Abstract
It is well known that cocaine blocks the dopamine transporter. This mechanism should lead to a general increase in dopaminergic neurotransmission, and yet dopamine D1 receptors (D1Rs) play a more significant role in the behavioral effects of cocaine than the other dopamine receptor subtypes. Cocaine also binds to σ-1 receptors, the physiological role of which is largely unknown. In the present study, D1R and σ1R were found to heteromerize in transfected cells, where cocaine robustly potentiated D1R-mediated adenylyl cyclase activation, induced MAPK activation per se and counteracted MAPK activation induced by D1R stimulation in a dopamine transporter-independent and σ1R-dependent manner. Some of these effects were also demonstrated in murine striatal slices and were absent in σ1R KO mice, providing evidence for the existence of σ1R-D1R heteromers in the brain. Therefore, these results provide a molecular explanation for which D1R plays a more significant role in the behavioral effects of cocaine, through σ1R-D1R heteromerization, and provide a unique perspective toward understanding the molecular basis of cocaine addiction.
Keywords: receptor heteromer, drug addiction
A key molecular mechanism contributing to the development of addiction by drugs of abuse consist of the increase of the extracellular levels of dopamine in the striatum, particularly in its ventral portion, the nucleus accumbens (1, 2). Cocaine causes a rapid and strong increase in striatal extracellular dopamine by its ability to bind with high affinity to the dopamine transporter (DAT) and to inhibit its function (3–5). In the striatum, dopamine signaling is mediated mainly by dopamine D1 and D2 receptors (D1Rs and D2Rs, respectively), which are mostly segregated in two phenotypically different subtypes of GABAergic medium-sized spiny neurons (MSNs) (6). Activation of D1Rs is an absolute requirement for the induction of many of the cellular and behavioral responses to cocaine, as deduced from studies performed in D1R KO mice and from experiments with transgenic mice in which D1R- or D2R-expressing MSNs are visualized by the expression of fluorescent proteins (7–11).
The σ-1 receptor, originally proposed as a subtype of opioid receptors, is now considered to be a nonopioid receptor with two transmembrane domains, one extracellular loop and cytosolic N and C termini (12). The σ1R is highly expressed in the brain, including the striatum, and its association with neurons is well established (12, 13). However, its biological function and even its main endogenous neurotransmitter remain enigmatic (12). Cocaine interacts with σ1Rs at pharmacologically relevant concentrations (12, 14). In fact, reducing brain σ1R levels with antisense oligonucleotides attenuates the convulsive and locomotor stimulant actions of cocaine (15, 16), and σ1R antagonists mitigate the actions of cocaine in animal models (12, 14). A recent study showed that σ1R agonists not only potentiate the reinforcing effects of cocaine, but they may be self-administered (17). In the current study, we explored the existence of molecular and functional interactions between σ1R and D1R, which could underlie these pharmacological interactions.
Using bioluminescence resonance energy transfer-based techniques, we report a molecular interaction in living cells between σ1R and D1R. Cocaine was able to bind to a receptor heteromer constituted by at least one σ1R and two D1R units and promoted structural changes in the heteromer that led to significant modifications in D1R function. Cocaine effects on D1R function did not occur in cells transfected with σ1R siRNA or in striatal slices of σ1R KO mice. Altogether, the findings indicate that σ1R-D1R heteromer-mediated alterations of dopaminergic neurotransmission constitutes a previously uncharacterized mechanism of cocaine action.
Results
Heteromerization of σ1R and D1R.
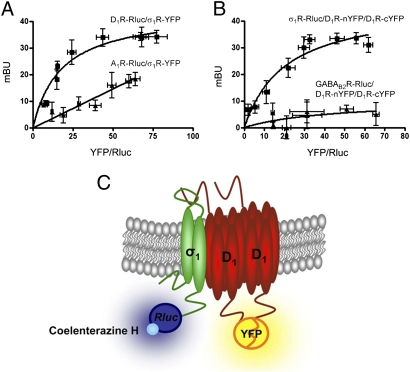
We explored the possibility that σ1R might interact directly with D1R. BRET measurements were performed in HEK-293T cells expressing a constant amount of D1R fused to Renilla Luciferase (Rluc) and increasing amounts of σ1R fused to yellow fluorescence protein (YFP). A positive and saturable BRET signal was obtained (BRETmax, 44 ± 4; BRET50, 18 ± 3; Fig. 1A). The pair constituted by the adenosine A1 receptor fused to Rluc, and the σ1R-YFP was used as a negative control. As shown in Fig. 1A, the negative control gave a linear nonspecific BRET signal, thus confirming the specificity of the interaction between D1R–Rluc and σ1R–YFP. Because one of the limitations of BRET is that it cannot distinguish between two or three interacting proteins, and because homomerization seems to be a requirement for the normal membrane expression of D1R (18), we investigated the possible formation of receptor heteromers constituted by σ1R and D1R homomers by combining BRET with bimolecular fluorescence complementation (BiFC, Fig.1C) (19). Cells were cotransfected with cDNAs for σ1R–Rluc, D1R–nYFP and D1R–cYFP, and BRET between σ1R-Rluc receptor as donor and reconstituted D1R–nYFP–D1R–cYFP homomer as acceptor was evaluated. σ1R–D1R–D1R heterotrimerization could be demonstrated by a positive and saturable BRET signal (BRETmax, 46 ± 6; BRET50, 21 ± 5; Fig. 1B). Cells expressing σ1R, D1R–cYFP and nYFP or σ1R, D1R–nYFP and cYFP did not provide any significant fluorescent signal or positive BRET. An additional negative control was performed using GABAB2 receptor fused to Rluc, which did not interact with D1R homodimers (Fig. 1B). Collectively, these results indicate that σ1R–D1R heteromers occur in cells coexpressing both receptors.
Fig. 1.
Heteromerization of D1R and σ1R in living cells. (A) BRET saturation experiments performed with HEK-293T cells transfected with D1R–Rluc cDNA (0.6 μg; ■) or A1R–Rluc cDNA as negative control (0.4 μg; ▲) and increasing amounts of σ1R-YFP cDNA (0.2–2 μg cDNA). (B) BRET saturation curve was obtained using HEK-293T cells cotransfected with σ1R-Rluc cDNA (0.4 μg, ■) or GABAB2R-Rluc cDNA as negative control (0.5 μg; ▲) and increasing equal amounts of D1R–nYFP and D1R–cYFP cDNAs (0.5–4 μg cDNA). BRET data are expressed as means ± SD of five to six different experiments grouped as a function of the amount of BRET acceptor. (C) Schematic representation of BiFC. A receptor-Rluc acts as BRET donor and, as BRET acceptor, one receptor is fused to an YFP N-terminal fragment (nYFP) and another receptor is fused to the remaining YFP C-terminal fragment (cYFP). Upon coexpression, fluorescence indicates reconstitution of YFP from both fragments and therefore a close receptor–receptor interaction.
Cocaine Induces Modifications of Subcellular Distribution of σ1R.
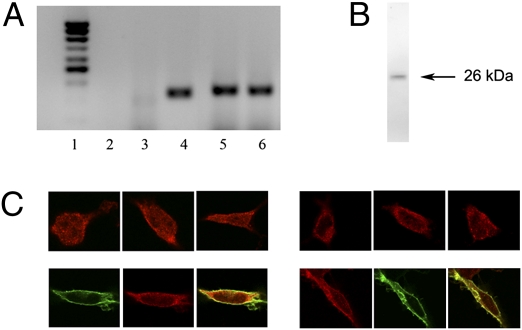
It is known that the majority of σ1R are found in the endoplasmic reticulum membrane (12). The possibility that cocaine binding to the σ1R may alter the cell surface levels of putative σ1R-D1R heteromers was therefore explored. HEK-293T cells were used in the assays, because they constitutively express σ1R, but not DAT (Fig. 2 A and B). By means of immunofluorescence a punctate σ1R staining in naïve HEK cells was detected, which is the expected pattern for an endoplasmic reticulum-associated protein (Fig. 2C, Left, top images). Expression of D1R induced in HEK-293T cells an increase in the localization of σ1R at the plasma membrane (Fig. 2C, Left, bottom images), suggesting that heteromerization with D1R facilitates translocation of σ1R to the plasma membrane. Cocaine (150 μM; 30 min) produced an increase of σ1R expression at the plasma membrane in nontransfected cells (Fig. 2C, Right, top images) and an increase in the colocalization of σ1Rs and D1Rs in transfected cells (Fig. 2C, Right, bottom images), suggesting that cocaine induces an increase in the amount of σ1R-D1R heteromers at the plasma membrane.
Fig. 2.
Expression and subcellular distribution of σ1R. (A) RT-PCR was performed using total RNA from HEK-293T cells (lanes 2, 3, 5, and 6) or RNA from human striatum as DAT positive control (lane 4), and primers specific for the human σ1R gene (lane 5), for the human DAT gene (lanes 3 and 4), or for human GADPH (lane 6). RNA from cells without primers (lane 2) was included as negative control. Molecular mass markers are shown in lane 1. (B) HEK cell membranes were analyzed by SDS/PAGE and immunoblotted with the anti-σ1R antibody. (C) Confocal microscopy images of HEK-293T cells transfected (Lower) or not transfected (Upper) with D1R–YFP cDNA, treated (right images) or not treated (left images) with 150 μM cocaine for 30 min. The σ1R (red) and D1R (green) were identified by immunocytochemistry. Colocalization is shown in yellow.
Cocaine Induces Modifications of Quaternary Structure of D1R Homomers in σ1R-D1R Heteromer.
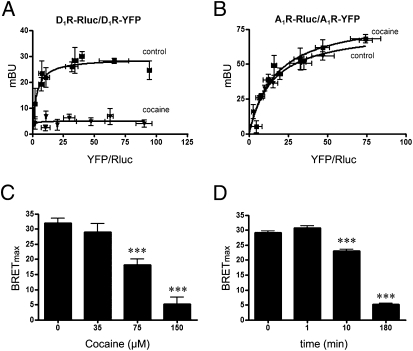
The observed changes in the plasma membrane expression of σ1R-D1R heteromers in the presence of cocaine suggested that cocaine binding might be altering the interaction between D1Rs and σ1Rs. Such a change should be detectable using an energy transfer-based approach. In HEK-293T cells expressing D1R–Rluc and D1R–YFP, the BRET saturation curve corresponding to D1R–Rluc–D1R–YFP pair was drastically reduced in the presence of cocaine (Fig. 3A). This effect was specific for the D1R, because it did not occur for the A1R-Rluc and A1R-YFP pair (Fig. 3B) and was dose dependent (Fig. 3C) and time dependent (Fig. 3d). Although the BRET signal for the D1R–Rluc–D1R–YFP pair was negligible at 180 min of cocaine treatment, there was no real disruption of D1R homomerization, because cocaine did not modify the amount of fluorescence in HEK 293 cells expressing D1R–cYFP–D1R–nYFP dimers. These results strongly suggest that cocaine binding to σ1R alters the quaternary structure of the σ1R–D1R–D1R heteromer, resulting from separation of the C-termini of the D1R protomers fused to Rluc and YFP. The participation of σ1R on the cocaine-mediated alteration of the quaternary structure of D1R was demonstrated in experiments performed in cells the σ1R expression of which was knocked down using an RNAi approach. By RNA interference (RNAi), using a specific small interfering RNA (siRNA), a robust silencing of σ1R expression was obtained without significantly altering the expression of D1R (Figs. 4 A and B). The treatment with the specific siRNA completely abolished the effect of cocaine on the BRET saturation curve obtained with D1R–Rluc and D1R–YFP (Fig. 4C). Finally, the selective σ1R agonist PRE084 also modified the BRET saturation curve corresponding to D1R–Rluc–D1R–YFP pair (200 nM; 10 min) (Fig. S1).
Fig. 3.
Effects of cocaine on D1R homomers. BRET was measured in HEK-293T cells cotransfected with D1R–Rluc cDNA (0.6 μg) and increasing amounts of D1R–YFP cDNA (A) or A1R-Rluc cDNA (0.4 μg) and increasing amounts of A1R-YFP cDNA (B), treated (▼) or not treated (■) with 150 μM cocaine for 180 min. BRET data are expressed as means ± SD of four to six different experiments grouped as a function of the amount of BRET acceptor. (C) Cells were treated for 180 min with the indicated concentrations of cocaine before the determination of BRET. (D) Cells were treated with 150 μM cocaine for the indicated times before the determination of BRET. BRETmax data are expressed as means ± SEM of four to six different experiments. ***Significantly different (P < 0.001) compared with cocaine 0 μM or 0 min (one-way ANOVA followed by Bonferroni post hoc tests).
Fig. 4.
Effect of cocaine on D1R homomers was mediated by σ1R. (A and B) HEK-293T cells were transfected or not transfected (wt, nontranfected cells) with σ1R siRNA, irrelevant oligonucleotides (oligo) and/or D1R cDNA (D1). Cell membranes were analyzed by SDS/PAGE and immunoblotted with the anti-σ1R (A) or D1R (B) antibody. Values are mean ± SEM of three experiments, and a representative Western blot for σ1R (A) or D1R (B) is shown. ***P < 0.001 compared with D1R cDNA transfected cells (one-way ANOVA followed by Bonferroni post hoc tests). (C) BRET saturation experiments were performed in HEK-293T cells cotransfected with σ1R siRNA (50 pmol), D1R–Rluc receptor cDNA (0.5 μg), and increasing amounts of D1R–YFP cDNA (0.3–3 μg cDNA), treated (open symbols) or not (filled symbols) with 150 μM cocaine for 30 min. BRET data are expressed as mean ± SD of four to six different experiments grouped as a function of the amount of BRET acceptor.
Cocaine Binding to σ1R Modulates D1R Function in Living Cells.
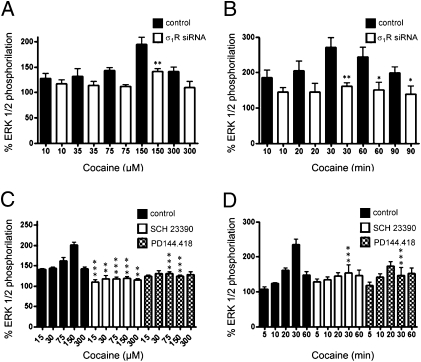
To study how cocaine affects D1R–mediated signaling, CHO cells were used, as they provided a lower baseline of signaling for which to detect downstream changes. CHO cells were also shown to constitutively express σ1Rs but not DAT or D1Rs (Fig. 5 A and B). As expected, in CHO cells expressing D1Rs, the full D1R agonist SKF 81297 dose-dependently increased cAMP production (Fig. 5C). Treatment with cocaine (150 μM; 10 min) did not induce a significant increase in cAMP, but robustly enhanced D1R agonist-induced cAMP accumulation (Fig. 5C). This was completely counteracted by silencing expression of σ1R via RNAi (Fig. 5C), indicating that this effect of cocaine was mediated by σ1R. SKF 81297 also produced a dose-dependent MAPK activation (ERK1/2 phosphorylation; Fig. 6A) with a maximum response at 2 min (Fig. 6B). SKF 81297-induced ERK1/2 phosphorylation was inhibited by the D1R antagonist SCH 23390 (10 μM) and also by the σ1R antagonist PD144.418 (1 μM; Figs. 6 A and B), indicating that σ1R modulates a D1R-mediated MAP kinase pathway in addition to the cAMP pathway.
Fig. 5.
Effect of cocaine on D1R-mediated cAMP production. (A) RT-PCR was performed using total RNA from CHO cells (lanes 1–4) and primers for Chinese hamster σ1R (lane 2), DAT (lane 3), or GAPDH (lane 4). RNA from cells without primers (lane 1) was included as negative control. (B) CHO cell membranes were analyzed by SDS/PAGE and immunoblotted with the anti-σ1R antibody (top blot) or anti-D1R antibody (bottom blot, lanes 1 and 2: cells transfected or not transfected with D1R cDNA, respectively). (C) CHO cells transfected with D1R cDNA (1.5 μg, filled bars) or cotransfected with D1 receptor cDNA and 125 pmol σ1R siRNA (open bars) were treated with increasing concentrations of D1R agonist SKF 81297 for 10 min in the absence or presence of 150 μM cocaine or with cocaine alone. Results are mean ± SEM of three to six independent experiments performed in triplicate. Bifactorial ANOVA of results of samples without or with siRNA transfection showed significant effect of SKF (P < 0.0001 and P < 0.001, respectively), but only in samples without siRNA transfection was there a highly significant effect of cocaine (***P < 0.0001, compared with samples with the same concentration of SKF 81297 and without RNAi transfection and in the absence of cocaine; Bonferroni post hoc tests).
Fig. 6.
Effect of σ1R ligands on D1R-mediated ERK1/2 phosphorylation. CHO cells transfected with D1R cDNA (1.5 μg) were stimulated with increasing concentrations of the D1R agonist SKF 81297 for 2 min (A) or with 100 nM SKF 81297 for increasing periods of time (B) in the absence (filled bars) or presence of 10 μM D1R antagonist SCH 23390 (open bars) or 1 μM σ1R specific ligand PD144.418 (cross-hatched bars). ERK1/2 phosphorylation is represented as percentage over basal levels (100%). Results are a mean ± SEM of four independent experiments performed in duplicate. Bifactorial ANOVA showed a significant effect of SKF 81297 (P < 0.0001 in A and P < 0.001 in B), and Bonferroni post hoc tests showed a significant SCH 23390-mediated or PD144.418-mediated counteraction of the effect SKF 81297 (*P < 0.05 and ***P < 0.001, compared with control samples with the same concentration and exposure time of SKF 81297).
Importantly, cocaine per se dose-dependently (Fig. 7A) and time-dependently (Fig. 7B) activated ERK1/2 phosphorylation. Again, this effect was mediated by σ1R, as it was strongly diminished in cells transfected with the σ1R siRNA (Fig. 7 A and B). Furthermore, a similar effect could be obtained with the selective σ1R agonist PRE084 (Fig. S1). Cocaine-induced ERK1/2 phosphorylation seemed to be dependent on D1R expression, because the increase in ERK1/2 phosphorylation was not found in CHO cells lacking D1R expression (Fig. S2). Moreover, cocaine-induced ERK1/2 phosphorylation in cells expressing σ1R and D1R was not only counteracted by PD144.418 (1 μM), which therefore acted as a σ1R antagonist, but also by SCH 23390 (10 μM, Fig. 7 C and D). All of these results suggest that cocaine binding to σ1R or SKF 81297 binding to D1R in the D1R–σ1R heteromer induce ERK1/2 phosphorylation that is equally counteracted by σ1R or D1R antagonists. Finally, we found a strong and reciprocal antagonistic interaction between σ1R and D1R on MAPK signaling. Thus, SKF 81297-induced ERK1/2 phosphorylation was drastically counteracted by increasing concentrations of cocaine (Fig. 8A), and cocaine-induced ERK1/2 phosphorylation was also counteracted in the presence of increasing concentrations of SKF 81297 (Fig. 8B). Again the same qualitative effects were obtained with the selective σ1R agonist PRE084 (Fig. S1).
Fig. 7.
Cocaine-induced σ1R-mediated ERK1/2 phosphorylation. CHO cells transfected with D1 receptor cDNA (1.5 μg, filled bars) or cotransfected (open bars) with D1R cDNA and σ1R siRNA (125 pmol) were incubated with increasing concentrations of cocaine for 30 min (A) or with 150 μM cocaine for increasing time periods (B). (C and D) CHO cells were transfected only with D1 receptor cDNA (1.5 μg) and were treated (30 min) with increasing concentrations of cocaine (C) or with 150 μM cocaine for different periods of time (D), in the absence (filled bars) or presence of 10 μM of the D1R antagonist SCH 23390 (open bars) or 1 μM σ1R antagonist PD144.418 (cross-hatched bars). ERK1/2 phosphorylation is represented as percentage over basal levels (100%). Results are mean ± SEM of four to seven independent experiments performed in duplicate. In all samples, bifactorial ANOVA showed a significant (P < 0.0001 in A–C; P < 0.001 in D) effect of cocaine, and Bonferroni post hoc tests showed a significant counteraction of cocaine effect by siRNA (A and B, *P < 0.05 and **P < 0.01 compared with sample with the same treatment and without siRNA transfection) and a significant SCH 23390-mediated or PD144.418-mediated counteraction of the cocaine effect for some concentrations and exposure times (C and D, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control samples with the same treatment).
Fig. 8.
Antagonistic interaction between cocaine and the D1R agonist SKF 81297 on ERK1/2 phosphorylation. (A and B) CHO cells transfected with D1R cDNA (1.5 μg) were treated or not treated for 30 min with increasing concentrations of cocaine (A) or with 150 μM cocaine (B) and, during the last 2 min, the additon of 100 nM (A) or increasing concentrations (B) of D1 receptor agonist SKF 81297. ERK1/2 phosphorylation is represented as percentage over basal levels (100%). Results are mean ± SEM of four independent experiments performed in duplicate. One-way ANOVA followed by Bonferroni post hoc tests showed a significant cocaine-mediated counteraction of SKF 81297 and a significant SKF 81297-mediated counteraction of cocaine-induced ERK1/2 phosphorylation (*P < 0.05, **P < 0.01, and ***P < 0.001 compared with control, without cocaine or SKF 81297 exposure). (C) WT (filled bars) and σ1R KO (open bars) mouse striatal slices were treated with SKF 81297 for 10 min, with cocaine for 30 min or with cocaine for 30 min and, during the last 10 min, the addition of SKF 81297. Immunoreactive bands from six slices obtained from five WT or five KO animals were quantified for each condition. Values represent mean ± SEM of percentage of phosphorylation relative to basal levels found in untreated slices. Significant differences respect to corresponding treatment in WT mouse slices were calculated by bifactorial ANOVA followed by post hoc Bonferroni tests (**P < 0.01).
Cocaine Binding to σ1R Modulates D1R Function in Mouse Brain Striatum.
To explore whether our results above using cultured cells could be extrapolated to the level of the organism, we took tissue from WT and s1R KO mice and examined the effects of cocaine on signaling. Previous in vivo studies have shown that pharmacologically significant doses of cocaine produce striatal levels of the drug at a low micromolar range (20). Those measurements reflect free, rather than bound, concentrations of cocaine, and it is well established that higher drug concentrations need to be applied in brain slice preparations, to allow diffusion into the tissue. Because, in cotransfected CHO cells, a strong and significant effect of cocaine was observed at 30 μM (Fig. 8A), a fivefold higher concentration, 150 μM, was then used to see clear effects in slices of mouse striatum (Fig. 8C). On one hand, both the D1R agonist SKF 81297 (1 μM) and cocaine (150 μM) induced ERK1/2 phosphorylation in striatal slices of WT mice after 10-min activation (Fig. 8C). On the other hand, in striatal slices of WT mice, SKF 81297-induced ERK1/2 phosphorylation was significantly reduced with pretreatment with cocaine for 30 min (Fig. 8C). The antagonistic interaction between σ1R and D1R on MAPK signaling is therefore detected in cotransfected cells and in striatal samples from WT mice. When similar experiments were performed in striatal slices from mice lacking the σ1R, cocaine was unable to induce ERK1/2 phosphorylation (Fig. 8C) and SKF 81297-induced ERK1/2 phosphorylation was not modified by pretreatment with cocaine (Fig. 8C). These results strongly support the existence of σ1R-D1R heteromers in the brain and indicate that all detected cocaine effects are dependent on σ1R.
Discussion
The role of σ1R in cell-signaling is not well understood and its main endogenous ligand has not been identified (12, 15). It has been suggested that σ1R may possess a constitutive biological activity, and that σ1R ligands may just be modulators of its innate activity (12). The best- characterized acute effects of σ1R ligands at the cellular level are their ability to modulate the function of several ion channels (K+ channels, NMDA receptors, IP3 receptors) (12). In the present study a mechanism by which σ1R modulates the function of a G-protein–coupled receptor, the D1R, is reported. This modulation depends on protein–protein interactions, which were detected by BRET assays. In agreement with the oligomeric nature of D1R (18), the existence of heteromers constituted by a minimum of a D1R homodimer and a σ1R was demonstrated by BRET/BiFC.
The σ1R, which is found mainly at the membrane of the endoplasmic reticulum, may modulate the activity of plasma membrane-located ion channels by its ability to translocate to the plasma membrane (12, 21). Coexpression of σ1R and D1R resulted in an alteration of σ1R subcellular distribution because, in the presence of D1R, σ1R was more abundant at the plasma membrane that in intracellular membranes. Importantly, coexpression of σ1R and D1R also led to heteromerization of the receptors, as measured by energy transfer in the absence of ligands. Acute administration of σ1R ligands, including cocaine, without coactivation of other receptors or channels may cause σ1R translocation to the plasma membrane (12). Apart from the increase in plasma membrane σ1R expression, cocaine led to an increase of σ1R-D1R colocalization. Taken together, these data suggest that heteromerization occurs between these receptors at steady state in the absence of ligands, but the presence of cocaine might induce an increase of the amount of receptor heteromers constituted by σ1Rs and D1Rs homomers at the level of the plasma membrane, perhaps through some stabilization of a given receptor conformation.
Although further studies will be required to understand how cocaine acts on the receptor monomers, homomers or heteromers and the specific effects at a protein level, we were able to observe that cocaine binding to σ1R led to a structural modification, detected as a separation between the C termini of the D1Rs in the σ1R–D1R–D1R heterotrimer. This was evidenced by a pronounced decrease (Fig. 3C) in the BRET signal due to a decrease in the energy transfer between Rluc and YFP (located in the C-terminal domains of D1Rs). These structural changes, which did not result from dimer disruption, correlated with changes in D1R function, as demonstrated by means of assays performed in both heterologous cells and in slices from mouse striatum. Importantly, cocaine binding to σ1R robustly enhanced D1R agonist-induced cAMP accumulation. This synergy is probably underlying the predominant role of D1R versus D2R in the behavioral effects of cocaine (discussed earlier here). These results are also strong evidence that cocaine effects are not adequately addressed by assuming that the drug is just increasing the synaptic dopamine concentration by a DAT-dependent mechanism. In fact, the reported effects were not dependent on DAT, because cell lines lacking this protein were used. It is thus expected that cocaine is acting by at least two different but interrelated mechanisms, one dependent on DAT and leading to an increase in dopamine levels and another dependent on σ1R and leading to an enhancement of D1R-mediated neurotransmission.
Unexpectedly, cocaine was able to induce ERK1/2 phosphorylation per se, although this effect depended on the presence of both the D1R and σ1R. As these particular effects were reproduced by the selective σ1R agonist (17) and counteracted by the putative σ1R antagonist PD144.418 (22), these results indicate that cocaine acts as a σ1R agonist. In living cells, cocaine-induced ERK1/2 phosphorylation was seen at short times of cocaine exposure (10 min); but the maximum effect was reached at 30 min, suggesting an involvement of cocaine-induced translocation of σ1R to the plasma membrane, with a consequent increase in cell surface σ1R-D1R heteromers. Both cocaine-induced and D1R-mediated ERK1/2 phosphorylation were counteracted by D1R or σ1R antagonists. The ability of an antagonist of one of the receptors in a receptor heteromer to block signals originated by stimulation of the partner receptor is a biochemical characteristic that has been described for other receptor heteromers, such as the D1R-histamine H3 receptor heteromer (23). Importantly, cocaine-induced ERK1/2 phosphorylation could also be demonstrated in mouse striatal slices, but not in striatal slices from σ1R KO mice. Because cocaine-induced ERK1/2 phosphorylation seems to be a biochemical characteristic of σ1R-D1R heteromers, these results provide evidence for the presence of these heteromers in the brain. Furthermore, we also found reciprocal antagonistic interactions between σ1R and D1R on MAPK activation, both in transfected cells and in mouse striatal slices. The D1R agonist-induced ERK1/2 phosphorylation was counteracted when agonist stimulation was performed in slices pretreated with cocaine and, conversely, cocaine-induced ERK1/2 phosporylation was counteracted by D1R agonist treatment. The cocaine-induced antagonistic modulation of D1R-mediated MAPK activation was shown to be dependent on σ1R, as demonstrated in cells transfected with σ1R siRNA and in striatal slices of σ1R KO mice. The qualitative similar results observed in transfected cells and in striatal slices support again the existence of σ1R-D1R heteromers in the brain.
We have described a previously uncharacterized mechanism by which cocaine binding to σ1R may significantly influence dopaminergic neurotransmission. Our results show that σ1R and D1R heteromerize in living cells and strongly suggest that σ1R-D1R heteromers are present in the striatum. Furthermore, our results shed light on the mechanisms behind the behavioral effects of cocaine that are dependent on σ1R. These data suggest that σ1R-D1R heteromers may be considered as targets for the treatment of cocaine addiction and that σ1R antagonists could counteract some of the behavioral and perhaps the addictive properties of cocaine. It will be important to determine the molecular determinants responsible for this heteromerization. This would allow the development of transgenic animals with mutated receptors not able to form σ1R-D1R receptor heteromers and therefore would allow one to better determine the role of σ1R-D1R receptor heteromerization in cocaine addiction.
Materials and Methods
Fusion Proteins and Expression Vectors.
The N-terminal truncated (nYFP) and the C-terminal truncated (cYFP) version of YFP were made as previously indicated (24). Human cDNAs for D1R, A1R, GABAB2R, or σ1R cloned in pcDNA3.1 were amplified without their stop codons and subcloned in an Rluc-expressing vector (pRluc-N1; PerkinElmer), or in a variant of GFP (EYFP-N3; enhanced yellow variant of GFP; Clontech), to give the plasmids that express D1R, A1R, GABAB2R or σ1R fused to either Rluc or YFP on the C-terminal end of the receptor (D1R–Rluc, D1R–YFP, σ1R–Rluc, σ1R–YFP, A1R–Rluc, A1R–YFP or GABAB2R–Rluc). Human cDNA for D1R was subcloned in pcDNA3.1–cYFP or pcDNA3.1–nYFP to give the plasmids that express D1R fused to either nYFP or cYFP on the C-terminal end of the receptor (D1R–cYFP and D1R–nYFP). When analyzed by confocal microscopy, it was observed that all fusion proteins showed similar subcellular distribution than naïve receptors. Fusion of Rluc and YFP to D1R did not modify receptor function as previously determined by cAMP assays.
Cell Culture and Transient Transfection.
HEK-293T and CHO cells, grown as previously described (23, 24), were transiently transfected with the corresponding cDNAs by PEI (PolyEthylenImine; Sigma) method as previously described (25) or with siRNA by lipofectamine (Invitrogen) method following the instructions of the supplier. Human and Chinese hamster σ1R siRNA and scrambled siRNA were designed and synthesized by Invitrogen (HSS 145543). Cells were used 48 h after transfection. To control for cell number, sample protein concentration was determined by a Bradford assay kit (Bio-Rad).
Immunostaining.
Immunocytochemistry assays were performed as previously described (24) using the primary antibodies mouse monoclonal anti-σ1R (1/200; Chemicon) or rat anti-D1R (1/200; Chemicon) and stained with the secondary antibodies Cyn3 donkey anti-mouse (1/100; Jackson Immunoresearch Laboratories) or Cyn2 goat antirat (1/100; Jackson Immunoresearch Laboratories). D1R fused to YFP protein was detected by its fluorescence properties. Samples were observed in a Leica SP2 confocal microscope (Leica Microsystems). Heterodimers of receptors fused to complementary fragments of YFP were detected directly by their fluorescence properties using a Zeiss 510 Meta confocal microscope.
RT-PCR.
Total cellular RNA was isolated from HEK-293T or CHO cells using QuickPrep Total RNA Extraction Kit (Amersham Biosciences). Total RNA (1 μg) was reverse transcribed by random priming using M-MLV Reverse Transcriptase, RNase H Minus, Point Mutant, following the protocol of Two-Step RT-PCR provided by the manufacturer (Promega). The resulting single-stranded cDNA was used to perform PCR amplification for σ1R, DAT and GAPDH as an internal control of PCR technique using Taq DNA Polymerase (Promega). Common primers to amplify human and Chinese hamster σ1R gene were used: 5′-CCTGGCTGTCGCAGCGGTGCTG-3′ (forward) and 5′-GGTGCCAGAGATGATGGTATCC-3′ (reverse). To amplify human and Chinese hamster DAT, the primers used were 5′-TTCATCATCTACCCGGAAGC-3′ (forward) and 5′-CACCATAGAACCAGGCCACT-3′ (reverse). To amplify human GAPDH, the primers used were 5′-TTCATCATCTACCCGGAAGC-3′ (forward) and 5′-CACCATAGAACCAGGCCACT-3′ (reverse). To amplify Chinese hamster GAPDH, the primers used were 5′-TTCATCATCTACCCGGAAGC-3′ (forward) and 5′- CACCATAGAACCAGGCCACT-3′ (reverse). RNA without reverse transcriptions did not yield any amplicons, indicating that there was no genomic DNA contamination.
BRET Assays.
HEK-293T cells were cotransfected with a constant amount of cDNA encoding for the receptor fused to Rluc and with increasingly amounts of cDNA encoding to the receptor fused to YFP to measure BRET, as previously described (25). For BRET assays with bimolecular fluorescence-complemented proteins, HEK-293T cells were cotransfected with a constant amount of cDNA encoding for σ1R-Rluc or GABAB2R-Rluc receptors and with increasingly equal amounts of cDNA corresponding to D1R–nYFP and D1R–cYFP, and fluorescence complementation and BRET were determined as previously indicated (25, 26). Both fluorescence and luminescence for each sample were measured before every experiment to confirm similar donor expressions (≈100,000 bioluminescence units) while monitoring the increase in acceptor expression (1,000–10,000 fluorescence units). In each BRET saturation curve, the relative amount of acceptor is given as the ratio between the fluorescence of the acceptor (YFP) and the luciferase activity of the donor (Rluc).
cAMP Determination.
CHO cells were treated for 10 min with the indicated concentrations of D1R agonist SKF 81297 (Sigma), in the absence or presence of 150 μM cocaine (cocaine-HCl, Spanish Agencia del Medicamento no: 2003C00220) or with cocaine alone and cAMP was determined by cAMP (3H) assay kit (Amersham Biosciences).
ERK1/2 Phosphorylation Assays.
Brains from WT littermates and σ1R KO CD1 male albino Swiss mice (8 wk of age, 25 g) were generously provided by Laboratorios Esteve (Barcelona, Spain) (27). Striatal slices were obtained as previously indicated (28), treated with the indicated concentrations of ligands for the indicated time, frozen on dry ice, and stored at −80 °C. Transfected CHO cells were cultured in serum-free medium for 16 h before the addition of the indicated concentration of ligands for the indicated time. Both cells and slices were lysed in ice-cold lysis buffer (24, 28), and ERK1/2 phosphorylation was determined as indicated elsewhere (24, 28).
Supplementary Material
Acknowledgments
We thank Hanna Hoffmann (Universitat Autónoma de Barcelona) for assistance with brain striatal slices and Jasmina Jiménez (University of Barcelona) for technical assistance. Brains from σ1R KO and wild-type littermates CD1 albino Swiss male mice were generously provided by Laboratorios Esteve (Barcelona, Spain). This study was supported by Grants SAF2008-00146, SAF2008-03229-E, and SAF2009-07276 from Spanish Ministerio de Ciencia y Tecnología, Grant 060110 from Fundació La Marató de TV3, and by Intramural Funds of the National Institute on Drug Abuse.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008911107/-/DCSupplemental.
References
- 1.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 2.Di Chiara G, Bassareo V. Reward system and addiction: What dopamine does and doesn't do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 4.Chen R, et al. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuming T, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerfen CR. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. Amsterdam: Elsevier Academic Press; 2004. pp. 445–508. [Google Scholar]
- 7.Xu M, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 8.Bertran-Gonzalez J, et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss F, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 10.Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- 11.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: Implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Su TP. The sigma receptor: Evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol. 2005;3:267–280. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso G, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: Potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto RR, et al. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol. 2001;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: Evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42:1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 17.Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–524. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong MM, Fan T, Varghese G, O'dowd BF, George SR. Agonist-induced cell surface trafficking of an intracellularly sequestered D1 dopamine receptor homo-oligomer. Mol Pharmacol. 2006;70:78–89. doi: 10.1124/mol.105.021246. [DOI] [PubMed] [Google Scholar]
- 19.Gandía J, Lluís C, Ferré S, Franco R, Ciruela F. Light resonance energy transfer-based methods in the study of G protein-coupled receptor oligomerization. Bioessays. 2008;30:82–89. doi: 10.1002/bies.20682. [DOI] [PubMed] [Google Scholar]
- 20.Pettit HO, Pan HT, Parsons LH, Justice JB., Jr. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- 21.Su TP, Hayashi T. Cocaine affects the dynamics of cytoskeletal proteins via sigma(1) receptors. Trends Pharmacol Sci. 2001;22:456–458. doi: 10.1016/s0165-6147(00)01740-5. [DOI] [PubMed] [Google Scholar]
- 22.Akunne HC, et al. The pharmacology of the novel and selective sigma ligand, PD 144418. Neuropharmacology. 1997;36:51–62. doi: 10.1016/s0028-3908(96)00161-x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrada C, et al. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br J Pharmacol. 2009;157:64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro G, et al. Interactions between calmodulin, adenosine A2A, and dopamine D2 receptors. J Biol Chem. 2009;284:28058–28068. doi: 10.1074/jbc.M109.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carriba P, et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods. 2008;5:727–733. doi: 10.1038/nmeth.1229. [DOI] [PubMed] [Google Scholar]
- 26.Gandia J, et al. Detection of higher-order G protein-coupled receptor oligomers by a combined BRET-BiFC technique. FEBS Lett. 2008;582:2979–2984. doi: 10.1016/j.febslet.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 27.Langa F, et al. Generation and phenotypic analysis of sigma receptor type I (sigma 1) KO mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 28.Navarro G, et al. Interactions between intracellular domains as key determinants of the quaternary structure and function of receptor heteromers. J Biol Chem. 2010;285:27346–27359. doi: 10.1074/jbc.M110.115634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.