Abstract
Breast cancer is one of the most frequent of human malignacies, and it is therefore fundamental to identify the underlying molecular mechanisms leading to cancer transformation. Among other causative agents in the development of breast cancers, an important role for reactive oxygen species (ROS) has emerged. However, most studies on the role of ROS in cancer have not reached specific conclusions, and many issues remain controversial. In the present study, we show that methionine sulfoxide reductase A (MsrA), which is known to protect proteins from oxidation and which acts as a ROS scavenger, is down-regulated in a number of breast cancers. Moreover, levels of MsrA correlate with advanced tumor grade. We therefore investigated the functional role of MsrA in breast cancer cells. Our data show that reduction of MsrA levels results in increased cell proliferation and extracellular matrix degradation, and consequently in a more aggressive cellular phenotype, both in vivo and in vitro. We also show that the underlying molecular mechanisms involve increased ROS levels, resulting in reduction of phosphatase and tensin homolog deleted on chromosome ten protein (PTEN), and activation of the phosphoinositide 3-kinase pathway. In addition, MsrA down-regulation results in up-regulation of VEGF, providing additional support for tumor growth in vivo.
Keywords: matrix degradation, oxidative damage
Cancer cells show increased reactive oxygen species (ROS) generation, which might promote cell proliferation and which, in many cases, might be coupled to redox adaptation promoting cell survival and drug resistance (1). Under persistent intrinsic oxidative stress, many cancer cells become well adapted to such stress and develop an enhanced, endogenous antioxidant capacity, which can make malignant cells resistant to exogenous stress (2–4). In addition, this adaptation can contribute to malignant transformation, metastasis, and resistance to anticancer drugs (5–7).
Animal studies using either gene KO or transgenic overexpression approaches have demonstrated potential roles for antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidases (GPx), and peroxiredoxin as tumor suppressors (8–10). In contrast, studies using primary cancer tissues have revealed increased activities of certain ROS-scavenging enzymes and elevated expression of antioxidant molecules (11–13). Among the several adaptative responses, even under high oxygen conditions, increased aerobic glycolysis (known as the Warburg effect) is commonly seen across a wide spectrum of human cancers (14). Enhanced glycolysis protects cells from oxidative stress and it has been suggested that the Warburg effect reflects a mechanism for escaping the restrictions to cell life span resulting from oxidative stress (15).
The mitochondria and the Nox system of NADPH oxidases are a major source of ROS production (16, 17). Several reports have demonstrated strong correlations between Nox isoenzyme overexpression, H2O2 generation, and tumorigenicity. Indeed, transfection of Nox1 into a prostate cancer cell line dramatically enhanced tumor growth (18), and Nox1 has been shown to be highly expressed in human colon and prostate cancers (19). H2O2 can alter the redox state of the cell by reacting directly with thiol amino acid residues, i.e., cysteine and/or methionine, within redox-sensitive proteins. The methionine sulfoxide reductase (Msr) system is a multigene family of the constitutively expressed proteins MsrA, MsrB1, MsrB2, MsrB3a, and MsrB3b, which are involved in regeneration of methionine from its oxidized form, Met(O). In addition to its established role in protein repair, the Msr system has been proposed to act as a ROS scavenger (20, 21) and, for this reason, is considered to be an important component of the defense mechanisms against oxidative damage. MsrA is one of the major enzymes involved in reduction of methionine sulfoxide moieties, and it is involved in the regulation of signaling pathways, such as phosphorylation. Calcium/calmodulin-dependent protein kinase II (CaMKII) is a pivotal signaling molecule activated by ROS, and its methionine oxidation is reversed by MsrA (22). MsrA has been localized to several cell organelles, including the mithocondria, nuclei, and endoplasmic reticulum (21, 23), and various studies have shown that MsrA is down-regulated in several human tumors. As an example, leukemia and lymphoma cell lines do not express MsrA mRNA, whereas normal peripheral blood leukocytes do (24). In addition, it has been shown that both MsrA mRNA and protein are decreased in hepatocellular carcinoma (25). Finally we have recently shown significantly lower MsrA expression in breast cancer MCF7 cells, compared with HEK-293 cells (26). Altogether, these reports support a posible role for MsrA in breast cancer carcinogenesis.
Here, we shown that MsrA gene expression is down-regulated in human breast cancers, and that silencing MsrA in a breast cancer cell line results in increased aggressiveness of these cells. This is due at least in part to increased ROS levels, which in turn cause extracellular matrix degradation. Finally, we show that this is linked to alterations of the PI3K pathway. Moreover, MsrA silencing affects VEGF expression, promoting accelerated xenograft growth in vivo.
Results
MsrA Expression Is Down-Regulated in Human Breast Cancer.
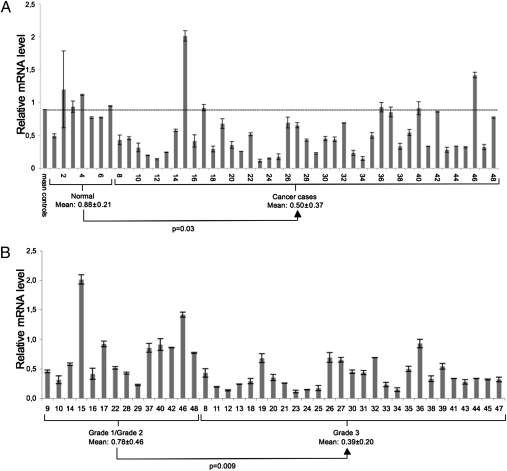
MsrA down-regualtion has been reported in only a few cancer systems (24, 25, 27). To investigate whether this can be considered a common charateristic of cancer cells, we evaluated MsrA gene expression using a real-time PCR-based tissue array of human breast tumors. Overall, 48 samples were analyzed, seven from normal mammary tissue and the remaining 41 from breast cancer tissue. MsrA expression was down-regulated in the breast cancer samples as compared with the normal tissue. As shown in Fig. 1A, in 34 of 41 breast cancer samples, MsrA expression levels were below the mean value of the MsrA transcript detected in normal tissues. The expression of MsrA mRNA significantly correlated with histological nuclear grade (Nottingham G1/G3). Cancer samples from patients with a tumor classified as G1 or G2 showed higher levels of MsrA expression, as compared with patients with a G3 tumor grade (Fig. 1B).
Fig. 1.
MsrA expression is down-regulated in a subset of human breast cancer tissues. (A) Breast cancer TissueScan real-time qPCR array containing seven normal cDNAs and 41 human breast cancer cDNAs, analyzed for MsrA expression by real-time PCR. Mean (±SD) relative MsrA expression levels from three independent plates are shown. MsrA mRNA levels are markedly down-regulated in breast cancer tissues. (B) Same data as in A were grouped according to tumor grading. Grade 3 tumors show a statistically different level of expression as compared with grades 1 and 2 tumors. Data are mean ± SD. Statistical significance was evaluated by t tests for unpaired data (two-tailed).
Knockdown of MsrA Increases H2O2 Levels and Protein Oxidation in MDA-MB231 Breast Cancer Cells.
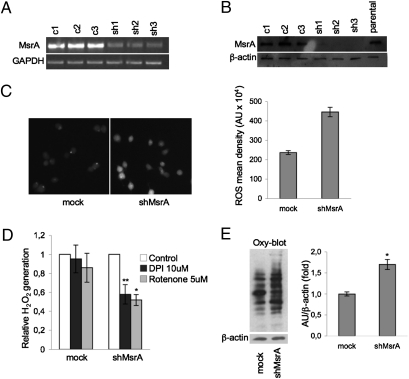
To gain further insight into the specific function of MsrA in cancer, we developed an shRNA interference approach to down-regulate MsrA expression in MDA-MB231 breast cancer cells. As shown in Fig. 2 A and B, MsrA expression was decreased by 90% in silenced MDA-MB231 cells (sh1, sh2, and sh3), as compared with parental and control transfected (c1, c2, and c3) cells. Based on these data, we selected two clones, sh1-MsrA and sh2-MsrA. Both of these clones were used in this study and produced identical results: thus, we present here the data from only the sh1-MsrA clone (shMsrA). Stable transfection of MDA-MB231 cells with an unrelated shRNA (directed against GFP) had no effects on MsrA mRNA levels (Fig. S1A).
Fig. 2.
Knockdown of MsrA increases H2O2 levels and protein oxidation in MDA-MB231 breast cancer cells. MsrA transcript (A) and protein (B) levels were determined by RT-PCR and Western blotting, respectively, in empty vector-transfected cells (c1, c2, c3) and in cells transfected with shRNA against MsrA (sh1, sh2, sh3). GAPDH and β-actin were used as loading controls for PCR and Western blotting, respectively. (C) Representative images (left) of cells stained with DCFDA ROS fluorescent probe to detect endogenous ROS levels in cells transfected with shRNA against MsrA (shMsrA) or with a control vector (mock). ShMsrA MDA-MB231 cells show increased staining, indicating increased ROS levels. Densitometric analysis of DCFDA-stained cells (right). Data are mean ± SEM. *P < 0.001. (D) Effects of DPI and rotenone on generation of ROS in MDA-MB231 cells. Cells (2 ×104) were treated with 10 μM DPI or 5 μM rotenone for 1 h, then incubated with 10 μM DCFDA for an additional 30 min and washed twice with PBS. Intracellular levels of ROS were measured using a SPECTRAmax GEMINI spectrofluorometer (Molecular Devices). Data are mean ± SD (n = 3). *P < 0.01, **P < 0.02. (E) Representative OxyBlot analysis of mock and shMsrA cells, for protein oxidation detected as total carbonyls. Densitometric analyses of protein carbonylation (right). Data are mean ± SD. *P < 0.05.
The Msr enzymes might have important roles in ROS scavenging through the “repairing” of oxidized methionine residues (20, 21). Thus, we investigated the possibility that MsrA is a regulator of ROS effects and levels in cancer cells. Indeed, in immunofluorescence studies, we observed that MsrA-deficient cells (shMsrA) had higher ROS levels compared with mock MDA-MB231 cells (Fig. 2C). These data were confirmed by flow cytometry (Fig. S1B). This increase in intracellular oxidants revealed by the ROS-sensitive fluorescent dye dichlorofluorescin-diacetate (DCFDA) is most likely due to the production of hydrogen peroxide, to which this probe is selectively sensitive. A general ROS-quenching agent N-acetyl cysteine (NAC) reduced ROS levels induced by the silencing of MsrA (Fig. S1).
In addition to mitochondria, ROS generated through the extramitochondrial NADPH oxidase system are also implicated in mitogenic signaling (28). To identify the source of ROS generation, we evaluated the effects of the Nox inhibitor diphenyliodonium (DPI) and of the mitochondrial complex I inhibitor rotenone on H2O2 levels in shMsrA and mock MDA-MB231 cells. Both DPI and rotenone inhibited ROS generation in MsrA silenced cells. These inhibitors had almost no effect on mock cells (Fig. 2D). Taken together, these data suggest the involvement of the Nox system and the mitochondria in the increased production of ROS observed in cells in which MsrA was down-regulated.
We then examined whether suppression of MsrA could be compensated for by altered expression of enzymes involved in the “classical” antioxidant defense system. However, there were no differences in the expression levels of SOD, catalase and GPx in shMsrA MDA-MB231 cells (Fig. S2). In addition, MsrB1, MsrB2, MsrB3a, and MsrB3b expression levels were not influenced by MsrA silencing (Fig. S3). These data suggest a direct involvement of MsrA in the control of cellular H2O2 levels in breast cancer cells.
MsrA KO mice are more sensitive to oxidative stress and accumulate higher levels of carbonylated proteins (29). Therefore, to investigate whether MsrA silencing leads to nonreversible posttranslational modifications, we examined the protein-carbonyl status of the shMsrA MDA-MB231 cells vs. control cells. Our data show that silencing of MsrA results in a significative increase of the cell protein carbonylation status (Fig. 2E).
MsrA Down-Regulation Increases Cell Invasion.
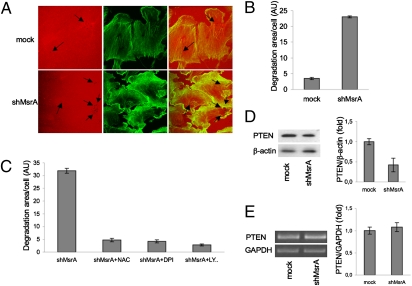
To determine whether MsrA down-regulation affects cell invasion, we investigated its effects on the degradation of the extracellular matrix (ECM), an event representative of cell invasion ability. In shMsrA MDA-MB231 cells, the ECM degradation area was sixfold greater than in mock-transfected cells (Fig. 3 A and B). Therefore the presence of MsrA appears to protect MDA-MB231 cells from acquiring a more invasive phenotype. In addition, these shMsrA MDA-MB231 cells also showed less organized actin filaments (Fig. 3A), which could again correlate with the acquisition of a more malignant phenotype, as reported previously (30). To investigate whether the increased ECM degradation was dependent on the increase in ROS levels, we performed ECM degradation assays in cells treated with the general ROS-quenching agent NAC and with the NADPH oxidase inhibitor DPI. Both compounds reduced the degradative activity of shMsrA-treated cells by more than sixfold, as compared with vehicle-treated cells (Fig. 3C).
Fig. 3.
MsrA down-regulation increases cell invasion ability. (A) Representative double staining of control (mock) and MsrA-deficient (shMsrA) cells grown on crosslinked-rhodamine conjugated gelatin (red) overnight and then fixed and labeled with Alexa Fluor 488-phalloidin (green) for F-actin staining. Dark holes on gelatin matrix (black arrows) represent areas of degradation. (B) Quantification of degradation areas in at least 100 cells using LSM510-3.2 software (Zeiss); data are mean of three independent experiments. (C) MsrA-deficient (shMsrA) cells were plated onto crosslinked rhodamine-conjugated gelatin for 24 h in the presence of BB94, a broad-spectrum metalloprotease inhibitor, and pretreated with vehicle alone (untreated) or with NAC (10 mM), DPI (10 μM), or LY294002 (10 μM, added during the last 30 min). Following BB94 washout, cells were treated overnight with vehicle alone (untreated) or with NAC, DPI or LY294002 at the same concentrations as above. Samples were then fixed and processed for F-actin staining. Degradation area per cell was quantified in at least 100 cells using LSM510-3.2 software (Zeiss); data are mean of three independent experiments. (D) Representative Western blot of PTEN protein levels. β-Actin was used as loading control. Data are mean ± SD (n = 3). P < 0.02. (Right) Quantification using densitometric analysis. (E) Representative RT-PCR analysis of PTEN transcript levels. Data are mean ± SD (n = 3). (Right) Quantification using PCR densitometric analysis.
Phosphatase and tensin homolog deleted on chromosome ten protein (PTEN) is considered to be the main negative regulator of the PI3K pathway, and it is frequently inactivated in human cancers; previous studies have indicated that ROS can affect phosphatidylinositol 3,4,5-trisphosphate (PtdInsP3) levels in cells by oxidizing PTEN (31, 32). Thus, we asked whether the observed increase in ECM degradation depends on this signaling pathway. Treatment of cells with the PI3K inhibitor LY294002 results in a greater than eightfold decrease of the degradation activity of shMsrA MDA-MB231 cells (Fig. 3C). We next examined PTEN protein levels in shMsrA MDA-MB231 cells and control cells. As shown in Fig. 3D, silencing of MsrA significantly decreases endogenous PTEN protein levels, whereas no effects are seen on PTEN mRNA levels (Fig. 3E), suggesting that MsrA regulates PTEN at the posttranscriptional level.
Silencing MsrA Enhances 3D Growth of MDA-MB231 Cells.
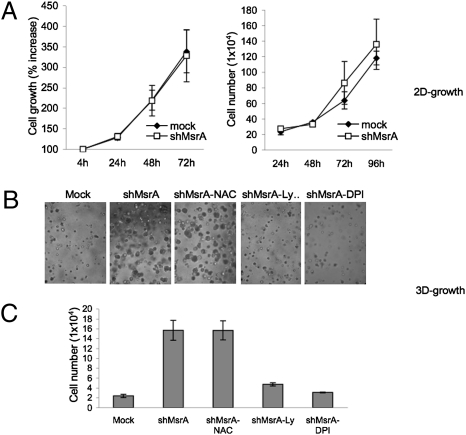
The silencing the the MsrA gene in “normal” cells, such as melanocytes, has been shown to result in reduced cell viability (33). To investigate the functional effects of the down-regulation of MsrA expression in breast cancer cells, we evaluated cell proliferation both by methyl thiazolyl tetrazolium (MTT) assay and by direct cell counting. The number of shMsrA MDA-MB231 cells was similar to the number of mock MDA-MB231 cells, and a similar result was seen for cell viability using the MTT assay (Fig. 4A), suggesting that MsrA down-regulation does not affect breast cancer cell viability or growth.
Fig. 4.
Silencing of MsrA enhances 3D growth of MDA-MB231 cells. (A) Effects of MsrA suppression on 2D growth of MDA-MB231 cells. Mock (◆) and shMsrA (□) cells were plated in 96-well dishes (1 ×103 per well) and cultured in complete medium. Cell proliferation was determined after 4, 24, 48, and 72 h, using MTT (left); cell counts were evaluated after 24, 48, 72, and 96 h (right). Data are mean of three independent experiments ± SD. (B) Representative phase-contrast microscopy images of MDA-MB231 mock and shMsrA cells in Matrigel (BD Biosciences) seeded at 2 ×104 cells per well in 24-well plates. Cells were grown for 1 wk in the presence of vehicle, 5 mM NAC, 10 μM LY294002, and 1 μM DPI. (C) Total cell numbers determined after Dispase digestion. Data are mean ± SD of three independent experiments.
Many studies have indicated that basal membranes can serve as barriers to prevent tumor cell entry into adjacent tissues (34, 35). As reported above, the growth of shMsrA MDA-MB231 cells was similar to mock MDA-MB231 cells on plastic surfaces. However, to determine whether growth differences might be observed in a more complex microenvironment, more similar to basal membranes, we evaluated the growth of shMsrA MDA-MB231 cells cultured in Matrigel (BD Biosciences). Under these experimental conditions, shMsrA MDA-MB231 cells produced much larger colonies than control cells (Fig. 4B); moreover, direct cell counting showed higher numbers of cells for shMsrA MDA-MB231 cells than for mock cells (Fig. 4C). Interestingly, DPI treatment significantly reduced the size and numbers of colonies grown on Matrigel (Fig. 4B), although, surprisingly, NAC had no effect on the size of the colonies on Matrigel. In line with these data, it has been recently reported that ROS generation, rather than ROS accumulation, is essential for the aggressive cell phenotype of prostate cancer cells (36). Indeed, in shMsrA MDA-MB231 breast cancer cells, inhibition of ROS generation (DPI) blocked cell growth, whereas the ROS accumulation inhibitor NAC did not (Fig. 4B). The same treatments had no effect on the growth of control cells (Fig. S4). These data suggest that MsrA down-regulation can facilitate the growth of breast cancer cells at tissue boundaries, accelerating the entry of tumor cells into adjacent tissues via a growth-related mechanism that is dependent on Nox generation of H2O2.
To determine whether the signaling mechanisms involved in increased cell invasion capacity is also implicated in the enhanced growth of shMsrA MDA-MB231 cells, we performed 3D growth experiments in the presence of the PI3K inhibitor LY294002. LY294002 markedly blocked shMsrA MDA-MB231 colony growth on Matrigel (Fig. 4B and Fig. S4), thus suggesting that PI3K-dependent activation is critically involved in promoting growth of shMsrA MDA-MB231 cells in this 3D culture system.
MsrA Silencing Induces VEGF Production in MDA-MB231 Cells and Promotes Growth of Tumor Xenografts in Nude Mice.
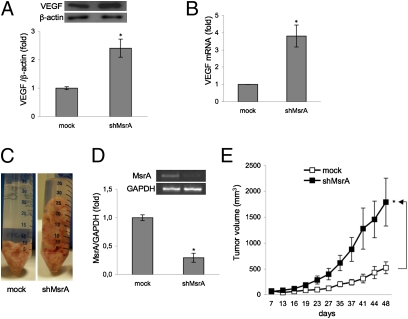
In human ovarian cancer cells, ROS hyperproduction was shown to positively regulate VEGF trancription and expression, suggesting that endogenous ROS levels are very important for inducing angiogenesis and consequently tumor development (37). We therefor investigated whether modulation of MsrA affects VEGF expression. Our results show that down-regulation of MsrA results in increased VEGF protein (Fig.5A) and mRNA levels (Fig. 5B), and thus potentially also angiogenesis and tumor growth in vivo.
Fig. 5.
MsrA silencing induces VEGF production in MDA MB-231 and promotes growth of tumor xenografts in nude mice. (A) Representative VEGF protein expression levels in mock and shMsrA MDA-MB231 cells, detected by Western blotting. (Lower) Densitometric analysis. (B) VEGF mRNA levels evaluated by real-time PCR. Data are mean ± SD (n = 3). *P < 0.01. (C) Representative tumors recovered from athymic nude mice injected s.c. into right and left flanks with either mock cells or shMsrA MDA-MB231 cells (8 ×106) (n = 10 per treatment group). (D) MsrA transcript levels were determined by RT-PCR in tumors recovered from mice injected with either mock or shMsrA MDA-MB231 cells. (E) Tumor growth in mice over time (as days after implantation) evaluated using a caliper in live mice. Data are mean ± SEM. *P < 0.05.
Finally, to determine whether MsrA silencing has similar effects in vivo, we used a xenograft tumor model in athymic mice. Subcutaneous injections in CD-1 nude mice were administered with mock-transfected and shMsrA MDA-MB231 cells, to evaluate the formation of solid tumor xenografts. Indeed, the mice had to be killed 48 d after the injections due to the rapid growth of the shMsrA MDA-MB231 cell tumors, which were dramatically larger than those formed by mock-transfected cells (Fig. 5 C and E). The effective silencing of MsrA gene was verified in tumors recovered from mice injected with shMsrA MDA-MB231 cells (Fig. 5D). These data demonstrate that down-regulation of MsrA dramatically enhances the growth of tumors in vivo.
Discussion
In the present study, we provide evidence supporting the idea that loss of MsrA is a favorable condition for tumor cell growth. Although it has been reported that the knockdown of MsrA in melanocytes results in reduced cell viability (33), we did not detect growth variations between MsrA-silenced and control breast cancer cells. It is important to specify that this finding is only true when cells were grown as 2D monolayers. Culturing cells in 3D provides another dimension for external mechanical input and for cell adhesion, which affects integrin ligation, cell contraction, and associated intracellular signaling (38). In agreement with this, MsrA-silenced breast cancer cells showed dramatic increases in growth under 3D conditions.
Normal and cancer cells have different oxidative metabolism systems. Cancer cells have a higher rate of glycolysis than normal cells (i.e., the Warburg effect). This leads to hyperactivation of the pentose phosphate pathway, generating high levels of NADPH (39). NADPH is crucial for glutathione reductase activity as well as for other enzymes involved in oxidative defense mechanisms. On the other hand, NADPH is a substrate for ROS generation by the Nox system. These two different pathways need to be carefully balanced in cancers because ROS hyperproduction will kill the cancer cells (40), whereas a modest ROS increase represents a favorable condition for tumor growth. Indeed, here, we have demonstrated that MsrA silencing promotes a modest increase in ROS levels (doubled), thus favoring the Nox-dependent 3D growth of MDA-MB231 cells.
One of the most important causes of poor prognosis in cancer patients is tumor cell invasion of distal organs, with the formation of metastases. The complex process of metastasization requires the integration of several events, including the dissociation of cells from the primary tumor in association with local remodeling/degradation of the ECM (41). Invasive cancer cells cultured on physiological substrates can degrade the underlying matrix through specialized membrane protrusions that are rich in actin filaments and are known as invadopodia. These invadopodia are thought to be necessary for carcinoma cell invasion, through the local remodeling of the ECM structures in the path of invading cells (42, 43). Interestingly, it has recently been shown that ROS generated by the Nox system are necessary for invadopodia formation and ECM degradation (44). In agreement with this, MsrA-silenced breast cancer cells showed a more invasive ROS-dependent phenotype. We also provide evidence that the increased 3D growth of shMsrA MDA-MB231 cells and their greater invasive behavior dependend on PI3K activity; indeed, a specific inhibitor of this pathway reduced cell proliferation and invasion capacity. The lipid products of PI3K, such as PtdInsP3, can provide anchors for assembling signaling proteins at specific locations in the membrane in response to cell stimulation. These signaling proteins coordinate complex events that can lead to changes in cell growth, movement, and survival (45). We have shown higher ROS levels in shMsrA MDA-MB231 cells, as compared with control cells, and thus MsrA down-regulation in cancer could potentiate the H2O2 signaling pathway. Recently, the role of inherent oxidative stress in prostate cancer progression has been characterized in vitro, and it was demonstrated to be required for a more aggressive phenotype (36). Here, we show that both of the major H2O2 producing pathways, as the mitochondria and the Nox system, are involved in ROS hyperproduction in shMsrA MDA-MB231 cells. H2O2 generated by Nox at the membranes might activate the survival pathway, contributing to cancer progression. Mouse MsrA is N-myristoylated in vivo (46), and myristoylated proteins are often targeted to the plasma membrane. The decrease in MsrA levels in the membrane microenvironment might increase the levels of methionine sulfoxide-containing proteins, thus altering the local H2O2 quencing capacity of this enzyme.
The tumor suppressor PTEN is a plasma-membrane lipid phosphatase that antagonizes the PI3K signaling pathway (47, 48). In cancers, the catalytic activity of PTEN is modulated by ROS (31, 32, 49), and PtdInsP3 accumulation occurs at the plasma membrane in response to ROS (32, 50). It is thus reasonable to imagine a regulatory role for MsrA in the modulation of PTEN function in cancer. MsrA down-regulation might indirectly inhibit PTEN activity through a local enhancement of H2O2 levels and/or directly by influencing PTEN methionine oxidation status. Here, we provide evidence that MsrA silencing can modulate PTEN protein levels at the posttranscriptional level, probably by protecting PTEN from degradation. PTEN contains nine methionine residues, and future studies will be required to determine whether there is a direct relationship between MsrA and PTEN methionine oxidation and/or reduction.
PTEN oxidation has been shown to enhance PI3K signaling, leading to increased expression of the key regulator of angiogenesis, VEGF (51). In line with previous studies, we found that MsrA silencing in breast cancer cells leads to a dramatic increase in both the VEGF mRNA and protein. To determine the effects of this inhibition of MsrA expression on the tumorigenic phenotype of human breast cancer cells, and especially on its role in controlling tumor growth, we investigated tumor xenograft growth in nude mice. Tumors derived from shMsrA MDA-MB231 cells were larger than those derived from control cells, confirming in vivo the increased growth detected under our 3D in vitro conditions. Thus, our data suggest that the MsrA down-regulation observed in primary tumors is essential for acceleration of tumor growth (24, 25).
In conclusion, we show that MsrA is down-regulated in human breast cancers and that its down-regulation results in a clear growth advantage for these cells both in vitro and in vivo, with a more aggressive cellular phenotype seen. Moreover, we show that loss of MsrA exerts its effects through a ROS-dependent activation of the PI3K pathway, as well as increased production of VEGF. Interestingly, we demonstrate that lower MsrA levels correlate with higher tumor grade (G1/G2 vs.. G3). These findings suggest that MsrA has antitumor effects, thus suggesting a promising strategy for the treatment of breast cancer.
Materials and Methods
Quantitative Real-Time PCR (Disease Tissue qPCR Arrays).
To evaluate MsrA expression levels, we used a real-time PCR-based TissueScan Breast Tissue qPCR Array, containing seven normal cDNAs and 41 human breast cancer cDNAs, normalized using the house-keeping gene β-actin (BCRT101, OriGeneTechnologies). Real-time PCR was performed using a TaqMan Universal PCR Master Mix (Applied Biosystems), according to the manufacturer's instructions. The human-specific probe set for MsrA was obtained from ABI (HS00737166-m1). VEGF real-time PCR details are reported in SI Materials and Methods.
Silencing of MsrA in MDA-MB231 Cells.
MDA-MB231 human breast cancer cells (obtained from ATCC) were maintained in a humidified atmosphere containing 5% CO2 at 37 °C, in DMEM containing high glucose (4.5 g/L, or 25 mM) supplemented with 10% FBS, 50 units/mL penicillin, and 50 mg/mL streptomycin.
Cells were transfected using Lipofectamine 2000 (Invitrogen) with the pRS vector (Origene # TR20003), pRS-shGFP (Origene # TR30003, encoding noneffective 29-mer shGFP cassette as a negative control), or with specific MsrA-directed 29-mer oligos (Origene; MsrA-shRNA; Table S1), according to the manufacturer's instructions. Stable clone selection procedures are reported in SI Materials and Methods.
Western Blotting.
Western blotting details and antibodies used in this study are reported in SI Materials and Methods.
Determination of Cellular ROS.
The ROS-fluorescent probe DCFDA (2,7-dichlorofluorescein diacetate, 287810; Calbiochem) was used to detect endogenous ROS levels. The cells were plated in 24-well plates and incubated overnight at 37 °C. The cells were then washed twice with PBS and incubated with 10 μM DCFDA in PBS for 30 min at 37 °C in the dark. Imaging was performed using an Inverted Nikon Eclipse TE2000-U wide-field fluorescence microscope.
Changes in ROS production in stably transfected cells were measured using DCFDA. Cells (2 ×104) were cultured in 96-well plates at 37 °C overnight and then treated with 10 μM DPI or 5 μM rotenone for 1 h. The cells were incubated with 10 μM DCFDA for 30 min at 37 °C. ROS levels were measured using a SPECTRAmax GEMINI spectrofluorometer (Molecular Devices, excitation wavelength, 490 nm; emission wavelength, 535 nm).
Protein Carbonylation Detection.
Protein oxidation was revealed using OxyBlot Protein Oxidation Detection kits (Chemicon International). Briefly, 2,4-dinitrophenylhydrazine was added to whole-cell extracts to derivatize the carbonyl groups in the protein side chains to 2,4-dinitrophenylhydrazone (DNP), which was then revealed by Western blotting with anti-DNP antibodies.
ECM-Degradation Assay.
Details of the fluorophore-conjugated gelatin preparation and the quantification of ECM degradation are reported in SI Materials and Methods.
2D Cell-Proliferation Assay and Cell Growth.
The proliferation of cells was compared using CellTiter 96 AQueous One (Promega). Details are provided in SI Materials and Methods.
3D Cell Culture.
To study cell behavior in three dimensions, the cells were embedded in Growth Factor Reduced Matrigel (BD Biosciences), according to the manufacturer's recommendations. Details are given in SI Materials and Methods.
Nude Mice Xenograft Analysis.
All procedures involving nude mice were conducted in compliance with institutional guidelines and with national (D.L. No. 116, G.U., Suppl. 40, February 18, 1992; Circolare No. 8, G.U., July 1994) and international (U.K.CCCR Guidelines for the Welfare of Animals in Experimental Neoplasia; EEC Council Directive 86/609, OJ L 358. 1, December 12, 1987) laws and policies.
Here, 8 × 106 cells resuspended in 200 μL PBS were injected s.c. into the flanks of 6- to 8-wk-old CD-1 athymic nude mice (Charles River Laboratories). Tumor growth was monitored using calipers. Tumor volume was determined using the following equation: volume = (width2 × length)/2. The tumors were recovered and photographed following necropsy.
Supplementary Material
Acknowledgments
We thank Dr. Monica Franciosi for help with the statistical analysis, and Professor Mauro Piantelli for helpful suggestions. This study was supported in part by grants from Fondi Ricerca Scientifica di Ateneo (ex 60%) (to B.F and C.D.I.), Associazione Italiana per la Ricerca sul Cancro, and Ministero dell'Istruzione Università e Ricerca (to V.D.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010171107/-/DCSupplemental.
References
- 1.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 2.Pervaiz S, Clement MV. Tumor intracellular redox status and drug resistance—serendipity or a causal relationship? Curr Pharm Des. 2004;10:1969–1977. doi: 10.2174/1381612043384411. [DOI] [PubMed] [Google Scholar]
- 3.Tiligada E. Chemotherapy: Induction of stress responses. Endocr Relat Cancer. 2006;13(Suppl 1):S115–S124. doi: 10.1677/erc.1.01272. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan R, Graham CH. Chemosensitization of cancer by nitric oxide. Curr Pharm Des. 2008;14:1113–1123. doi: 10.2174/138161208784246225. [DOI] [PubMed] [Google Scholar]
- 5.Schneider BL, Kulesz-Martin M. Destructive cycles: The role of genomic instability and adaptation in carcinogenesis. Carcinogenesis. 2004;25:2033–2044. doi: 10.1093/carcin/bgh204. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Sánchez G, Giuliani A. Cellular redox status regulates hypoxia inducible factor-1 activity. Role in tumour development. J Exp Clin Cancer Res. 2007;26:39–50. [PubMed] [Google Scholar]
- 7.Chen EI, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67:1472–1486. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- 8.Elchuri S, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 9.Egler RA, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 10.Lu YP, et al. Enhanced skin carcinogenesis in transgenic mice with high expression of glutathione peroxidase or both glutathione peroxidase and superoxide dismutase. Cancer Res. 1997;57:1468–1474. [PubMed] [Google Scholar]
- 11.Ray G, et al. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–170. doi: 10.1023/a:1006357330486. [DOI] [PubMed] [Google Scholar]
- 12.Skrzydlewska E, Stankiewicz A, Sulkowska M, Sulkowski S, Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health A. 2001;64:213–222. doi: 10.1080/15287390152543690. [DOI] [PubMed] [Google Scholar]
- 13.Oltra AM, Carbonell F, Tormos C, Iradi A, Sáez GT. Antioxidant enzyme activities and the production of MDA and 8-oxo-dG in chronic lymphocytic leukemia. Free Radic Biol Med. 2001;30:1286–1292. doi: 10.1016/s0891-5849(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 14.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 15.Kondoh H, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–185. [PubMed] [Google Scholar]
- 16.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 17.Arnold RS, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci USA. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbiser JL, et al. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim SD, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XH, Weissbach H. Origin and evolution of the protein-repairing enzymes methionine sulphoxide reductases. Biol Rev Camb Philos Soc. 2008;83:249–257. doi: 10.1111/j.1469-185X.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee BC, Dikiy A, Kim HY, Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790:1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson JR, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HY, Gladyshev VN. Alternative first exon splicing regulates subcellular distribution of methionine sulfoxide reductases. BMC Mol Biol. 2006;7:11. doi: 10.1186/1471-2199-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuschel L, et al. Molecular cloning and functional expression of a human peptide methionine sulfoxide reductase (hMsrA) FEBS Lett. 1999;456:17–21. doi: 10.1016/s0014-5793(99)00917-5. [DOI] [PubMed] [Google Scholar]
- 25.Lei KF, et al. Identification of MSRA gene on chromosome 8p as a candidate metastasis suppressor for human hepatitis B virus-positive hepatocellular carcinoma. BMC Cancer. 2007;7:172. doi: 10.1186/1471-2407-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Luca A, Sacchetta P, Di Ilio C, Favaloro B. Identification and analysis of the promoter region of the human methionine sulphoxide reductase A gene. Biochem J. 2006;393:321–329. doi: 10.1042/BJ20050973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, et al. HTPAP gene on chromosome 8p is a candidate metastasis suppressor for human hepatocellular carcinoma. Oncogene. 2006;25:1832–1840. doi: 10.1038/sj.onc.1209191. [DOI] [PubMed] [Google Scholar]
- 28.Brar SS, et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 29.Moskovitz J, et al. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98:12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman E, Verderame M, Winawer S, Pollack R. Actin cytoskeletal organization loss in the benign-to-malignant tumor transition in cultured human colonic epithelial cells. Cancer Res. 1984;44:3040–3050. [PubMed] [Google Scholar]
- 31.Lee SR, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 32.Leslie NR, et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Z, et al. Decreased methionine sulphoxide reductase A expression renders melanocytes more sensitive to oxidative stress: A possible cause for melanocyte loss in vitiligo. Br J Dermatol. 2009;161:504–509. doi: 10.1111/j.1365-2133.2009.09288.x. [DOI] [PubMed] [Google Scholar]
- 34.Durko M, Navab R, Shibata HR, Brodt P. Suppression of basement membrane type IV collagen degradation and cell invasion in human melanoma cells expressing an antisense RNA for MMP-1. Biochim Biophys Acta. 1997;1356:271–280. doi: 10.1016/s0167-4889(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 35.Yamamura K, Kibbey MC, Jun SH, Kleinman HK. Effect of Matrigel and laminin peptide YIGSR on tumor growth and metastasis. Semin Cancer Biol. 1993;4:259–265. [PubMed] [Google Scholar]
- 36.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 37.Xia C, et al. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 38.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein H, et al. Patchy field defects of apoptosis resistance and dedifferentiation in flat mucosa of colon resections from colon cancer patients. Ann Surg Oncol. 2002;9:505–517. doi: 10.1007/BF02557276. [DOI] [PubMed] [Google Scholar]
- 40.Marchetti M, et al. Sulindac enhances the killing of cancer cells exposed to oxidative stress. PLoS ONE. 2009;4(6):e5804. doi: 10.1371/journal.pone.0005804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 42.Buccione R, Caldieri G, Ayala I. Invadopodia: Specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–149. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 43.Stylli SS, Kaye AH, Lock P. Invadopodia: At the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–737. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Diaz B, et al. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2(88):ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 46.Kim G, Cole NB, Lim JC, Zhao H, Levine RL. Dual sites of protein initiation control the localization and myristoylation of methionine sulfoxide reductase A. J Biol Chem. 2010;285:18085–18094. doi: 10.1074/jbc.M110.119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 48.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 49.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Downes CP, et al. Acute regulation of the tumour suppressor phosphatase, PTEN, by anionic lipids and reactive oxygen species. Biochem Soc Trans. 2004;32:338–342. doi: 10.1042/bst0320338. [DOI] [PubMed] [Google Scholar]
- 51.Connor KM, et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.