Abstract
The Great Barrier Reef (GBR) provides a globally significant demonstration of the effectiveness of large-scale networks of marine reserves in contributing to integrated, adaptive management. Comprehensive review of available evidence shows major, rapid benefits of no-take areas for targeted fish and sharks, in both reef and nonreef habitats, with potential benefits for fisheries as well as biodiversity conservation. Large, mobile species like sharks benefit less than smaller, site-attached fish. Critically, reserves also appear to benefit overall ecosystem health and resilience: outbreaks of coral-eating, crown-of-thorns starfish appear less frequent on no-take reefs, which consequently have higher abundance of coral, the very foundation of reef ecosystems. Effective marine reserves require regular review of compliance: fish abundances in no-entry zones suggest that even no-take zones may be significantly depleted due to poaching. Spatial analyses comparing zoning with seabed biodiversity or dugong distributions illustrate significant benefits from application of best-practice conservation principles in data-poor situations. Increases in the marine reserve network in 2004 affected fishers, but preliminary economic analysis suggests considerable net benefits, in terms of protecting environmental and tourism values. Relative to the revenue generated by reef tourism, current expenditure on protection is minor. Recent implementation of an Outlook Report provides regular, formal review of environmental condition and management and links to policy responses, key aspects of adaptive management. Given the major threat posed by climate change, the expanded network of marine reserves provides a critical and cost-effective contribution to enhancing the resilience of the Great Barrier Reef.
Keywords: biodiversity protection, spatial planning and zoning, social and ecological resilience, coral reefs, economic cost benefit analysis
The Great Barrier Reef (GBR) is a marine ecosystem of globally significant biodiversity, exceptional environmental, cultural, social, and economic value, and extraordinary beauty. Those values are recognized in its listing as a World Heritage Area and national Marine Park. Coral reefs are exceptional reservoirs of marine biodiversity (1), but the GBR also includes a wide range of other ecosystems, from coastal seagrass beds to a wide range of diverse seafloor habitats (2). However, as for many marine ecosystems globally, those values are under serious threat from a range of human causes, with climate change at the fore (3–5). Responding to those threats demands a portfolio of diverse and adaptive conservation strategies, in turn requiring review of the effects and effectiveness of those different approaches (6–8).
The Great Barrier Reef as a Regional-Scale Case Study of Marine Reserve Management
Networks of marine protected areas are a prominent strategy in marine conservation, and current paradigms suggest numerous benefits for biodiversity and fisheries, especially as part of an integrated package of management approaches (e.g., consensus statement in ref. 9; also refs. 3, 10). As the world's largest network of marine reserves, the GBR provides a unique opportunity to test those paradigms at large spatial scales and under best-practice circumstances, with broad relevance to the science and management of marine conservation. The Great Barrier Reef Zoning Plan 2003, implemented in 2004, serves as a benchmark for process and outcomes in marine reserve networks. Based on best-practice in design and implementation (11, 12; SI Section 1), it also provides the only set of comparisons, which include: (i) replication, across a large range of latitudes and other gradients; (ii) some before–after comparisons; (iii) a range of treatment levels (zones) beyond fished and no-take reserves (Table S1); and (iv) information on compliance and enforcement.
This review synthesizes available information, including extensive previously unpublished results and gray literature, on the effects of zoning and spatial management on the GBR, with an emphasis on the 2004 Zoning Plan and in the context of adaptive management of the GBR Marine Park. The paper examines direct effects of the zoning on target fish and sharks on no-take and no-entry coral reefs, indirect effects on corals, crown-of-thorns starfish, and reef food webs, and effects for nonreef habitats and species of conservation concern. These ecological insights are complemented by an examination of compliance and enforcement within the network and social and economic costs and benefits. Finally, the implications of this information both for marine reserve management and for the science to underpin that management are discussed. Only the most significant results are included in the main paper; many results and background information on the GBR, zoning, and monitoring are included in SI Text.
Effects of Spatial Zoning and Marine Reserves in the Great Barrier Reef
Direct Biological and Ecological Effects of Zoning on Coral Reefs: Changes in Reef Fish and Sharks.
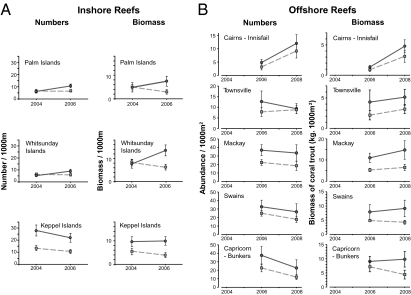
There is now very strong evidence that no-take zones on the Great Barrier Reef benefit fish stocks within those zones. The strongest results so far come from visual surveys of abundance and size of target fish, principally coral trout (Plectropomus spp., the major target of line fishing on the GBR), using comparisons of fished and no-take reefs (Fig. 1) (13). Throughout this paper, “fished” is used to refer to areas legally open to fishing and does not include areas that may have illegal fishing. Monitoring has documented very fast and sustained recovery, with up to 2-fold increases in both numbers and size of fish on many no-take reefs. Significantly, this basic pattern holds across ≈1,000 km north–south and for both inshore and offshore reefs, despite strong environmental differences among those reefs (Fig. S1A).
Fig. 1.
Abundance and biomass of coral trout on fished and no-take reefs spread across ≈1,000 km of the Great Barrier Reef (see map in Fig. S1). Solid lines are no-take zones; dashed lines are fished reefs. Data are means ±SEM from scuba-based, visual transects of reefs zoned in 2004, updated from ref. 13. Data for inshore reefs (A) include data from before zoning implementation. Note different vertical axes and periods (dates) for A and B.
These increases appear to reflect genuine recovery of exploited fish populations on no-take reefs, rather than declines in abundance on fished reefs due to displaced fishing effort (13); note that other changes to fisheries management occurred simultaneously (14). In one of very few before–after comparisons available for GBR zoning, data from inshore reefs show that on most of those reefs, the differences primarily reflected increases in fish on protected reefs, with little decrease on fished reefs (Fig. 1A). The rate of the increases is also particularly noteworthy, with 2-fold increases in coral trout biomass appearing within 2 years of the implementation of the new zoning plan (13). Many of the protected reefs had previously been fished heavily. Although the basic pattern of elevated stocks in no-take areas was remarkably consistent, there is nonetheless notable variation between regions and cross-shelf locations, likely to reflect differences in both ecology and intensity of exploitation (15). The increased mean size of fish in no-take zones is particularly important as large fish are disproportionately more fecund and therefore contribute greatly to future fish populations (e.g., ref. 16), potentially including stocks in fished zones.
A recent series of surveys of deep, reef-base habitats also found distinct benefits to targeted fish species, using baited, remote, underwater video surveys. These patterns were strongest in coral-dominated habitats, where coral trout (Plectropomus spp.), red emperor (Lutjanus sebae), and redthroat emperor (Lethrinus miniatus) were all more abundant on no-take reefs. However, the patterns varied considerably among species and habitats. Differences between zones were less clear-cut than those for shallow reefs, perhaps due to lower fishing effort at these depths and/or continuity of habitat between zones, allowing fish unrestricted passage out of protected zones (17).
There is also a range of strong evidence for the benefits of no-take zones based on comparisons of zones in place before the 2004 rezoning (detailed description in SI Section 2; zoning history in Table S2). A large scale manipulative study of offshore reefs found that no-take reefs generally, but not always, had more, larger, and older fish for the two main target species than did reefs open to fishing (Fig. S1 B–D) (14, 15). Surveys of inshore reefs of the central and southern GBR found that coral trout and stripey seaperch (Lutjanus carponotatus) were generally less abundant and smaller on fished reefs than on no-take reefs implemented in 1987 (Fig. S2) (18, 19). Significantly, the evidence suggests that coral trout stocks on inshore reefs generally were markedly depleted by 1984, before reserve implementation (Fig. S2).
The effects of no-entry zones are markedly stronger still than those of no-take zones. Comparing long-term (pre-2004) fished, no-take, and no-entry zones confirmed the benefits of no-take zones, but also showed that coral trout, the redthroat emperor (L. miniatus), and lutjanids (tropical snappers) were markedly more abundant and coral trout were larger in no-entry zones than in no-take zones (Fig. S3) (20). Although the data for no-entry zones have some limitations, this is a critical result because it raises the possibility that lower abundance in no-take zones is due to incomplete compliance (no-entry zones are much simpler to enforce, and hence have more effective compliance; further explanation, SI Section 2). It also suggests that baseline populations of target fish may have been significantly more abundant than previously recognized, with stocks in most areas significantly depleted in comparison with that baseline.
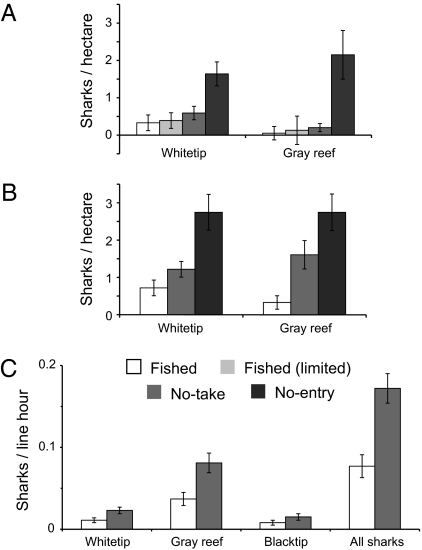
Populations of reef sharks, the main apex predator in coral reef ecosystems, show even stronger effects of zoning, with the largest benefits found in no-entry zones (Fig. 2). In surveys of reefs zoned before 1992, whitetip (Triaenodon obesus) and gray reef (Carcharhinus amblyrhynchos) sharks respectively were ≈4 and 8 times more abundant on no-entry reefs than on fished reefs in the central GBR (20). Gray reef sharks were up to 30 times more abundant on no-entry reefs than on fished reefs in the northern GBR (Fig. 2A) (21). Abundance in no-take zones was intermediate in the central GBR (Fig. 2B) (20), but Robbins et al. (21) found numbers in no-take zones were closer to those in fished zones than no-entry zones, especially for gray reef sharks. Line fishing surveys of sharks found that catch rates of sharks on reefs historically open to fishing were less than half those on reefs that had been closed to fishing since the late 1980s (Fig. 2C) (22). Note that all three of these shark studies compared zones implemented before 1992. Surveys of deep, reef-base habitats in the southern GBR using baited underwater video found higher numbers of gray reef sharks in newly created (2004) no-take zones than fished zones (17).
Fig. 2.
Abundance of reef sharks in different zones in the northern and central GBR. Abundance of sharks based on scuba-based, visual transects for A (from ref. 20) and for B (from ref. 21). (C) Catch rates of sharks using commercial line fishing, disaggregated from ref. 20. All data are means ±SEM.
The studies by Robbins et al. (21) and Ayling and Choat (20) demonstrate the value of expanding simple fished/no-take contrasts to include a range of different zones (c.f. 23 for temperate examples). Abundances in no-entry zones, markedly higher than for no-take zones, again suggest that no-take zones do not provide a reliable baseline for undisturbed shark abundances and suggest possible compliance problems (20, 21), although these interpretations again require caution (SI Section 2). Robbins et al. (21) also surveyed zones with limited fishing (Conservation Park), intermediate in protection between no-take zones and zones open to fishing (General Use). The effects of limited fishing zones on shark abundances were minor and not statistically significant compared to open fishing zones, although shark abundances ranked consistently higher with increased protection.
Potential Effects on Ecosystem-Wide Fish Populations.
An important aspect of the effectiveness of no-take reserves is their benefits not only to fish populations within individual no-take reserves, but also their contributions to overall fish populations across the ecosystem, including both other no-take reserves within the network and contributions to fished areas. With 32% of GBR reef area in no-take reefs, and fish densities about two times greater on those reefs, fish populations across the ecosystem have increased considerably (14). Contributions beyond a reserve depend on adult and larval connectivity both among no-take reefs, and between no-take and fished reefs (e.g., refs. 7, 10, 24, 25). Although evidence exists for some export of adult fish from no-take zones to fished areas (26, 27), adult coral trout rarely move between individual coral reefs on the GBR (26, 28) and current no-take zones generally include entire reefs. The lack of adult movement between reefs clearly enhances the effectiveness and measurability of protection for fish populations within reserves. However, it also means that increased biomass of coral trout in no-take zones will have little direct (conservation or fisheries) benefits through export of adult fishes to the two-thirds of reef area that is open to fishing.
However, reproductive output from no-take reefs may be of enormous significance, due to disproportionately higher output per unit area from the more plentiful, larger fishes in reserves (SI Section 3). Evidence from the GBR and elsewhere suggests that populations within marine reserves are at least partially self-sustaining between generations (29, 30), but that there is also considerable larval exchange between reefs (SI Section 3). Larval export from no-take zones is important both for connectivity within the no-take network and for sustaining both conservation and fishery values of the larger area of fished reefs on the GBR. The extent of such export depends on three factors: the extent of larval transport between reefs, the relative reproductive output of no-take and fished reefs, and the dispersal distances from no-take reefs to other reefs. Larval transport and relative output are considered in SI Section 3; for the main target species, no-take reefs likely have the capacity to provide substantial proportions of ecosystem-wide larval supply.
Recent work has recommended that networks of marine reserves should aim to preserve the natural distribution of dispersal distances and in particular maximize the proportion of reefs within 15–30 km of a potential source reef (7, 24, 25). Spatial analysis of dispersal distances between no-take reefs suggests that the 2004 rezoning of the GBR successfully maintained the naturally occurring spectrum of dispersal distances between reefs within the no-take network (Fig. S4). Under the 2004 rezoning, the distribution of nearest-neighbor distances between no-take reefs closely matches that of all GBR reefs, and more than 99.5% of no-take reefs have a no-take reef within 14 km. Analysis of distances between no-take reefs and fished reefs show that more than 75% of fished reefs have a no-take reef within 16 km and more than 90% within 22 km, indicating that the no-take network has the capacity to provide substantial larval subsidies to the fished reefs.
Indirect Effects of Zoning on Coral Reefs: Effects on Corals, Crown-of-Thorns Starfish, and Prey Fish.
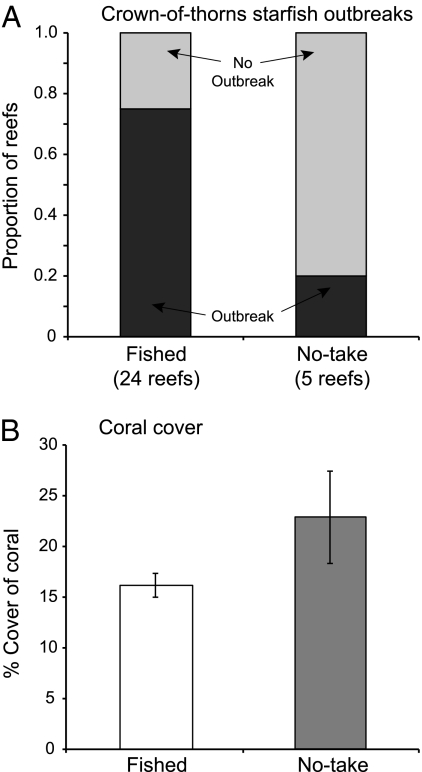
Zoning benefits for target, predatory fish species are important, but the potential effects on broader biodiversity, and on reef-building corals in particular, are of greater ecological and economic significance, because the entire reef ecosystem depends on the structure provided by corals. One of the most ecologically important effects documented for GBR zoning is the decreased frequency of outbreaks of the coral-eating crown-of-thorns starfish in no-take zones (31) (Fig. 3A; pre-2004 zones; further detail in SI Section 4). This starfish has been the major cause of coral mortality on the Great Barrier Reef. The relative frequency of outbreaks on midshelf reefs that were open to fishing was 3.75 times higher than that on no-take reefs. Most outbreaks occur on the midshelf region. If all reefs across the shelf were included, outbreak frequency was seven times greater on fished reefs (31).
Fig. 3.
Effects of zoning on coral-eating starfish and hence on coral populations. (A) Frequency of outbreaks of crown-of-thorns starfish on no-take and fished midshelf reefs in regions with active outbreaks present. Data are for 1994–2004, redrawn from ref. 31; note low numbers of no-take reefs were available pre-2004; further background in SI Section 4. (B) Abundance of hard corals on midshelf reefs after crown-of-thorns starfish outbreaks. Data, previously unpublished, are means ±SEM of percent cover; details of methods in SI Section 4.
Importantly, the reduction in starfish outbreaks appears to have direct benefits for coral populations (Fig. 3B). The cover of coral on midshelf reefs after outbreak periods appears to be markedly higher in no-take zones than in fished zones. These results are ecologically very important because they show a strong connection between a specific management strategy (reserves) and the major historical cause of mortality for reef-building corals on the GBR, with likely consequences both for overall biodiversity and for tourism value of the reefs.
Although the effect on starfish outbreaks is clear, the ecological mechanism causing this pattern remains uncertain. The major target species affected by the zoning on the central GBR are not considered to be direct predators on crown-of-thorns starfish. Sweatman (31) speculated that reductions in coral trout may cause trophic cascades, resulting in a decrease in invertebrate predators of starfish juveniles. The effects on corals (Fig. 3B) are consistent with results of independent surveys of inshore reefs (18, 19, 32) (details in SI Section 4, although crown-of-thorns starfish are unusual on inshore reefs). More detailed information being collected under the current zoning monitoring should help understand the where, when, and how of zoning effects on coral populations. Whatever the mechanism, reduced frequency of a major source of coral mortality will have major consequences for reef resilience.
Reserves also appear to have some impacts on food web structure on GBR coral reefs, but those impacts are not generally consistent with simplistic, top-down effects of removal of predatory fish. In particular, if abundance of prey fish depends primarily on top-down control, then recovery of fish populations within no-take zones might be expected to reduce abundance of prey fish. Although such changes have been recorded, they are far from consistent (SI Section 4 and Fig. S5).
Nonreef Habitats and Trawling Effects.
Although nonreef habitats occupy around 95% of the area of the GBR Marine Park, and include an extraordinary diversity of habitats and taxa, only recently have there been even basic biological surveys for most of these habitats (2). For most habitats, there is negligible direct information on the biological effects of zoning or other management initiatives (except for shoals: see below). Given this lack of biological information for seabed areas, development of the bioregions underpinning the 2004 zoning had to be largely interpolated from physical information, such as bathymetry and sediment data. However, this also prompted a major survey of seabed biodiversity, with 1,380 sites covering 200,000 km2 (the Seabed Biodiversity Project, ref. 2). This new, vastly more detailed information provided the means both to assess the effectiveness of the 2004 zoning in protecting biodiversity and thereby to test the effectiveness of using physical proxies for patterns of biodiversity. Such analysis indicated that both the approach and the outcome had been very effective, substantially increasing protection at a range of levels, including species, species groups, assemblages, and habitat types (SI Section 5) (33). For each level, 20% or more of biomass or area was protected in zones that do not allow trawling.
The effects of prawn trawling in the GBR have been studied directly (34, 35), allowing zoning effects on trawling impacts to be modeled and analyzed (35). Although potentially destructive to seabed habitats and responsible for the majority of discarded catch in the GBR fisheries (8), trawling is only allowed in 33% of the GBR Marine Park area (General Use zones). Available evidence suggests that there is relatively good compliance with zoning and that current trawling predominantly occurs within areas of seabed where scope for damage is limited. Seagrass beds in particular are not considered vulnerable (36). Pitcher et al. (35) suggested that very few species have been significantly affected by trawling and that overall management changes have largely reversed previous trends for damage to bottom habitats (further detail in SI Section 5). Remaining concerns about incidental catch of species of conservation concern may be partially ameliorated by bycatch reduction devices (SI Section 5).
The only data available for direct effects of zoning on nonreef habitats are for shoals, areas where hard substrata outcrop from the seabed in deeper water (generally >20 m). Monitoring zoning effects on these habitats involves considerable challenges, including confounded comparisons between zones (SI Section 1), lack of background information, and the need to develop new monitoring techniques (SI Section 5). The clearest results for shoal monitoring come from well-defined, deepwater shoals in the southern GBR, where mean abundance indices for targeted fish on no-take shoals were twice those of fished shoals, with ratios of up to 11 (Fig. S6) (37). However, some targeted species did not show benefits of protection. Results from shoals in the central GBR are less clear, largely due to the lack of clearly comparable fished and no-take zoned shoals (SI Section 1). In some cases, some target fish were more abundant on no-take shoals, but in other cases, the reverse was true (38).
Species of Conservation Concern: Dugong and Marine Turtles.
The biology, scale of ecological function, population status, and appropriate management and monitoring approaches for dugongs (Dugong dugon) provide a marked contrast to those of reef-attached fish. Dugongs are considered at serious risk, have a relatively low reproductive capacity (39, 40), are highly mobile at scales greater than that of most no-take zones (41), and are considered part of a single stock in the GBR (42). Population estimates for dugong at the scales of no-take zones have high uncertainty, due to the animals’ spatially heterogeneous distribution and their predominant occurrence in turbid waters, which makes them challenging to survey, even from the air (43). Thus assessment of dugong management effectiveness is more complex than simple comparisons of density within and outside no-take areas. Further background on dugong status and management are given in SI Section 5.
In addition to the greatly enhanced area protected by the 2004 zoning, management agencies use a suite of complementary measures to protect dugongs in the GBR. These include bycatch reduction and gear changes, a voluntary moratorium on Indigenous hunting in the southern two-thirds of the GBR, and dugong protection areas (DPAs) introduced in 1998 to protect specific areas of high conservation value (8, 40, 44, 45). Although the rezoning in 2004 protected 42% of high-priority dugong habitat in no-take reserves, doubling the previous proportion protected, this nonetheless fell short of the 50% recommended by experts as part of the Biophysical Operating Principles (45).
Overall, marine reserves and other measures appear to be providing critical but insufficient contributions to protecting GBR dugongs. A time series of aerial surveys suggests that populations on the inhabited coast are now so low that recovery will require zero human-induced mortality (40). By overlaying the population distribution models with spatial information on ranked threats to dugongs, based on expert assessments, Grech and Marsh (46) provided a rapid assessment of risks to GBR dugong. They estimated that since the 2004 rezoning, ≈96% of habitat of high conservation value for dugongs and 93% with medium conservation value, is at low risk from human activities (either due to spatial protection or to low levels of human activities). This is a considerable improvement on the prezoning situation, especially with respect to fishing bycatch (47). Grech and Marsh (46) also concluded that the protection afforded by the current ecosystem-scale network of marine reserves is limited by the inability of reserves per se to mitigate all of the factors that threaten the marine environment, including activities in the adjacent coastal catchments.
Marine turtle protection involves similar issues of scale and biology to those for dugong. Globally significant populations of several listed threatened species inhabit the Marine Park and evidence suggests populations of several species are in decline, with mortality due to fishing bycatch as a major threat. The design principles for the 2004 zoning included incorporation of marine turtle internesting (areas adjacent to nesting beaches) and foraging habitats in no-take areas, specifically including all very high-priority nesting sites and 20% of foraging areas. These principles were not fully achieved, but protection of identified internesting sites increased from 23.4 to 56.5% and foraging habitat increased from 7.1 to 29% (48, 49). Other key strategies include mandatory use of turtle excluder devices on trawl nets. A case study of iterative management responses to survey data for loggerhead turtles is given in SI Section 5. As for dugong, spatial zoning alone may not provide sufficient protection for marine turtles, but can be highly effective in concert with other measures.
Zoning Management, Compliance, and Enforcement.
The ecological effectiveness of marine reserves depends critically on compliance, without which reserves are protected in name only. Monitoring of compliance (reviewed in SI Section 6 and Fig. S7) provides valuable information to support and direct enforcement, but may be strongly confounded and should be integrated with data on target species, to assess the effectiveness of management. For the GBR, the combination of compliance data and the patterns of abundance of target fish between fished, no-take, and no-entry zones (Fig. 2 and Fig. S3) (20, 21) indicate that compliance with zoning regulations is not complete. That no-take zones generally achieve markedly higher fish biomasses than fished zones shows that overall compliance is considerable. However, the large differences between no-entry and no-take zones most likely indicate significant poaching within many no-take zones (where effective enforcement is more difficult, SI Section 6).
Social and Economic Effects of Zoning.
Importantly, the ecological benefits of the zoning appear to have only entailed limited social or economic costs, and some significant benefits. The increased abundance of corals and fish are likely to have major flow-on, long-term benefits for the major human use (tourism) and potentially for fisheries (8). Recognition of the conservation value of the zoning changes seems widespread within the broader community, even within sectors directly affected by the changes, although some concerns remain among fishers. There have of course been significant changes in locations for both recreational and commercial fishing. Available evidence on social effects is reviewed in SI Section 7.
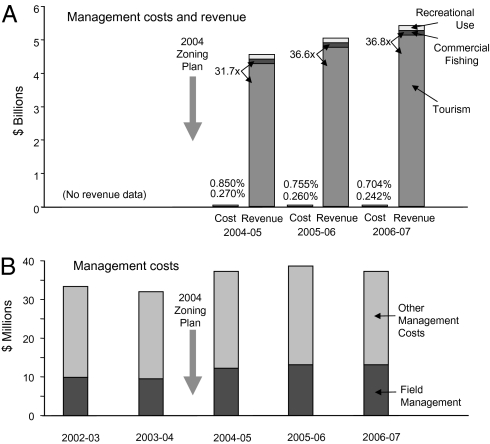
The economic value of a healthy GBR to Australia is enormous, currently estimated to be about A$5.5 billion annually and increasing steadily (Fig. 4) (50–52) (estimates only include use values and so underestimate total economic value), although comparable data are not available before 2004. The contribution to employment is estimated at 53,800 full time jobs. Tourism accounts for the vast majority of reef-based income and employment. Although such estimates are necessarily approximate, income from tourism is estimated to be about 36 times greater than commercial fishing and that ratio is increasing. Since 2005–2006, recreational use (mostly fishing) is estimated to contribute marginally more than commercial fishing. Significantly, these contributions accrue to both private industry and government sectors (through taxation and reduced unemployment welfare payments).
Fig. 4.
Economic costs and benefits for the Great Barrier Reef. (A) Economic value of the GBR to the Australian economy (50–52), compared to expenditure on management of the Great Barrier Reef Marine Park (65–67). Tourism provides the vast majority of economic benefits (numbers indicate ratio of tourism to commercial fishery estimates). Percentages above costs give expenditure as percentage of revenue, respectively, for overall expenditure on management of the Marine Park and for field management. (B) Costs of field management (including enforcement) and other management of the GBR (65–69). All values in Australian dollars.
The major economic cost associated with the rezoning was a once-off, structural adjustment package for commercial fishing industries, which totalled A$211 million at July 2009 [funds from Australian Government but not Great Barrier Reef Marine Park Authority (GBRMPA); data courtesy of the Department of the Environment, Water, Heritage and the Arts; also ref. 53]. In January 2004 an Australian Government policy statement was released, outlining assistance to fishers, fishing-related businesses, and fishing-dependent communities subsequent to declaration or rezoning of marine protected areas (54, 55). Estimates of likely economic impact and of financial assistance are not directly comparable (56), but a priori estimates of the costs of GBR zoning to fisheries were approximately A$14 million per annum (gross value of production; or A$0.5–2.59 million value added; refs. 57–59) with industry estimates as high as A$23 million per annum (60). Review of the initial business exit component of this package suggested a number of potential changes to improve outcomes and cost effectiveness (61) and a further review is currently underway. Given the considerable final investment, more cost-effective environmental and socioeconomic outcomes might have been achieved if initial strategic planning had been able to formally incorporate social and economic information, the need for industry structural adjustment, and cross-jurisdictional coordination of economic impacts (56).
Evidence for economic effects on businesses in the recreational fishing industry is very limited, but does not indicate major impacts. For example, recreational vessel registration data show no sign of changes due to the zoning plan (Fig. S8).
Expenditure on zoning enforcement, and on overall Marine Park management, has been relatively stable, with only minor increases in 2004 (∼32% and 15%, respectively) in response to the more than 7-fold increase in highly protected zones (Fig. 4B; excludes special initiatives). Estimated current investment in field management and compliance is A$47 per km2 no-take zone per year, plus an estimated A$30 per km2 per year for surveillance by the Australian Customs (Coastwatch). Implementation of the new zoning plan involved a once-off communication and awareness program of A$4.3 million over 5 years funded under a special initiative by the Australian Government (data courtesy GBRMPA, all figures in Australian dollars).
Importantly, expenditure on zoning and on overall management of the Marine Park are relatively minor when compared to the estimated economic value of the GBR (Fig. 4A). Proportional to economic returns, since 2004 annual investment in overall management of the Marine Park has been consistently less than 0.9% and decreasing, and expenditure on field management (predominantly zoning compliance) has been consistently less than 0.3% and decreasing (strictly such comparisons should use net value of the GBR, rather than gross output values, but net measures are not available; precise allocation of zoning and other field management costs is not possible). Even the costs of structural adjustment only amount to about 3.9% of the economic returns from the GBR in a single year (2006–2007 financial year).
Marine Reserve Paradigms: Insights from the Great Barrier Reef
Overall, zoning of the GBR marine reserve network appears to be making major contributions to the protection of biodiversity, ecosystem resilience, and social and economic values of the GBR Marine Park. The breadth and regional scale of these benefits provide important validation and extension of emerging ideas about the value of reserve networks (e.g., consensus statement in ref. 9), particularly given that the GBR is the first large network designed systematically at a regional scale and provides scope for rigorous comparisons (12, 62) (see Introduction and SI Section 1). The results demonstrate the value of reserves both for active restoration of ecosystem structure (e.g., the widespread recovery of depleted fish stocks within the new no-take network), and for preventing ongoing degradation (the stated primary goal of the 2004 zoning; e.g., reduced coral mortality). However, it must also be emphasized that the GBR sits within an exceptional context, in terms of biogeography, scale, governance, and economics, so that emerging lessons should not be assumed relevant across all circumstances. For example, the extent of the 2004 zoning network may not be feasible in regions that lack centralized governance arrangements or that lack resources for effective enforcement. Further, this paper focuses on the effects of zoning, but those results must be seen in the context of broader, complementary management and monitoring initiatives (see below). Insights into the specific scientific challenges of assessing the effects of marine reserves are discussed in SI Section 8.
The breadth and extent of benefits reflect very well on the scientific and engagement processes involved in the development and implementation of the 2004 Zoning Plan (11), especially the value of larger reserve size and high proportion of overall area in reserves to provide margins of error. For example, the protection of natural patterns of reef separation (Fig. S4) was not incorporated in the design in its own right, but is an outcome of the robust and comprehensive design principles (11). Similarly, comprehensive protection of minimum levels of seabed biodiversity (SI Section 5) is an outcome of those same principles and demonstrates the effective use of physical data as proxies where prior knowledge of biodiversity is limited. The benefit to the entire ecosystem of enhanced fish populations, or reduced coral mortality, clearly increases with increased proportional area of reserves.
Scientifically, effects such as increased biomass of target fish in protected areas are not novel. However, results from the GBR demonstrate those benefits over larger scales and provide concrete examples of the value of monitoring for evaluating management effectiveness and for community acceptance (8, 9) (SI Sections 1 and 7). The breadth and scale of GBR monitoring also illustrate the considerable variability inherent in the effects of reserves, variability among regions (Fig. 1 and Fig. S1 B and C) and among species with different life-history traits or vulnerability to fishing (e.g., target fish cf. sharks and dugongs cf. prey species). Reserve effects also depend strongly on the extent of fishing pressure and compliance within a region.
The demonstration of indirect benefits on corals, through crown-of-thorns starfish (Fig. 3), is especially important in demonstrating the value of reserves in maintaining ecosystem structure and function (9). Because corals construct the very habitat of coral reefs, these effects are highly relevant to long-term community structure and resilience and hence to socioeconomic value. Previous demonstrations of such benefits for no-take reserves on coral reefs have generally involved effects on fishing for herbivores and/or habitat-destructive fishing practices (e.g., refs. 63, 64), neither of which is significant on GBR reefs.
Many of the benefits of high proportions of protected habitats will not be limited to the protected zones, but may be diffused across zones, due to strong ecological connectivity between zones (e.g., highly mobile species, ecosystem-wide larval supply, and biodiversity). Benefits to fish stocks seem likely to accrue in part to the entire ecosystem, through larval subsidies (SI Section 3). Such ecosystem-wide benefits may be very real, but very difficult to measure reliably, as they are not amenable to simple comparisons of fished and no-take zones.
Overall, the ecological benefits appear to bring net social and economic benefits. Broad community opinion appears to support the zoning (SI Section 7), and the economic costs, which are being addressed through structural adjustment arrangements, are greatly outweighed by the economic benefits of a healthy reef (Fig. 4). These results show the considerable value of direct assessments of social and economic costs and benefits, assessments that are often advocated but less often implemented (9). Critics of marine reserves within the broader community and media often assert major social and economic costs of implementation. However, monitoring and survey data for the GBR suggest those costs are lower than asserted and minor compared to the social and economic values of the Marine Park. Further, understanding the costs that do occur provides insights into how they can be avoided or mitigated in the future (e.g., ensuring that fishers feel engaged in planning processes, etc., SI Section 7). Such lessons are valuable both for on-going management of the GBR and for the design and implementation of marine reserves elsewhere.
However, review of the GBR zoning also provides some clear cautionary insights. No-take networks alone do not provide sufficient protection for some taxa, even in a network as extensive as the GBR. By incorporating entire reefs within protected zones, the present system provides strong protection for taxa such as coral trout, which occupy single reefs throughout their adult lives. However, taxa such as sharks, dugongs, and marine turtles, that operate over larger scales and range between protected and open zones, are likely to benefit but to a much lesser extent. As widely recommended (e.g., ref. 9), GBR zoning is complemented by a great deal of nonspatial management, including explicit management of fisheries within fished zones and bycatch reduction efforts (SI Section 1). The results for dugongs and marine turtles show the importance of such complementary management (SI Section 5).
The dramatic differences between fished and no-take zones (Figs. 1 and 2 and Figs. S1–S3), suggest that, even on one of the best managed marine systems in the world, a large proportion of reefs are significantly depleted in predatory fish and sharks. However, the stark differences between no-take and no-entry zones (Fig. 2 and Fig. S3) indicate that that depletion is much more serious than indicated by abundances in no-take zones alone, potentially affecting most reefs (no-entry zones only account for 0.2% of area). The ecological consequences of this depletion are probably exacerbated by associated depletion of by-catch species and may be more serious in terms of ecosystem structure than fisheries impacts. On this basis, the large proportion of new no-take zones, although very positive, nonetheless seems insufficient to restore ecosystem-wide stocks of target fish to undepleted levels. Interpretation of no-take reserves as baselines (c.f. ref. 9) requires rigorous compliance within those reserves: GBR no-entry zones, as “full compliance” no-take zones, are critical in preventing the shifting baseline phenomenon of perceiving depleted stocks as normal.
Effective compliance and enforcement are critical to the overall ecological effectiveness of marine reserve networks. The evidence for notable noncompliance in GBR no-take zones, although limited, is a distinct concern and demonstrates the importance of monitoring to assess compliance (above and SI Section 6). Even limited noncompliance may have major ecological consequences, especially because poaching in no-take zones will tend to have dramatically higher catch rates and to catch the largest (and hence most fecund) fish and sharks (Fig. S3). Improved compliance could involve increased investment in education and awareness to improve voluntary compliance, increased investment in enforcement, and increased penalties to ensure real disincentives for noncompliance (SI Section 6). Given the environmental and economic value of the GBR, and the relatively minor current expenditure on zoning compliance (Fig. 4), there seems a strong case for increasing investment in compliance to protect such a valuable asset and revenue source.
In summary, the network of marine reserves on the GBR has brought major, sustained ecological benefits, including enhanced populations of target fish, sharks, and even corals, the foundation of the coral reef ecosystem. Although it is not possible to directly measure effects on seabed biodiversity, analyses indicate enhanced protection within no-trawl zones under the new network. Risk assessments even indicate some benefits to dugongs and marine turtles, despite protected zones being much smaller than the ranges of these species. These ecological benefits are likely to bring significant, long-term benefits for human uses of the Marine Park, and social and economic costs of the 2004 zoning appear limited in comparison with the large and growing economic return from a healthy GBR. Overall, the available evidence suggests that the large-scale network of marine reserves on the GBR is proving to be an excellent investment in social, economic, and environmental terms.
Supplementary Material
Acknowledgments
The assistance and data provided by the Great Barrier Reef Marine Park Authority (GBRMPA) and numerous staff is greatly appreciated. S. Gaines, K. Grorud-Colvert, S. Lester, N. Stoeckl, J. Quiggan, and G. Lange provided valuable comments. The authors acknowledge the traditional owners of the sea country of the Great Barrier Reef. Shoals monitoring results are courtesy of the Marine and Tropical Sciences Research Facility (MTSRF) and especially P. Speare, M. Stowar, and P. Doherty. This work was supported by a Pew Fellowship in Marine Conservation (to L.Mc.C.), the Australian Research Council Centre of Excellence for Coral Reef Studies, the MTSRF/Reef and Rainforest Research Centre, the GBRMPA, and the Australian Institute of Marine Science. The Effects of Line Fishing Experiment was supported by the Cooperative Research Centre for the Great Barrier Reef, the Fisheries Research and Development Corporation, the GBRMPA, Queensland Fisheries Management Authority, Commonwealth Scientific and Industrial Research Organisation Marine and Atmospheric Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.D.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909335107/DCSupplemental.
References
- 1.Roberts CM, et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science. 2002;295:1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- 2.Pitcher CR, et al. Seabed Biodiversity on the Continental Shelf of the Great Barrier Reef World Heritage Area: CRC Reef Research Task Final Report. Cleveland, QLD: CSIRO Marine and Atmospheric Research; 2007. [Google Scholar]
- 3.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 4.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson C. Status of Coral Reefs of the World: 2008. Townsville: Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre; 2008. [Google Scholar]
- 6.McCook LJ, et al. Ecological resilience, climate change and the Great Barrier Reef. In: Johnson J, Marshall P, editors. Climate Change and the Great Barrier Reef. Townsville: Great Barrier Reef Marine Park Authority; 2007. pp. 75–96. [Google Scholar]
- 7.McCook LJ, et al. Management under uncertainty: guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs. 2009;28:353–366. [Google Scholar]
- 8.Great Barrier Reef Marine Park Authority . Great Barrier Reef Outlook Report. Townsville: Great Barrier Reef Marine Park Authority; 2009. [Google Scholar]
- 9.Lubchenco J, Palumbi SR, Gaines SD, Andelman S. Plugging a hole in the ocean: the emerging science of marine reserves. Ecol Appl. 2003;13:S3–S7. [Google Scholar]
- 10.Russ GR. Marine reserves as reef fisheries management tools: yet another review. In: Sale PF, editor. Coral Reef Fishes Dynamics and Diversity in a Complex Ecosystem. San Diego: Academic Press; 2002. pp. 421–443. [Google Scholar]
- 11.Fernandes L, et al. Establishing representative no-take areas in the Great Barrier Reef: Large-scale implementation of theory on marine protected areas. Conserv Biol. 2005;19:1733–1744. [Google Scholar]
- 12.Hughes TP, et al. Adaptive management of the Great Barrier Reef and the Grand Canyon World Heritage Areas. Ambio. 2007;7:586–592. doi: 10.1579/0044-7447(2007)36[586:amotgb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Russ GR, et al. Rapid increase in fish numbers follows creation of world's largest marine reserve network. Curr Biol. 2008;18:R514–R515. doi: 10.1016/j.cub.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Mapstone BD, et al. Management strategy evaluation for line fishing in the Great Barrier Reef: Balancing conservation and multi-sector fishery objectives. Fish Res. 2008;94:315–329. [Google Scholar]
- 15.Mapstone BD, et al. The Effects of Line Fishing on the Great Barrier Reef and Evaluations of Alternative Potential Management Strategies. Townsville: CRC Reef Research Centre; 2004. Technical Report No. 52. [Google Scholar]
- 16.Birkeland C, Dayton PK. The importance in fishery management of leaving the big ones. Trends Ecol Evol. 2005;20:356–358. doi: 10.1016/j.tree.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Cappo M, MacNeil A, Stowar M, Doherty P. The Influence of Zoning (Closure to Fishing) on Fish Communities of the Deep Reef Bases of the Southern Great Barrier Reef Marine Park. Part 1. Baited Video Surveys of the Pompeys, Swains, and Capricorn-Bunker Groups off Mackay and Gladstone. Report to the Marine and Tropical Sciences Research Facility. Townsville: Reef and Rainforest Research Centre Limited, Cairns and Australian Institute of Marine Science; 2008. [Google Scholar]
- 18.Williamson DH, Russ GR, Ayling AM. No-take marine reserves increase abundance and biomass of reef fish on inshore fringing reefs of the Great Barrier Reef. Environ Conserv. 2004;31:149–159. [Google Scholar]
- 19.Evans RD, Russ GR. Larger biomass of targeted reef fish in no-take marine reserves on the Great Barrier Reef, Australia. Aquatic Conservation. 2004;14:505–519. [Google Scholar]
- 20.Ayling AM, Choat JH. Abundance Patterns of Reef Sharks and Predatory Fishes on Differently Zoned Reefs in the Offshore Townsville Region. Townsville: Great Barrier Reef Marine Park Authority; 2008. Research Publication No. 91. [Google Scholar]
- 21.Robbins WD, Hisano M, Connolly SR, Choat JH. Ongoing collapse of coral-reef shark populations. Curr Biol. 2006;16:2314–2319. doi: 10.1016/j.cub.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Heupel M, et al. Effects of fishing on tropical reef associated shark populations on the Great Barrier Reef. Fish Res. 2009;95:350–361. [Google Scholar]
- 23.Guidetti P. Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol Appl. 2006;16:963–976. doi: 10.1890/1051-0761(2006)016[0963:mrrlpi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Jones GP, et al. Larval retention and connectivity among populations of corals and reef fishes: History, advances and challenges. Coral Reefs. 2007;28:307–325. [Google Scholar]
- 25.Almany GR, et al. Connectivity, biodiversity con-servation and the design of marine reserve networks for coral reefs. Coral Reefs. 2009;28:339–351. [Google Scholar]
- 26.Zeller DC, Russ GR. Marine reserves: Patterns of adult movement of the coral trout Plectropomus leopardus (Serranidae) Can J Fish Aquat Sci. 1998;55:917–924. [Google Scholar]
- 27.Zeller D, Stoute SL, Russ GR. Movements of reef fishes across marine reserve boundaries: Effects of manipulating a density gradient. Mar Ecol Prog Ser. 2003;254:269–280. [Google Scholar]
- 28.Davies CR. Inter-Reef Movement of the Common Coral Trout Plectropomus leopardus. Townsville: Great Barrier Reef Marine Park Authority; 2000. Research Publication No. 61. [Google Scholar]
- 29.Jones GP, Milicich MJ, Emslie MJ, Lunow C. Self-recruitment in a coral reef fish population. Nature. 1999;402:802–804. [Google Scholar]
- 30.Jones GP, Planes S, Thorrold SR. Coral reef fish larvae settle close to home. Curr Biol. 2005;15:1314–1318. doi: 10.1016/j.cub.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 31.Sweatman H. No-take reserves protect coral reefs from predatory starfish. Curr Biol. 2008;18:R598–R599. doi: 10.1016/j.cub.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 32.Graham NAJ, Evans RD, Russ GR. The effects of marine reserve protection on the trophic relationships of reef fishes on the Great Barrier Reef. Environ Conserv. 2003;30:200–208. [Google Scholar]
- 33.Pitcher R, Venables B, Browne M, Doherty P, De'ath G. Indicators of Protection Levels for Seabed Habitats, Species and Assemblages on the Continental Shelf of the Great Barrier Reef World Heritage Area. Report to the Marine and Tropical Sciences Research Facility. Cairns: Reef and Rainforest Research Centre Limited; 2007. [Google Scholar]
- 34.Poiner IR, et al. The environmental effects of prawn trawling in the far northern section of the Great Barrier Reef Marine Park: 1991-1996. Cleveland, QLD: CSIRO and Queensland Department of Primary Industries; 1998. Final Report to GBRMPA and FRDC. [Google Scholar]
- 35.Pitcher CR, et al. Recovery of seabed habitat from the impact of prawn trawling in the Far Northern Section of the Great Barrier Reef Marine Park. Cleveland, QLD: CSIRO; 2008. [Google Scholar]
- 36.Coles R, Grech A, Dew K, Zeller B, McKenzie L. A Preliminary Report on the Adequacy of Protection Provided to Species and Benthic Habitats in the East Coast Otter Trawl Fishery by the Current System of Closures. Brisbane: Department of Primary Industries and Fisheries; 2008. [Google Scholar]
- 37.Stowar M, et al. Influence of Zoning on Midshelf Shoals from the Southern Great Barrier Reef. Report to the Marine and Tropical Sciences Research Facility. Cairns: Reef and Rainforest Research Centre Limited; 2008. [Google Scholar]
- 38.Speare P, Stowar M, Johansson C. Temporal Monitoring of Northern Shoals off Cardwell and Townsville. Report to the Marine and Tropical Sciences Research Facility. Cairns: Reef and Rainforest Research Centre Limited; 2008. [Google Scholar]
- 39.Marsh H, Eros C, Corkeron P, Breen B. A conservation strategy for dugongs: Implications of Australian research. Mar Freshw Res. 1999;50:979–990. [Google Scholar]
- 40.Marsh H, De'ath G, Gribble N, Lane B. Historical marine population estimates: Triggers or targets for conservation? The dugong case study. Ecol Appl. 2005;15:481–492. [Google Scholar]
- 41.Sheppard J, Preen AR, Marsh H, Lawler IR, Jones RE. Movement heterogeneity of dugongs, Dugong dugon (Müller) over large spatial scales. J Exp Mar Biol Ecol. 2007;334:64–83. [Google Scholar]
- 42.McDonald B. PhD thesis. Townsville: James Cook University; 2006. Population genetics of dugongs around Australia; Implications for contemporary management. [Google Scholar]
- 43.Pollock K, Marsh H, Lawler I, Alldredge M. Modelling availability and perception processes for strip and line transects: An application to dugong aerial surveys. J Wildl Manage. 2006;70:255–262. [Google Scholar]
- 44.Marsh H. Evaluating management initiatives aimed at reducing the mortality of dugongs in gill and mesh nets in the Great Barrier Reef World Heritage Area. Mar Mamm Sci. 2000;16:684–694. [Google Scholar]
- 45.Dobbs K, et al. Incorporating dugong habitats into the marine protected area design for the Great Barrier Reef Marine Park, Queensland, Australia. Ocean Coast Manage. 2008;51:368–375. [Google Scholar]
- 46.Grech A, Marsh H. Rapid assessment of risks to a mobile marine mammal in an ecosystem-scale marine protected area. Conserv Biol. 2008;22:711–720. doi: 10.1111/j.1523-1739.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- 47.Grech A, Marsh H, Coles R. A spatial assessment of the risk to a mobile marine mammal from bycatch. Aquatic Conservation. 2008;18:1127–1139. [Google Scholar]
- 48.Dobbs K, et al. Incorporating marine turtle habitats into the marine protected area design for the Great Barrier Reef Marine Park. Pac Conserv Biol. 2007;13:293–302. [Google Scholar]
- 49.Dryden J, Grech A, Moloney J, Hamann M. Rezoning of the Great Barrier Reef World Heritage Area: Does it afford greater protection for marine turtles? Wildl Res. 2008;35:477–485. [Google Scholar]
- 50.Access Economics Pty Ltd. Measuring the Economic and Financial Value of the Great Barrier Reef Marine Park. Townsville: Great Barrier Reef Marine Park Authority; 2006. [Google Scholar]
- 51.Access Economics Pty Ltd . Measuring the Economic and Financial Value of the Great Barrier Reef Marine Park, 2005-06. Townsville: Great Barrier Reef Marine Park Authority; 2007. [Google Scholar]
- 52.Access Economics Pty Ltd . Economic Contribution of the GBRMP, 2006-07. Townsville: Great Barrier Reef Marine Park Authority; 2008. [Google Scholar]
- 53.Department of the Environment Water Heritage and the Arts . Annual Report 2007-08. Canberra: Department of the Environment, Water, Heritage and the Arts; 2008. [Google Scholar]
- 54.Department of the Environment and Heritage . Annual Report 2004-05. Canberra: Department of the Environment and Heritage; 2005. [Google Scholar]
- 55.Australian Fisheries Management Authority . Environment Update 21. Canberra: Australian Fisheries Management Authority; 2004. [Google Scholar]
- 56.Department of the Environment and Heritage . Review of the Great Barrier Reef Marine Park Act 1975, Review Panel Report. Canberra, Australia: Department of the Environment and Heritage; 2006. [Google Scholar]
- 57.Hand T. Submitted to the Australian Parliament. Townsville: P. D. P. Australia Pty Ltd. and Great Barrier Reef Marine Park Authority; 2003. An economic and social evaluation of implementing the representative areas program by rezoning the Great Barrier Reef Marine Park: Report on the revised zoning plan. [Google Scholar]
- 58.Great Barrier Reef Marine Park Authority . Submitted to the Australian Parliament. Townsville: Great Barrier Reef Marine Park Authority; 2003. [accessed July 2009]. Explanatory statement: Great Barrier Reef Zoning Plan 2003. Available at http://kurrawa.gbrmpa.gov.au/corp_site/management/zoning/rap/rap/pdf/ES_25-11-03.pdf. [Google Scholar]
- 59.Bureau of Rural Sciences . Implementing the Representative Areas Program in the Great Barrier Reef Marine Park - BRS Assessment of Potential Social Impact on Commercial Fishing and Associated Communities. Canberra: Bureau of Rural Sciences; 2003. [Google Scholar]
- 60.Minnegal M, Dwyer PD. Mixed messages: Buying back Australia's fishing industry. Mar Policy. 2008;32:1063–1071. [Google Scholar]
- 61.Fisheries Economics Research Management Pty Ltd . A Review of the Business Exit (Licence Buyout) Assistance Component of the Great Barrier Reef Marine Park Structural Adjustment Package, Final Report. Canberra: Department of Environment and Water Resources; 2007. [Google Scholar]
- 62.Leslie HM. Synthesis of marine conservation planning approaches. Conserv Biol. 2005;19:1701–1713. [Google Scholar]
- 63.Mumby PJ, et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science. 2006;311:98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 64.McClanahan TR, Muthiga NA, Maina J, Kamukuru AT, Yahya SAS. Changes in northern Tanzania coral reefs during a period of increased fisheries management and climatic disturbance. Aquatic Conservation. 2009;89:161–182. [Google Scholar]
- 65.Great Barrier Reef Marine Park Authority . Annual Report 2004–2005. Townsville: Great Barrier Reef Marine Park Authority; 2005. [Google Scholar]
- 66.Great Barrier Reef Marine Park Authority . Annual Report 2005–2006. Townsville: Great Barrier Reef Marine Park Authority; 2006. [Google Scholar]
- 67.Great Barrier Reef Marine Park Authority . Annual Report 2006–2007. Townsville: Great Barrier Reef Marine Park Authority; 2007. [Google Scholar]
- 68.Great Barrier Reef Marine Park Authority . Annual Report 2002–2003. Townsville: Great Barrier Reef Marine Park Authority; 2003. [Google Scholar]
- 69.Great Barrier Reef Marine Park Authority . Annual Report 2003–2004. Townsville: Great Barrier Reef Marine Park Authority; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.