient sequence similarity, solo LTRs remain potential substrates for intrachromosomal recombination.
We have identified a class of extrachromosomal circles that arise upon homologous recombination between solo retrotransposon LTRs flanking the general amino acid permease gene GAP1. The resulting circular DNA elements contain GAP1, the active replication origin ARS1116, and a recombination product of the Ty1 solo LTRs YKRCδ11 and YKRCδ12. The extrachromosomal circle is the product of an intrachromosomal recombination event that results in a solo hybrid LTR at the chromosomal locus. Whereas wild-type yeast carry the chromosomal GAP1 allele, retention of the extrachromosomal GAP1 circle (GAP1circle) is selected under long-term l-glutamine limitation in chemostats. Deletion of GAP1 (gap1Δ) and loss of the resultant circular DNA are selected under various other nitrogen-limiting conditions in chemostats including ammonium, urea, and allantoin limitation. Thus, a single recombination event can generate two different GAP1 alleles that confer selective advantages under different nitrogen-limiting conditions. We suggest that this particular genomic architecture has been retained to facilitate evolvability of S. cerevisiae populations exposed to dramatically varying nitrogen conditions. We also provide evidence that the GAP1circle is able to reintegrate at the original locus, suggesting that GAP1circle formation is genetically reversible.
Results
Diverse GAP1 Alleles Are Recovered from Nitrogen-Limited Populations.
To study adaptive evolution of yeast in nitrogen-limited environments, we propagated isogenic populations of prototrophic haploid yeast in chemostats for several hundred generations. In these experiments, a single nitrogen source was present at a growth-limiting concentration (Table 1) while all other media components were present in excess. Steady-state populations of ≈1010 cells were established and maintained under continuous culture conditions for a period of about 2 mo. Samples were recovered from the population at regular intervals, and upon completion of the evolution experiment we isolated clones from each population for analysis.
Table 1.
Variation of GAP1 genotypes and fitness in populations subjected to long-term nitrogen limitation in chemostat cultures
| Nitrogen source | Generations | gap1 (%) | GAP1circle (%) | Genotypes of selected clones | Relative fitness of clone (gen−1) |
| Ammonium* | 400 | 70 | 0 | RB17 gap1Δ† | 1.09 |
| l-glutamine* | 250 | 15 | 12 | RB10 gap1Δ† | 0.99 |
| CDG23-1 GAP1 amplification† | 1.28 | ||||
| CDG23-2 GAP1 amplification† | 1.14 | ||||
| CDG23-3 GAP1 amplification† | 1.31 | ||||
| RB410 gap1Δ GAP1circle | n.d. | ||||
| RB411 gap1ΔGAP1circle | n.d. | ||||
| RB413 gap1Δ GAP1circle | n.d. | ||||
| RB414 gap1Δ GAP1circle | n.d. | ||||
| l-alanine | 250 | 94 | 0 | ||
| l-glutamate | 250 | 15 | n.d. | CDG25-2 gap1Δ† | 0.93 |
| CDG25-3 GAP1 amplification† | 1.44 | ||||
| Urea | 250 | 100 | n.d. | ||
| Allantoin | 250 | 15 | n.d. | CDG29-2 gap1Δ† | 1.64 |
n.d., no data.
*Two independent populations were propagated under ammonium and l-glutamine limitation. A single population was analyzed for each of the four additional nitrogen limitations.
†Genotype determined by array comparative genomics hybridization.
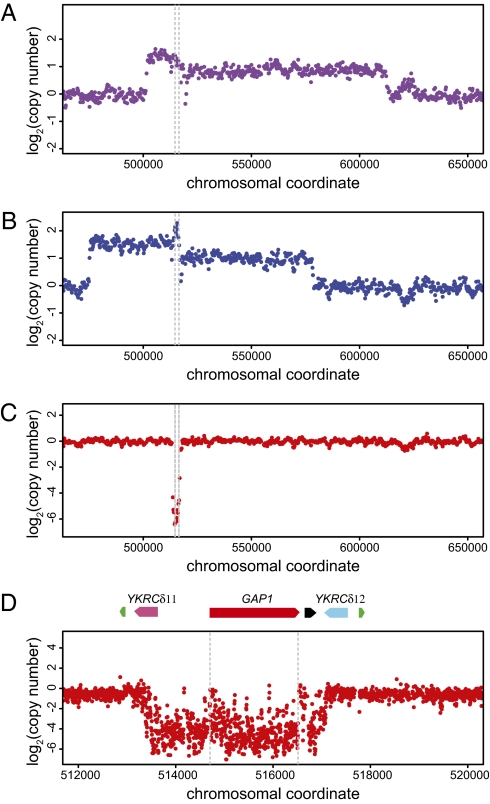
Previous studies have reported a high frequency of gene amplifications and gross chromosomal rearrangements in strains that have undergone adaptive evolution in nutrient-limited environments (5, 16, 17). Therefore, we performed array comparative genomics hybridization (aCGH) using a DNA microarray with probes spaced every ∼400 bases to determine whether adaptation to nitrogen limitation is associated with similar genomic variation. In our sample of 21 clonal isolates from 8 independent populations analyzed using aCGH, we observed a high frequency of copy-number variant alleles at the GAP1 locus (8 of 21 clones; Table 1). These alleles include large-scale amplifications involving many contiguous genes in addition to GAP1 in clones adapted to l-glutamine limitation (Fig. 1A) and l-glutamate limitation (Fig. 1B) and deletion alleles in several populations that appeared to include only GAP1 (Fig. 1C). Examination of the likely boundaries of the two amplification alleles did not show evidence of repeated sequences, and these alleles were not examined further.
Fig. 1.
Gene amplification and deletion alleles at the GAP1 locus identified by array comparative genomic hybridization. We identified frequent copy-number polymorphisms at the GAP1 locus in clones subjected to long-term adaptation in diverse nitrogen-limited chemostats. These included unique amplification alleles in clones adapted to l-glutamine (A) and l-glutamate (B). Each of these amplification alleles is ∼100 kb and includes GAP1 (bounded by gray dashed lines in all plots) and several flanking genes. In addition, we identified deletion events that include only the GAP1 gene in several clones, including one adapted to allantoin limitation (C). We hybridized DNA from the allantoin-adapted clone to an overlapping tiling microarray (D) and determined that the deletion event is bounded by two flanking long terminal repeats: YKRCδ11 and YKRCδ12. The locus also includes an origin of replication (black) and two tRNA genes (green) that lie distal to the LTRs.
The gap1 deletion allele appeared to be flanked by Ty1 solo LTRs YKRCδ11 and YKRCδ12 (Fig. 1D), which are 82% sequence-identical and in a tandem orientation. To precisely map the breakpoints of this deletion event, we hybridized genomic DNA to a high-density overlapping tiling array (average probe spacing ∼4 bp). We mapped the deletion of GAP1 to an ≈4.2-kb region spanning nucleotide coordinates 513174–517424 on chromosome XI that lie within the Ty1 solo LTRs YKRCδ11 and YKRCδ12 (Fig. 1D).
Loss-of-function mutations in GAP1, through either point mutation or deletion, result in resistance to the toxic amino acid d-histidine. We measured the fraction of gap1 mutants in each population by determining the proportion of d-histidine-resistant (d-HisR) cells using a plate assay. Loss of GAP1 function ranged from 15% to 94% in experimentally evolved populations (Table 1), indicating that one or more gap1 clones had rapidly increased in frequency in several nitrogen-limiting conditions. To investigate the fitness effect of different GAP1 alleles, evolved strains were mixed with the ancestral strain and their relative fitness was measured using competitive growth rate assays. GAP1 chromosomal amplification alleles are associated with higher fitness in clones adapted to l-glutamine and l-glutamate (Table 1). GAP1 deletion alleles (gap1Δ) are associated with higher fitness in clones recovered from ammonium, urea, and allantoin limitation; however, gap1Δ clones are not significantly fitter under l-glutamine and l-glutamate limitation (Table 1). This is surprising, given that d-HisR cells are recovered at significant frequency in both these populations.
GAP1 Deletion Occurs via a Homologous Recombination Event.
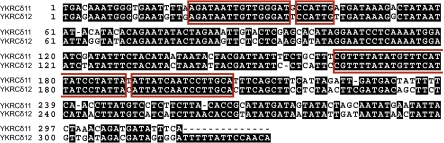
To determine the molecular basis of GAP1 deletion alleles, we designed PCR primers distal to the two LTRs and sequenced the deletion product in gap1Δ strains. These strains included one clone obtained from long-term ammonium limitation and four independent spontaneous gap1Δ alleles recovered by selection for resistance to d-histidine. Sequencing confirmed the presence of an intact solo LTR in all five clones that is a hybrid of the LTRs YKRCδ11 and YKRCδ12. We resolved cross-overs to regions of sequence identity of fewer than 20 bases (Fig. 2). The identification of a single LTR containing clearly defined breakpoints is consistent with homologous recombination underlying these deletion events.
Fig. 2.
Homologous recombination breakpoints in the two LTRs mediating intrachromosomal recombination at the GAP1 locus. Sequences of YKRδ11 (upper strand) and YKRδ12 (lower strand) are aligned, and identity between the two sequences is indicated in black. Recombination breakpoints in five different clones were identified on the basis of sequence differences between the two LTRs and resolved to the level of the regions defined by red-bounding boxes. The hybrid LTRs contain YKRδ11 5′ to the breakpoint and the YKRδ12 sequence in the subsequent 3′ region.
To determine the rate of GAP1 loss-of-function mutations, we performed a Luria–Delbrück fluctuation analysis by testing cultures for acquisition of resistance to d-histidine. We estimate that the rate of mutations inactivating GAP1 is 3.1 × 10−7 mutations per generation. This mutation rate is similar to other loci of similar target size in the genome (7) and suggests that under normal laboratory conditions—that is, growth in rich media—the rate of GAP1 loss-of-function mutation through either mutation or recombination is not increased relative to other loci in the genome.
Detection of Circular Extrachromosomal GAP1.
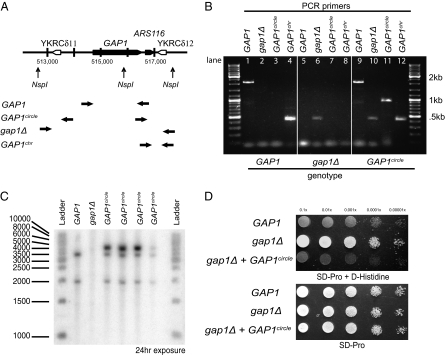
We noticed that an origin of DNA replication (ARS1116) lies proximal to the 3′ end of the GAP1-coding region. We hypothesized that deletion of GAP1 via LTR-mediated homologous recombination could result in a self-replicating extrachromosomal DNA molecule. To test for the existence of this circular DNA, we purified total DNA from clonal isolates recovered from l-alanine, l-glutamine, and ammonium sulfate limitations and used a PCR assay designed to produce a unique fragment only upon circularization of the GAP1 locus (Fig. 3A). In addition, we used PCR primers that unambiguously detect the presence of full-length GAP1, the gap1Δ allele, and the chromosomal location of GAP1 (Fig. 3A). Whereas the parental strain contains the full-length GAP1 at the chromosomal locus (Fig. 3B, lanes 1 and 4), d-HisR clones recovered from long-term ammonium limitation contain the GAP1 deletion (Fig. 3B, lane 6). DNA from a representative clone from the l-glutamine–limited evolution experiment produces a positive PCR product for the predicted circular product, substantiating the existence of the extrachromosomal GAP1circle (Fig. 3B, lane 11). Using PCR amplification, we determined that the clone containing the GAP1circle has a full-length copy of GAP1 and a deletion at the chromosomal GAP1 locus (Fig. 3B, lanes 9 and 10). Interestingly, our PCR assay also provides evidence that a chromosomal copy of GAP1 is contained in at least some of the cells containing the GAP1circle (Fig. 3B, lane 12).
Fig. 3.
Test for the presence of a circular extrachromosomal molecule containing a functional GAP1. (A) Schematic representation of the wild-type chromosomal allele of GAP1. Horizontal arrows represent annealing sites for PCR primers used for verification of the wild-type GAP1 ORF, GAP1circle, gap1Δ, and the chromosomal presence of GAP1. NspI restriction sites are indicated. (B) Agarose gel electrophoresis of PCR products obtained from three different genotypes of GAP1 (indicated below image). Wild-type GAP1 results in PCR amplification of the ORF and chromosomal fragment (lanes 1 and 4); deletion of GAP1 results in the deletion product only (lane 6); the presence of the GAP1circle results in a PCR product using primers that face the opposite direction in a chromosomal context (lane 11). In addition, PCR products corresponding to the ORF and deletion allele are detected (lanes 9 and 10). We also find evidence for the chromosomal location of GAP1 in a clone containing the GAP1circle (lane 12). (C) Southern blot analysis of the three different GAP1 genotypes. Two bands of expected size 3,213 bp and 1,929 bp are detected for the clone carrying the wild-type GAP1 allele. No signal is detected for the clone with the gap1Δ allele. The predicted GAP1circle is 4,252 bp and contains a single NspI site. We detect a single band corresponding to the expected size of the linearized product in four GAP1circle clones (Table 1). In addition, we detect bands corresponding to the wild-type GAP1 locus in all four GAP1circle-containing strains. (D) Growth of GAP1, gap1Δ, and gap1Δ transformed with the GAP1circle on minimal media containing proline and d-histidine. The presence of a wild-type GAP1 allele confers sensitivity to d-histidine, whereas loss of GAP1 (gap1Δ) results in resistance. Introduction of the plasmid DNA recovered from a GAP1circle strain into a gap1Δ strain confers sensitivity, confirming that the GAP1circle contains a functional copy of GAP1. All genotypes are able to grow in minimal media containing proline. Tenfold dilutions from an overnight culture are shown.
To confirm the presence of the GAP1circle, we performed a Southern blot analysis on complete genomic DNA digested with NspI using a probe that hybridizes to the entire GAP1 ORF (Fig. 3C). As Nsp1 cuts within GAP1 (Fig. 3A), we expected to identify two bands of 3,231 and 1,929 bases in clones carrying a chromosomal version of GAP1. We confirmed the detection of the expected bands and the absence of any hybridization signal in a gap1Δ strain (Fig. 3C). NspI is expected to linearize the GAP1circle, which is predicted to be 4,252 bp. We confirmed the presence of a band of this size in four different GAP1circle clones (Fig. 3C). Consistent with our PCR assay, we also detect the presence of the chromosomal GAP1 allele in clones containing the GAP1circle. One explanation for the presence of the GAP1circle and the corresponding chromosomal deletion in addition to the chromosomal copy of GAP1 in these haploid cells is the occurrence of reintegration events that restore the GAP1 chromosomal allele. Alternatively, the circular element may arise through an initial duplication of the GAP1 locus by unequal sister chromatid exchange and subsequent generation of a GAP1circle from one of the duplicated copies.
To determine whether the GAP1circle contains a functional copy of GAP1, a gap1Δ strain was transformed with DNA from the plasmid fraction recovered from a GAP1circle clone. As GAP1 is the sole transporter of l-citrulline, we were able to select transformants on minimal citrulline medium. We recovered cells able to grow on l-citrulline with reasonably high frequency. These transformants were subsequently tested for their ability to grow on d-histidine (Fig. 3D). Whereas gap1Δ cells grow on d-histidine, the parental GAP1 and the gap1Δ strain transformed with the GAP1circle are unable to grow on d-histidine. This confirms that the GAP1circle contains a functional copy of GAP1.
To gain insight into the frequency of the GAP1circle, PCR was performed on 192 descendants from each of l-alanine-, l-glutamine-, and ammonium-limited cultures. Twelve percent of the clones from an l-glutamine limitation experiment gave rise to the GAP1circle-specific PCR product, whereas one clone from ammonium limitation and none of the clones from l-alanine limitation experiments gave rise to this product.
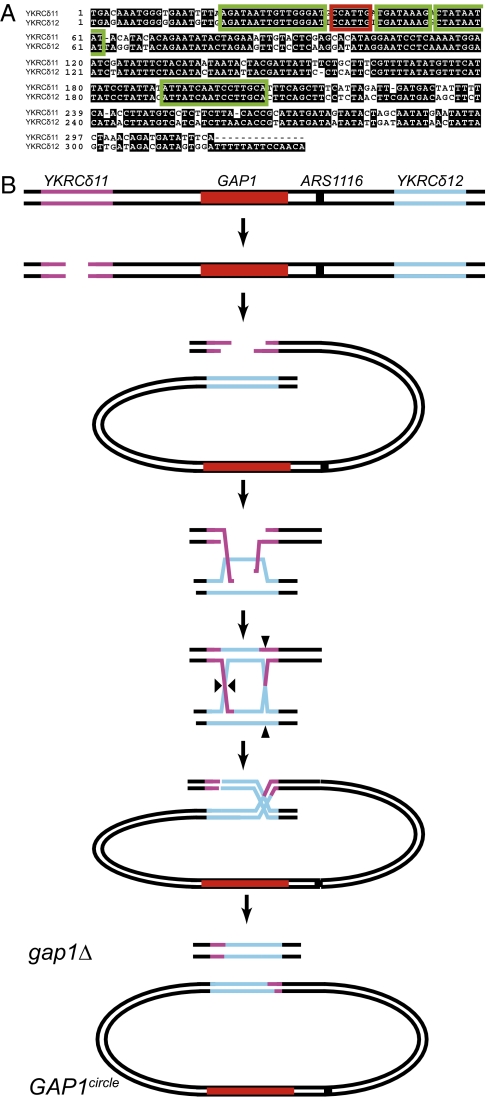
We sequenced the putative cross-over site in both the GAP1circle and gap1Δ alleles for the four GAP1circle clones from the l-glutamine–limited culture analyzed by Southern blot. All GAP1circle strains carried one hybrid LTR on the GAP1circle, suggesting that the circle had formed through recombination between YKRCδ11 and YKRCδ12. Sequence analysis indicated that the four GAP1circle alleles contained different cross-over sites within the GAP1circle (Fig. 4A). Sequence analysis of corresponding gap1Δ alleles in these four clones confirmed the presence of a single hybrid LTR at the chromosomal locus in all four clones. The cross-over site within the gap1Δ alleles was found at the same site in all four of the investigated clones (Fig. 4A). The cross-over sites in the GAP1circle are as close as 1–15 bases and as far as 148–170 bases from the chromosomal cross-over site (Fig. 4A). The precise location of the cross-over site is a function of the site of the initial double-strand DNA break and the extent of resection and branch migration (Fig. 4B). The identification of different cross-over sites in the GAP1circle of the four clones indicates that the GAP1circle alleles had arisen by four independent recombination events in the same l-glutamine–limited culture. We attribute the identical cross-over site in the gap1Δ alleles to chance, as alternate gap1Δ breakpoints are identified in strains containing only the gap1Δ (Fig. 2).
Fig. 4.
Recombination sites in the LTRs YKRCδ11 and YKRCδ12 generated by formation of gap1Δ and the corresponding GAP1circle alleles in four clones (described in Table 1 and Fig. 3). (A) Sequences of YKRCδ11 (upper strand) and YKRCδ12 (lower strand) are aligned, and identity between the two sequences is shown in black. The YKRCδ11-YKRCδ12 breakpoint within the GAP1circle is shown for four clones (green boxes) together with the corresponding YKRCδ11-YKRCδ12 breakpoint from the chromosomal gap1Δ deletions (red box) in each of the same four clones. (B) Model of LTR-mediated homologous recombination at the GAP1 locus resulting in generation of a circular DNA element and a chromosomal deletion. Black triangles indicate the sites of DNA cross-overs.
Discussion
We have identified a genomic architecture at the GAP1 locus that facilitates gene deletion and generation of an extrachromosomal circular DNA species. A single intrachromosomal recombination event between the solo LTRs YKRCδ11 and YKRCδ12 generates a circular self-replicating DNA element containing a functional GAP1 gene and results in a gap1Δ deletion on chromosome XI. Three lines of evidence suggest that this event occurs through homologous recombination: (i) the uneven breakpoints between YKRCδ11 and YKRCδ12 in the GAP1circle and gap1Δ alleles, which is consistent with variable DNA resection and branch migration resolving a double-strand DNA break during homologous recombination; (ii) the identification of a single hybrid LTR in the GAP1circle and gap1Δ alleles; and (iii) the fact that LTR pairs flanking Ty retrotransposons are excised from the genome through homologous recombination (18). Retrotransposons and retroviruses can be excised from eukaryotic genomes by homologous recombination, leaving an extrachromosomal circle with one LTR (15, 19). The formation of the GAP1circle containing a single LTR is likely to follow the same mechanism.
The different alleles of GAP1 have selective advantage under different nitrogen conditions (Table 1). The deletion allele gap1Δ is selected under long-term ammonium, allantoin, and urea limitation, whereas retention of the GAP1circle and large-scale genome amplification events that include GAP1 are found following long-term selection in l-glutamine and l-glutamate limitation. The strong selective advantage associated with increased GAP1 copy number in l-glutamine and l-glutamate limitation conflicted with our initial observation of gap1Δ alleles in l-glutamine and l-glutamate limitation. This was resolved by the identification of the GAP1circle in these populations, the generation of which produces a gap1Δ allele. The high frequency of the GAP1circle allele in populations adapted to l-glutamine limitation (Table 1) suggests that retention of the GAP1circle confers a strong fitness advantage. Self-replication of the GAP1circle is likely to be enabled by the presence of the replication origin, ARS1116, which has previously been shown to be an active replication site (20). The GAP1circle is lost after a few generations in rich media, suggesting that the GAP1circle confers a strong selective disadvantage under nitrogen-rich conditions.
GAP1 encodes a plasma membrane transporter for all 20 common amino acids (21). Expression of GAP1 mRNA is normally repressed under nitrogen-rich conditions and, when the gene is expressed, the protein is sorted to the plasma membrane only under particular conditions (22). Under nitrogen-limiting conditions in the chemostat, GAP1 mRNA is expressed at high levels regardless of the nitrogen source (23). Selective advantage of clones carrying GAP1circle or GAP1 amplifications in l-glutamine and l-glutamate limitation is likely due to increased uptake of the limiting nutrient through increased GAP1 transport capacity. Selective advantage of the gap1Δ in ammonium-, urea-, and allantoin-limited populations may be due to two causes. First, the expression of GAP1 in nitrogen-limited chemostats is high, but the inability of GAP1 to transport these nitrogen-containing molecules may create an unfavorable genetic load. Alternatively, as transport is driven by the electrochemical gradient across the plasma membrane, it is possible that GAP1 exports amino acids when the gradient of amino acids between cytosol and cell exterior is steeper than that of the electrochemical gradient. Recent data have shown that amino acids are excreted under particular conditions (24) consistent with this possibility. The loss and reimport of amino acids would generate a futile cycle, with a concomitant reduction in cellular fitness. We are currently unable to distinguish between these two underlying causations, although their relative importance can be experimentally tested.
Nitrogen availability plays a unique role in determining commitment to major processes in yeast such as meiosis and sporulation and differentiation into pseudohyphae. Sufficient nitrogen levels are also required for commitment to the cell-division cycle. Although the natural history of yeast is the subject of speculation, it seems likely that in its natural habitat yeast experience extreme fluctuations in nitrogen availability and source. We speculate that the genomic architecture at the GAP1 locus has been retained through evolution to facilitate adaptation to diverse nutrient-limited environments. It is interesting to note that intrachromosomal recombination between tandem repeats is stimulated by transcription of the intervening sequence (9). This is likely to be true for the GAP1 locus, and therefore poor nitrogen environments that result in increased GAP1 expression might increase the frequency at which gap1Δ and GAP1circle alleles are generated via intrachromosomal recombination. The generation of diverse GAP1 alleles of strong and opposing phenotypic effect at a high frequency may provide a population-level benefit in fluctuating environments akin to phase switching or phenotypic switching in bacteria.
Amplification of genes encoding nutrient transporters has also been observed in yeast populations evolved under glucose (5) and sulfate (16) limitation. In both cases, the amplified transporter genes are found at their endogenous locus. The LTR-mediated generation of a circular autonomously replicating DNA element described in this study is a unique mechanism by which gene amplifications are generated and selected. Three percent of the S. cerevisiae genome consists of retrotransposable elements (25), and any genomic fragment bounded by LTRs and containing an autonomously replicating sequence (ARS) has the potential to generate a self-replicating circular element. For example, the closely linked hexose transporters HXT3, HXT6, and HXT7 are bound by the LTRs YDRWδ25 and YDRWδ26 and contain two ARSs. We would predict that an extrachromosomal circle containing these genes would confer a selective advantage under hexose-limiting conditions.
A related example in yeast is known in which intact Ty1 elements recombine to form an extrachromosomal circle with a 39-kb fragment of chromosome II (26). The chromosome II element contains the histone genes HTA2-HTB2, a centromere, and origins of replication and can be selected for in HTA1-HTB1/hta1-htb1 heterozygotes. The HTA2-HTB2 circle is formed with high frequency and is thought to be induced in the absence of HTA1 and HTB1. The site within the 7.5-kb Ty1s in which recombination occurs is not known and likely cannot be known due to the sequence identity of the Ty1 elements. By contrast, the sequence differences between YKRCδ11 and YKRCδ12 enable mapping of the intrachromosomal recombination breakpoints with unprecedented resolution.
Eight and a half percent of the human genome consists of LTR elements (27). It is likely that the human genome has the ability to form extrachromosomal self-replicating circles through homologous recombination of LTRs. Extrachromosomal self-replicating circular DNA elements are found in tumor cells, where they are known as double minutes (28). Double minutes carry amplified oncogenes or genes involved in drug resistance such as the epidermal growth factor gene (EGFR) (29) and are thought to confer proliferative potential. The discovery of autonomously replicating circular DNA that confers a selective advantage to yeast in evolution experiments is analogous to this genomic phenomenon observed in human cancer cells (17) and provides a potential system for studying genetic and chemical approaches to inhibiting their formation.
Materials and Methods
Strains and Media.
Prototrophic haploid S. cerevisiae strains CEN.PK113-7D (MATα) and FY4 (MATa) were used as ancestors for long-term chemostat selection experiments. Descendants from evolution experiments were recovered by plating cells on rich solid media. Defined media were used for all experiments as described (23, 30) with a limiting nitrogen concentration of either 6 mM (l-alanine–, ammonium-, and l-glutamine–limited cultures of CEN.PK113-7D) or 800 μM (allantoin-, urea-, l-glutamate–, and l-glutamine–limited cultures of FY4). Minimal proline + d-histidine media were prepared as described (20).
Chemostat Cultivation.
Steady-state aerobic chemostat cultures were grown at 30 °C at a dilution rate of 0.2 (CEN.PK113-7D) or 0.12 (FY4) culture volumes per hour. For a subset of cultures, we measured residual nitrogen concentration every 50 generations by ion-exchange HPLC using a Waters 474 scanning fluorescence detector to ensure that cultures were indeed limited for nitrogen throughout the experiment. Populations of an initial size of 1010 cells were propagated for at least 250 generations.
DNA Microarray Analysis.
Comparative genomics hybridization was performed using Agilent 44k and Affymetrix Yeast Tiling Array 1.0R yeast microarrays as described (16).
Genotyping.
The status of the GAP1 locus was determined using the following PCR primer sets: primer set GAP1 (5′-TGAGACGATGTTTTTCTC-3′; 5′-TTAGTGCACGGAAATATA-3′) detects a 2.1-kb product corresponding to an intact GAP1, primer set gap1Δ (5′-GTCATCACTGTTGTTGGG-3′; 5′-AGGCGGCAACAAACGCG-3′) detects the deletion of GAP1 and produces a 0.5-kb PCR product, and primer set GAP1circle (5′-ACTGCGGCTCCGCTC-3′; 5′-GACCTGTGGAAGGTCG-3′) detects the GAP1circle producing a 0.9-kb PCR product. Primer set GAP1chr (5′-GGCTGTTCAATCTCCGG-3′; 5′-ACTGCGGCTCCGCTC-3′) detects the presence of GAP1 at its chromosomal location and produces a 0.5-kb product.
Southern Blot.
Purified genomic DNA was digested with NspI, separated on a 1.5% native gel, and probed with a 32P-labeled GAP1 probe. To prepare the probe, 50 ng of purified PCR product generated with primer set GAP1 (5′-TGAGACGATGTTTTTCTC-3′; 5′-TTAGTGCACGGAAATATA-3′) was labeled radioactively by random priming with [α-32P]dCTP (PerkinElmer) using a Prime-It II Random Primer Labeling Kit (Stratagene) and purified using illustra ProbeQuant G-50 micro columns (GE Healthcare). Southern blots were scanned by PhosphorImager (Molecular Dynamics) and analyzed with conventional Kodak BioMax MS film (PerkinElmer).
Fluctuation Analysis.
GAP1 mutation rates were determined by fluctuation analysis. CEN.PK113-7D cells were inoculated into 200 μL liquid YPD at a concentration leading to 0–1 cell per sample and grown to a saturation of ∼108 cells/mL. The 14 resultant cell lines were subsequently plated on (i) solid YPD to calculate the exact number of cell divisions in each cell line and (ii) MP + d-histidine to measure the proportion of gap1 mutants. Mutation rates were calculated using the P0 method of Luria and Delbrück (31).
Competitive Growth Assays.
Competition experiments were performed by coculturing the strain of interest with the corresponding parental strain in chemostat cultures. We measured the relative abundance of the two strains by determining the fraction of d-HisR cells on plates or the proportion of GAP1circle cells using PCR as described above. For some competition experiments, we competed the unlabeled adapted strain against a fluorescently labeled ancestral strain and measured their relative abundance using a FACS Analyzer (BD Biosciences). Fitness was estimated by determining the slope of the regression of ln(evolved/ancestral) against time in generations (16).
Acknowledgments
We thank Sefa Alizadeh for technical assistance. The work was supported by Danish Research Councils Grant 09-062220/FNU, by National Institutes of Health Research Grant GM046406, and by the National Institute of General Medical Sciences Center for Quantitative Biology (GM071508).
Footnotes
The authors declare no conflict of interest.
References
- 1.Steiner CC, Weber JN, Hoekstra HE. Adaptive variation in beach mice produced by two interacting pigmentation genes. PLoS Biol. 2007;5:e219. doi: 10.1371/journal.pbio.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrick JE, Lenski RE. Genome-wide mutational diversity in an evolving population of Escherichia coli. Cold Spring Harb Symp Quant Biol. 2009;74:119–129. doi: 10.1101/sqb.2009.74.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilke CM, Adams J. Fitness effects of Ty transposition in Saccharomyces cerevisiae. Genetics. 1992;131:31–42. doi: 10.1093/genetics/131.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aminetzach YT, Macpherson JM, Petrov DA. Pesticide resistance via transposition-mediated adaptive gene truncation in Drosophila. Science. 2005;309:764–767. doi: 10.1126/science.1112699. [DOI] [PubMed] [Google Scholar]
- 5.Brown CJ, Todd KM, Rosenzweig RF. Multiple duplications of yeast hexose transport genes in response to selection in a glucose-limited environment. Mol Biol Evol. 1998;15:931–942. doi: 10.1093/oxfordjournals.molbev.a026009. [DOI] [PubMed] [Google Scholar]
- 6.Perry GH, et al. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256–1260. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang GI, Murray AW. Estimating the per-base-pair mutation rate in the yeast Saccharomyces cerevisiae. Genetics. 2008;178:67–82. doi: 10.1534/genetics.107.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian Z, et al. Yeast Ty1 retrotransposition is stimulated by a synergistic interaction between mutations in chromatin assembly factor I and histone regulatory proteins. Mol Cell Biol. 1998;18:4783–4792. doi: 10.1128/mcb.18.8.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas BJ, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 10.Dorsey M, Peterson C, Bray K, Paquin CE. Spontaneous amplification of the ADH4 gene in Saccharomyces cerevisiae. Genetics. 1992;132:943–950. doi: 10.1093/genetics/132.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hourcade D, Dressler D, Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci USA. 1973;70:2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horowitz H, Haber JE. Identification of autonomously replicating circular subtelomeric Y′ elements in Saccharomyces cerevisiae. Mol Cell Biol. 1985;5:2369–2380. doi: 10.1128/mcb.5.9.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putnam CD, Pennaneach V, Kolodner RD. Saccharomyces cerevisiae as a model system to define the chromosomal instability phenotype. Mol Cell Biol. 2005;25:7226–7238. doi: 10.1128/MCB.25.16.7226-7238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitte C, Panaud O. LTR retrotransposons and flowering plant genome size: Emergence of the increase/decrease model. Cytogenet Genome Res. 2005;110:91–107. doi: 10.1159/000084941. [DOI] [PubMed] [Google Scholar]
- 15.Jordan IK, McDonald JF. Tempo and mode of Ty element evolution in Saccharomyces cerevisiae. Genetics. 1999;151:1341–1351. doi: 10.1093/genetics/151.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gresham D, et al. The repertoire and dynamics of evolutionary adaptations to controlled nutrient-limited environments in yeast. PLoS Genet. 2008;4:e1000303. doi: 10.1371/journal.pgen.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunham MJ, et al. Characteristic genome rearrangements in experimental evolution of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2002;99:16144–16149. doi: 10.1073/pnas.242624799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winston F, Durbin KJ, Fink GR. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell. 1984;39:675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- 19.Farnet CM, Haseltine WA. Circularization of human immunodeficiency virus type 1 DNA in vitro. J Virol. 1991;65:6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regenberg B, Hansen J. GAP1, a novel selection and counter-selection marker for multiple gene disruptions in Saccharomyces cerevisiae. Yeast. 2000;16:1111–1119. doi: 10.1002/1097-0061(20000915)16:12<1111::AID-YEA611>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Jauniaux JC, Grenson M. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae. Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur J Biochem. 1990;190:39–44. doi: 10.1111/j.1432-1033.1990.tb15542.x. [DOI] [PubMed] [Google Scholar]
- 22.Stanbrough M, Magasanik B. Two transcription factors, Gln3p and Nil1p, use the same GATAAG sites to activate the expression of GAP1 of Saccharomyces cerevisiae. J Bacteriol. 1996;178:2465–2468. doi: 10.1128/jb.178.8.2465-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usaite R, Patil KR, Grotkjaer T, Nielsen J, Regenberg B. Global transcriptional and physiological responses of Saccharomyces cerevisiae to ammonium, l-alanine, or l-glutamine limitation. Appl Environ Microbiol. 2006;72:6194–6203. doi: 10.1128/AEM.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess DC, Lu W, Rabinowitz JD, Botstein D. Ammonium toxicity and potassium limitation in yeast. PLoS Biol. 2006;4:e351. doi: 10.1371/journal.pbio.0040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garfinkel DJ. Genome evolution mediated by Ty elements in Saccharomyces. Cytogenet Genome Res. 2005;110:63–69. doi: 10.1159/000084939. [DOI] [PubMed] [Google Scholar]
- 26.Libuda DE, Winston F. Amplification of histone genes by circular chromosome formation in Saccharomyces cerevisiae. Nature. 2006;443:1003–1007. doi: 10.1038/nature05205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Druker R, Whitelaw E. Retrotransposon-derived elements in the mammalian genome: A potential source of disease. J Inherit Metab Dis. 2004;27:319–330. doi: 10.1023/B:BOLI.0000031096.81518.66. [DOI] [PubMed] [Google Scholar]
- 28.Hahn PJ. Molecular biology of double-minute chromosomes. Bioessays. 1993;15:477–484. doi: 10.1002/bies.950150707. [DOI] [PubMed] [Google Scholar]
- 29.Collins VP. Gene amplification in human gliomas. Glia. 1995;15:289–296. doi: 10.1002/glia.440150309. [DOI] [PubMed] [Google Scholar]
- 30.Brauer MJ, et al. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol Biol Cell. 2008;19:352–367. doi: 10.1091/mbc.E07-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delbrück M, Luria SE. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]