Abstract
Emissions of a broad range of greenhouse gases of varying lifetimes contribute to global climate change. Carbon dioxide displays exceptional persistence that renders its warming nearly irreversible for more than 1,000 y. Here we show that the warming due to non-CO2 greenhouse gases, although not irreversible, persists notably longer than the anthropogenic changes in the greenhouse gas concentrations themselves. We explore why the persistence of warming depends not just on the decay of a given greenhouse gas concentration but also on climate system behavior, particularly the timescales of heat transfer linked to the ocean. For carbon dioxide and methane, nonlinear optical absorption effects also play a smaller but significant role in prolonging the warming. In effect, dampening factors that slow temperature increase during periods of increasing concentration also slow the loss of energy from the Earth’s climate system if radiative forcing is reduced. Approaches to climate change mitigation options through reduction of greenhouse gas or aerosol emissions therefore should not be expected to decrease climate change impacts as rapidly as the gas or aerosol lifetime, even for short-lived species; such actions can have their greatest effect if undertaken soon enough to avoid transfer of heat to the deep ocean.
Keywords: atmosphere, dynamics, radiation
Carbon dioxide, methane, nitrous oxide, and other greenhouse gases increased over the course of the 20th century due to human activities. The human-caused increases in these gases are the primary forcing that accounts for much of the global warming of the past fifty years, with carbon dioxide being the most important single radiative forcing agent (1). Recent studies have shown that the human-caused warming linked to carbon dioxide is nearly irreversible for more than 1,000 y, even if emissions of the gas were to cease entirely (2–5). The importance of the ocean in taking up heat and slowing the response of the climate system to radiative forcing changes has been noted in many studies (e.g., refs. 6 and 7). The key role of the ocean’s thermal lag has also been highlighted by recent approaches to proposed metrics for comparing the warming of different greenhouse gases (8, 9). Among the observations attesting to the importance of these effects are those showing that climate changes caused by transient volcanic aerosol loading persist for more than 5 y (7, 10), and a portion can be expected to last more than a century in the ocean (11–13); clearly these signals persist far longer than the radiative forcing decay timescale of about 12–18 mo for the volcanic aerosol (14, 15). Thus the observed climate response to volcanic events suggests that some persistence of climate change should be expected even for quite short-lived radiative forcing perturbations. It follows that the climate changes induced by short-lived anthropogenic greenhouse gases such as methane or hydrofluorocarbons (HFCs) may not decrease in concert with decreases in concentration if the anthropogenic emissions of those gases were to be eliminated. In this paper, our primary goal is to show how different processes and timescales contribute to determining how long the climate changes due to various greenhouse gases could be expected to remain if anthropogenic emissions were to cease.
Advances in modeling have led to improved Atmosphere-Ocean General Circulation Models (AOGCMs) as well as to Earth Models of Intermediate Complexity (EMICs). Although a detailed representation of the climate system changes on regional scales can only be provided by AOGCMs, the simpler EMICs have been shown to be useful, particularly to examine phenomena on a global average basis. In this work, we use the Bern 2.5CC EMIC (see Materials and Methods and SI Text), which has been extensively intercompared to other EMICs and to complex AOGCMs (3, 4). It should be noted that, although the Bern 2.5CC EMIC includes a representation of the surface and deep ocean, it does not include processes such as ice sheet losses or changes in the Earth’s albedo linked to evolution of vegetation. However, it is noteworthy that this EMIC, although parameterized and simplified, includes 14 levels in the ocean; further, its global ocean heat uptake and climate sensitivity are near the mean of available complex models, and its computed timescales for uptake of tracers into the ocean have been shown to compare well to observations (16). A recent study (17) explored the response of one AOGCM to a sudden stop of all forcing, and the Bern 2.5CC EMIC shows broad similarities in computed warming to that study (see Fig. S1), although there are also differences in detail. The climate sensitivity (which characterizes the long-term absolute warming response to a doubling of atmospheric carbon dioxide concentrations) is 3 °C for the model used here. Our results should be considered illustrative and exploratory rather than fully quantitative given the limitations of the EMIC and the uncertainties in climate sensitivity.
Results
One Illustrative Scenario to 2050.
In the absence of mitigation policy, concentrations of the three major greenhouse gases, carbon dioxide, methane, and nitrous oxide can be expected to increase in this century. If emissions were to cease, anthropogenic CO2 would be removed from the atmosphere by a series of processes operating at different timescales (18). Over timescales of decades, both the land and upper ocean are important sinks. Over centuries to millennia, deep oceanic processes become dominant and are controlled by relatively well-understood physics and chemistry that provide broad consistency across models (see, for example, Fig. S2 showing how the removal of a pulse of carbon compares across a range of models). About 20% of the emitted anthropogenic carbon remains in the atmosphere for many thousands of years (with a range across models including the Bern 2.5CC model being about 19 ± 4% at year 1000 after a pulse emission; see ref. 19), until much slower weathering processes affect the carbonate balance in the ocean (e.g., ref. 18). Models with stronger carbon/climate feedbacks than the one considered here could display larger and more persistent warmings due to both CO2 and non-CO2 greenhouse gases, through reduced land and ocean uptake of carbon in a warmer world. Here our focus is not on the strength of carbon/climate feedbacks that can lead to differences in the carbon concentration decay, but rather on the factors that control the climate response to a given decay. The removal processes of other anthropogenic gases including methane and nitrous oxide are much more simply described by exponential decay constants of about 10 and 114 y, respectively (1), due mainly to known chemical reactions in the atmosphere. In this illustrative study, we do not include the feedback of changes in methane upon its own lifetime (20). We also do not account for potential interactions between CO2 and other gases, such as the production of carbon dioxide from methane oxidation (21), or changes to the carbon cycle through, e.g., methane/ozone chemistry (22).
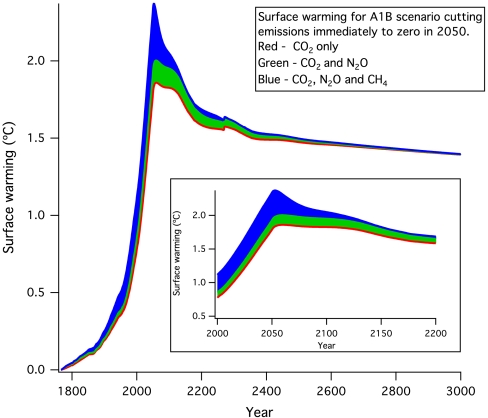
Fig. 1 shows the computed future global warming contributions for carbon dioxide, methane, and nitrous oxide for a midrange scenario (23) of projected future anthropogenic emissions of these gases to 2050. Radiative forcings for all three of these gases, and their spectral overlaps, are represented in this work using the expressions assessed in ref. 24. In 2050, the anthropogenic emissions are stopped entirely for illustration purposes. The figure shows nearly irreversible warming for at least 1,000 y due to the imposed carbon dioxide increases, as in previous work. All published studies to date, which use multiple EMICs and one AOGCM, show largely irreversible warming due to future carbon dioxide increases (to within about ± 0.5 °C) on a timescale of at least 1,000 y (3–5, 25, 26).
Fig. 1.
Computed surface warming obtained in the Bern 2.5CC model due to CO2, CH4, and N2O emission increases to 2050 following a “midrange” scenario (called A1B; see ref. 23) followed by zero anthropogenic emissions thereafter. The gases are changed sequentially in this calculation in order to explicitly separate the contributions of each. The bumps shown in the calculated warming are due to changes in ocean circulation, as in previous studies (5, 26, 39). The main panel shows the contributions to warming due to CO2, N2O, and CH4. The inset shows an expanded view of the warming from year 2000 to 2200.
Fig. 1 shows that the calculated future warmings due to anthropogenic CH4 and N2O also persist notably longer than the lifetimes of these gases. The figure illustrates that emissions of key non-CO2 greenhouse gases such as CH4 or N2O could lead to warming that both temporarily exceeds a given stabilization target (e.g., 2 °C as proposed by the G8 group of nations and in the Copenhagen goals) and remains present longer than the gas lifetimes even if emissions were to cease. A number of recent studies have underscored the important point that reductions of non-CO2 greenhouse gas emissions are an approach that can indeed reverse some past climate changes (e.g., ref. 27). Understanding how quickly such reversal could happen and why is an important policy and science question. Fig. 1 implies that the use of policy measures to reduce emissions of short-lived gases will be less effective as a rapid climate mitigation strategy than would be thought if based only upon the gas lifetime.
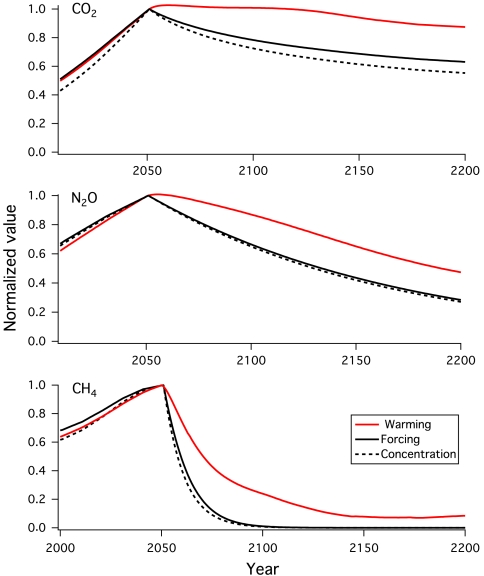
Fig. 2 illustrates the factors influencing the warming contributions of each gas for the test case in Fig. 1 in more detail, by showing normalized values (relative to one at their peaks) of the warming along with the radiative forcings and concentrations of CO2, N2O, and CH4. For example, about two-thirds of the calculated warming due to N2O is still present 114 y (one atmospheric lifetime) after emissions are halted, despite the fact that its excess concentration and associated radiative forcing at that time has dropped to about one-third of the peak value. Two factors contribute to the differences between decreases in concentrations of greenhouse gases and persistence of the resulting warming, discussed further below: (i) Radiative forcing may not simply follow concentration because of optical depth effects (for CO2 and CH4), and (ii) warming may not match decreases in radiative forcing because of climate inertia, particularly due to the ocean.
Fig. 2.
Relative changes in CO2 (Top), N2O (Middle) and CH4 (Bottom) concentrations, radiative forcings, and surface warmings, normalized to one at their peak values, for the A1B scenario to 2050, followed by an abrupt cessation of emissions (as in Fig. 1).
Climate Change Persistence: (I) Optical Depth Effects.
The physics of absorption spectroscopy dictate that radiative forcing will be linearly related to concentration changes for those gases whose atmospheric optical depth is thin, whereas nonlinear forcing occurs for thicker optical depths (24). Because CO2 absorption is not optically thin, the fractional increase in radiative forcing per parts per million by volume of CO2 increase becomes smaller for larger CO2 concentrations. Fig. 2 shows how this factor acts in the reverse sense during relaxation from a peak, enhancing the CO2 radiative forcing relative to the calculated concentration decrease. For example, for a 535 ppmv peak (as in the calculation in Fig. 1), the excess CO2 concentration above the preindustrial value of 278 ppmv remaining in the year 2200 is about 55% of the peak value, whereas the fractional radiative forcing remaining in that year is about 63% of the peak value (i.e., the relative change in forcing is greater than the relative change in concentration by about 14%).
Nonlinear optical effects grow as the concentration change grows. For example, for a peak of CO2 of 1,200 ppmv in the 21st century followed by a stop of emissions, the relative change in forcing compared to the relative change in concentration in the year 3000 is about 30%. Thus nonlinear spectroscopy, although not the dominant factor, contributes to rendering the warming from CO2 nearly irreversible, especially for larger values of peak concentration. Methane also displays significant nonlinearities in its radiative absorption, whereas these effects are very small for N2O (Fig. 2).
HFCs and perfluorocarbons absorb in the atmospheric window and are optically thin over the full range of plausible future concentrations; therefore, these gases display no nonlinear optical absorption. We find that nonlinear spectral effects exceed 10% contributions to the persistence of warming only for carbon dioxide and methane, and not for any of the other anthropogenic greenhouse gases.
Climate Change Persistence: (II) Physical Processes.
Climate change is linked to a range of phenomena displaying varying timescales (see, e.g., ref. 28). The atmosphere, clouds, and water vapor respond within a few months following a change in radiative forcing (29). The transfer of heat from the atmosphere to the ocean’s mixed layer (top 100 m or so) is thought to occur on timescales on the order of a decade or less (30), whereas multiple centuries are required to warm or cool the deep ocean (31), and changes in the great ice sheets and vegetation coverage may occur over many thousands of years (4).
Much of the energy that has been added to the Earth’s climate system in the 20th century through net radiative forcing has been taken up by the ocean (32). However, a large fraction of the energy that could be trapped due to the impact of radiative forcing has not been added to the climate system at all but rather has been lost to space, because the Earth has already warmed and therefore must radiate more energy. Observations and models both suggest that about two-thirds of the net radiative forcing (warming by anthropogenic greenhouse gases less cooling by stratospheric and tropospheric aerosols) of the past half century has been radiated to space, while about one-third has been absorbed by the ocean (33–35).
If anthropogenic radiative forcing were to be stabilized, atmospheric warming would continue for many centuries as the components of the climate system reach a balance. On the other hand, if such forcing were to abruptly cease, some energy would be expected to be lost rapidly through radiation to space, while some would be lost more slowly as the coupled ocean mixed layer/atmosphere system adjusts. Some of the energy loss would occur over centuries depending mainly upon the amount of heat that has been stored in the deep ocean. These processes are linked both to transient climate response and ocean heat uptake, and the uncertainties in these parameters are of order ± 50% between current state-of-the-art AOGCMs (4, 35). Ocean heat uptake and changes in ocean circulation are not well characterized by observations and contribute to the differences in future climate responses between models (3, 4, 31). Carbon cycle processes that may slowly release carbon back to the atmosphere in a warming world (e.g., through changes in forest cover and soil carbon dynamics) also affect the long-term behavior of warming and differ from model to model (3, 36).
Understanding the warming persistence from various forcing agents with different lifetimes and radiative forcing histories is aided by considering energy balance for a time horizon long enough for the Earth to return to its original temperature. The energy balance equation can be written as
| [1] |
where F is the added energy due to anthropogenic radiative forcing, N is the net heat flux, and λΔTsurface is the energy radiated to space by a warmer Earth. Earth loses energy via a surface and atmosphere that are warmer than their equilibrium values. The quantity λ expresses how much energy is lost per unit rise in temperature. In the long term, it is the inverse of the climate sensitivity because, at a new steady state, N becomes zero and ΔTsurface = F/λ.
If emissions are stopped and treturn is a time in the future when the Earth has returned to its initial temperature (including the average temperature of the oceans), then
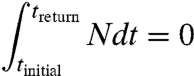 |
[2] |
Integrating both sides of Eq. 1 for this time interval and making the additional assumption that the radiative response is independent of time and the rate of warming over this timescale (i.e., λ is approximately constant, or uncorrelated with the forcing), then
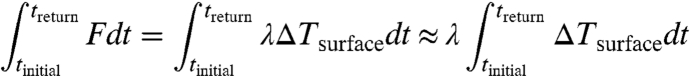 |
[3] |
The left-hand integral is just the energy trapped by the radiative forcing. Eq. 3 states that the time-integrated warming is approximately proportional to the integrated forcing, because the only way the Earth can get rid of trapped energy is to radiate it to outer space. Ocean heat uptake delays and spreads the warming out in time, and also defines the warming that must continue after emissions cease, i.e., the amount of time-integrated warming that must eventually occur before the Earth returns to its original temperature. Consequences for climate change, ecosystems, and people can depend on the time history: A long, modest increase in temperature is likely to be less harmful than a short pulse of extreme warming.
In practice, Eq. 3 is less useful for gases such as CO2 and SF6 that have such long lifetimes that the time horizon for their forcing to decay to zero and the Earth to return to an equilibrium temperature is many millennia. In those cases, a simplified way to view future warming persistence is that emissions of CO2 and a handful of other extremely long-lived gases imply warming that is essentially irreversible on human timescales without geoengineering or active sequestration. All shorter-lived gases and aerosols imply a transient warming whose time integral is approximately determined simply by the time integral of the forcing and the equilibrium climate sensitivity.
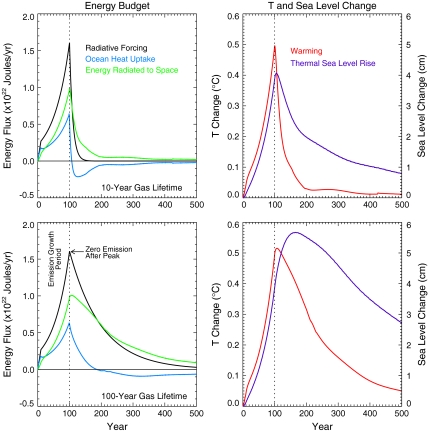
Fig. 3 shows how the energy budget of the earth–atmosphere system in the Bern 2.5CC EMIC would behave in response to increases in radiative forcing over 100 y followed by a stop of emissions for a greenhouse gas with a 10 or 100 y lifetime. The peak forcing in both cases is 1 W/m2. A linear increase is assumed for the first 10 y followed by a 2% per year increase from that time until year 100. After the peak, the forcing decays with the assumed lifetime. Of particular interest is the behavior of ocean heat uptake (Fig. 3, Left) as well as the atmospheric temperature and sea level rise (Fig. 3, Right). In the case of a gas with a 10-y lifetime, for example, energy is slowly stored in the ocean during the period when concentrations are elevated, and this energy is returned to the atmosphere from the ocean after emissions cease and radiative forcing decays, keeping atmospheric temperatures somewhat elevated for several decades. Elevated temperatures last longer for a gas with a 100-y lifetime because, in this case, radiative forcing and accompanying further ocean heat uptake continue long after emissions cease. As radiative forcing decays further, the energy is ultimately restored from the ocean to the atmosphere.
Fig. 3.
Energy budget terms calculated by the Bern 2.5CC model for an increase in radiative forcing from 0 to 1 W/m2 over 100 y and a subsequent decline in that forcing determined by a 10-y adopted gas lifetime (Upper) or 100-y gas lifetime (Lower). The left panels show instantaneous terms in the energy budget, whereas the right panels present the computed warming and sea level rise due to ocean thermal expansion only. The black lines show radiative forcing, whereas the pale-blue lines show ocean heat uptake. The difference between radiative forcing and ocean heat uptake is also shown as the pale-green lines, and this represents the energy emitted by the Earth–atmosphere system to space in response to the forcing. The modeled atmospheric temperature responses are represented by the red lines in the right panels, which track the instantaneous energy emitted by the Earth–atmosphere system.
Fig. 3 shows that the slow timescale of ocean heat uptake has two important effects. It limits the transfer of energy to the ocean if emissions and radiative forcing occur only for a few decades or a century. However, it also implies that any energy that is added to the ocean remains available to be transferred back to the atmosphere for centuries after cessation of emissions.
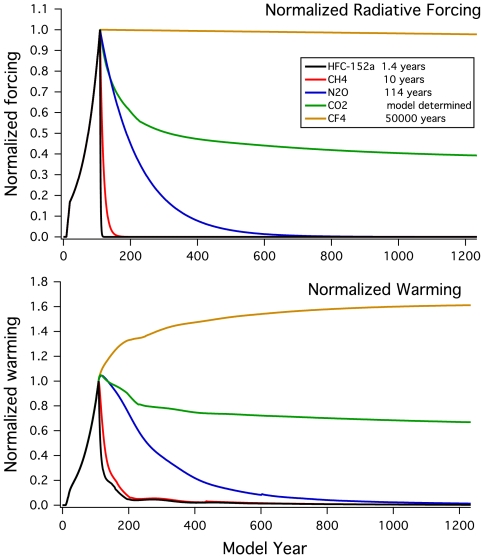
Fig. 4 further illustrates how the computed warmings due to a broader range of specific different greenhouse gases would evolve assuming an idealized 21st century ramp of emissions to 1 W/m2 in 100 y (as in Fig. 3), followed by cessation of emissions in the Bern 2.5CC model. If the rate of radiative forcing were to increase at 2% per year (about the average value observed over the past several decades for CO2), the computed warming or “realized” warming (33) in the Bern 2.5CC model is about 60% of the quasi-equilibrium value, similar to that of the range of models recently assessed (4). Put differently, the climate system response under increasing radiative forcing (even on the timescale of a century) will be smaller than the response would be if the forcing were maintained at a constant level and the system were to largely equilibrate. The smaller response is related to the transient climate response and to the considerations indicated above regarding the partitioning of energy flow between the ocean and loss to space under increasing forcing.
Fig. 4.
Relative changes in radiative forcing (Upper) and warming (Lower) in the Bern 2.5CC model, for the same assumed profile of increasing radiative forcing over 100 y, followed by a stop of emissions as in Fig. 3, for a range of greenhouse gases of varying lifetimes. The gases considered are HFC-152a (1.4-y lifetime), methane (≈10-y lifetime), N2O (114-y lifetime), carbon dioxide (see text), and CF4 (50,000-y lifetime). All quantities are normalized to one when emissions stop, in order to examine relative changes.
The simulations presented in Fig. 4 illustrate the importance of realized warming versus quasi-equilibrium warming. For a gas such as CF4 with a very long lifetime of about 50,000 y, concentrations and forcing remain essentially constant for more than 1,000 y following cessation of emissions (Fig. 4, Upper). But the warming due to CF4’s radiative forcing continues to increase slowly as the ocean and atmosphere adjust over centuries, reaching a quasi-equilibrium atmospheric warming that is about 60% larger than the transient value obtained when emissions stopped in this model for the test case considered here (and this value is approximately the inverse of the realized warming noted above). The same behavior would be expected if, for example, atmospheric concentrations of any gas were to be stabilized but, for shorter-lived gases, stabilization requires continued emission (in contrast to CF4).
Carbon dioxide concentrations display an initial fast decay for several decades in carbon cycle models after cessation of emissions, followed by a much slower subsequent decline (see Fig. S2), but temperatures remain nearly constant throughout as shown in Fig. 4. The above discussion of CF4 illuminates a key reason for this behavior. The near-irreversibility of the CO2-induced warming after emissions cease and concentrations peak is linked mainly to a near balance between concentration changes (which slowly decrease to a value that is about 40% of the peak of excess concentration above preindustrial, see Fig. 4) and the fact that the ratio of quasi-equilibrium to transient warming is about 1.6 in this model (compare the range of about 1.3–2.3 across models in ref. 4). Thus the decrease in CO2 concentration is roughly compensated by the way that the transient warming evolves to a near equilibrium warming (i.e., the warming is realized over time), together with a significant but lesser contribution due to the nonlinear dependence of radiative forcing on CO2 concentration. These long-term changes in both CO2 concentration and warming are robust across a broad range of coupled carbon/climate models (3, 4) and are both linked to the slow timescales of transport in the ocean.
For forcing agents shown in Fig. 4 with lifetimes of years to centuries, some forcing due to these gases will continue even as concentrations decay, leading to some persistence of the induced warming. Fig. 4 illustrates the persistence for HFC-152a, CH4, and N2O, and Fig. S3 shows the behavior calculated in the Bern 2.5CC model for a range of halocarbons with lifetimes ranging from years to centuries. An important qualitative conclusion of Fig. 4 is that the warming induced by even a very short-lived gas such as HFC-152a can persist longer than the gas itself and its associated forcing (see also Figs. 3 and 4). The extent to which warming is prolonged is linked to the competition between decay of the radiative forcing and ocean heat uptake and will also depend on the carbon cycle feedback; the carbon cycle feedback and ocean heat uptake will differ somewhat among models. Persistence of the induced climate change should be expected to be larger for gases with lifetimes long enough to transfer more heat to the ocean, i.e., several decades to centuries or more, and much smaller for gases with short lifetimes of a year to a decade. Similarly, the persistence of the warming will be greater if radiative forcing is maintained over longer periods through sustained anthropogenic emissions (17, 27); i.e., the longer humans continue to emit greenhouse gases, the longer the climate memory of that emission will become, even for very short-lived substances, due to ocean thermal inertia (9). This paper focuses on emissions over a century.
Discussion
An accurate assessment of the benefits of greenhouse gas mitigation options requires consideration of climate change impacts of these compounds, including not only their magnitude but also their persistence. Here we have probed how the duration of the warming effects of various greenhouse gases depends not just on the lifetimes of the gases as is sometimes emphasized in reporting, including in highly respected media (e.g., ref. 37), but is also strongly dependent upon climate system timescales. The analysis provided here is intended in part as an aid to communication on this important science/policy point. The persistence of the warming perturbation due to various gases can be described as a reflection of how the time history of radiative forcing interacts with ocean heat uptake. Maintaining a forcing for a longer period of time transfers more heat to the deep relative to the shallow ocean, with a correspondingly longer timescale for release of energy if emissions were to be halted (17), so that the net effect on climate change depends upon both ocean physics and how long the gas is retained in the atmosphere. Carbon dioxide and perfluorocarbons represent special cases, primarily because zero emissions do not lead to zero concentrations for thousands of years. Nonlinear optical depth effects are also significant for carbon dioxide and methane.
We have shown how ocean heat transport and its response to climate change is crucial to evaluation of the speed with which near-term warming would abate if emissions of various gases were to be reduced, including methane, nitrous oxide, and various hydrofluorocarbons; the same considerations apply to absorbing aerosols including black carbon. This study has explained why the slow timescales of the ocean imply that actions to mitigate the climate impacts of these warming agents would be most effective if undertaken sooner; conversely such actions would become less effective the longer the radiative forcing is maintained. Further study of these effects using a broader range of models is needed to better inform policy discussions of the short- and long-term climate mitigation potential of different greenhouse gases and the implications of trading between them.
Materials and Methods
The EMIC used in this study is the Bern 2.5CC EMIC described in refs. 3 and 38; the EMIC is compared to other models in refs. 3 and 4. This model is a coupled climate–carbon cycle model of intermediate complexity that consists of a zonally averaged dynamic ocean model, a one-layer atmospheric energy-moisture balance model, and interactive representations of the marine and terrestrial carbon cycles. The model includes 14 ocean levels and 4 ocean basins. A test case showing how carbon dioxide concentrations would change if anthropogenic emissions were to stop, along with the partitioning of carbon into the land and ocean reservoirs, is illustrated in the SI Text and shows good agreement across carbon cycle models especially on longer timescales of a century or more.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006282107/-/DCSupplemental.
References
- 1.Forster P, et al. Changes in atmospheric constituents and in radiative forcing. In: Solomon S, et al., editors. Climate Change 2007: The Physical Science Basis. Cambridge, UK: Cambridge Univ Press; 2007. pp. 747–845. [Google Scholar]
- 2.Matthews HD, Caldeira K. Stabilizing climate requires near-zero emissions. Geophys Res Lett. 2008;35:L04705. 10.1029/2007GL032388. [Google Scholar]
- 3.Plattner GK, et al. Long-term climate commitments projected with climate-carbon cycle models. J Climate. 2008;21:2721–2751. [Google Scholar]
- 4.Meehl GA, et al. Global climate projections. In: Solomon S, et al., editors. Climate Change 2007: The Physical Science Basis. Cambridge, UK: Cambridge Univ Press; 2007. pp. 747–845. [Google Scholar]
- 5.Solomon S, Plattner G-K, Knutti R, Friedlingstein P. Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci USA. 2009;106:1704–1709. doi: 10.1073/pnas.0812721106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider SH, Thompson S. Atmospheric CO2 and climate: The importance of the transient response. J Geophys Res. 1981;86:3135–3147. [Google Scholar]
- 7.Wigley ML, Ammann CM, Santer BD, Raper SCB. Effect of climate sensitivity on the response to volcanic forcing. J Geophys Res. 2005;110:D09107. [Google Scholar]
- 8.Shine KP, Fuglestvedt JS, Hailemariam K, Stuber N. Alternatives to the global warming potential for comparing climate impacts of emissions of greenhouse gases. Climatic Change. 2005;68:281–302. [Google Scholar]
- 9.Shine KP, Berntsen TK, Fuglestvedt JS, Skeie RB, Stuber N. Comparing the climate effect of emissions of short- and long-lived climate agents. Philos Trans Roy Soc A. 2007;365:1903–1914. doi: 10.1098/rsta.2007.2050. [DOI] [PubMed] [Google Scholar]
- 10.Thompson DWJ, Wallace JM, Jones PD, Kennedy JJ. Identifying signatures of natural climate variability in time series of global-mean surface temperature: methodology and insights. J Climate. 2009;22:6120–6141. [Google Scholar]
- 11.Gleckler PJ, et al. Krakatoa’s signature persists in the ocean. Nature. 2006;439:675. doi: 10.1038/439675a. [DOI] [PubMed] [Google Scholar]
- 12.Delworth TL, Ramaswamy V, Stenchikov GL. The impact of aerosols on simulated ocean temperature and heat content in the 20th century. Geophys Res Lett. 2005;32:L24709. [Google Scholar]
- 13.Church JA, White NJ, Arblaster JM. Significant decadal-scale impact of volcanic eruptions on sea level and ocean heat content. Nature. 2005;438:74–77. doi: 10.1038/nature04237. [DOI] [PubMed] [Google Scholar]
- 14.Deshler T. A review of global stratospheric aerosol: Measurements, importance, life cycle, and local stratospheric aerosol. Atmos Res. 2008;90:223–232. [Google Scholar]
- 15.Robock A. Volcanic eruptions and climate. Rev Geophys. 2000;38:191–219. [Google Scholar]
- 16.Matsumoto K, et al. Evaluation of ocean carbon cycle models with data-based metrics. Geophys Res Lett. 2004;31:L07303. [Google Scholar]
- 17.Held IM, et al. Probing the fast and slow components of global warming by returning abruptly to pre-industrial forcing. J Climate. 2010;23:2418–2427. [Google Scholar]
- 18.Archer D, Brovkin V. The millennial atmospheric lifetime of anthropogenic CO2. Climatic Change. 2008;90:283–297. [Google Scholar]
- 19.Cao L, et al. The role of ocean transport in the uptake of anthropogenic CO2. Biogeosciences. 2009;6:375–390. [Google Scholar]
- 20.Prather MJ. Time scales in atmospheric chemistry: Theory, GWPs for CH4 and CO, and runaway growth. Geophys Res Lett. 1996;23:2597–2600. [Google Scholar]
- 21.Boucher O, Friedlingstein P, Collins B, Shine KP. The indirect global warming potential and global temperature change potential due to methane oxidation. Environ Res Lett. 2009;4:044007. [Google Scholar]
- 22.Sitch S, Cox PM, Collins WJ, Huntingford C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature. 2007;448:791–794. doi: 10.1038/nature06059. [DOI] [PubMed] [Google Scholar]
- 23.Nakicenovic N, et al. Special Report on Emissions Scenarios: A Special Report of Working Group III of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2000. p. 599. [Google Scholar]
- 24.Ramaswamy V, et al. In: Radiative Forcing of Climate Change, in Climate Change 2001: The Scientific Basis. Houghton J, et al., editors. Cambridge, UK: Cambridge Univ Press; 2001. pp. 349–416. [Google Scholar]
- 25.Lowe JS, et al. How difficult is it to recover from dangerous levels of global warming? Environ Res Lett. 2009;4:1–9. [Google Scholar]
- 26.Eby M, et al. Lifetime of anthropogenic climate change: Millennial time-scales of potential CO2 and surface temperature perturbations. J Climate. 2009;22:2501–2511. [Google Scholar]
- 27.Froehlicher TL, Joos F. Reversible and irreversible impacts of greenhouse gas emissions in multi-century projections with the NCAR global coupled carbon cycle-climate model. Clim Dynam. 2010 10.1007/s00382-009-0727-0. [Google Scholar]
- 28.Knutti R, Kraehenmann S, Frame DJ, Allen MR. Comment on heat capacity, time constant, and sensitivity of Earth’s climate system by S. E. Schwartz. J Geophys Res. 2008;113:D15103. 10.1029/2007JD009473. [Google Scholar]
- 29.Gregory G, Webb M. Tropospheric adjustment induces a cloud component in CO2 forcing. J Climate. 2008;21:58–71. [Google Scholar]
- 30.Hansen J, et al. Climate Processes and Climate Sensitivity. Am Geophysical Union; 1984. Climate sensitivity: Analysis of feedback mechanisms; pp. 130–163. (Geophysical Monograph 29). [Google Scholar]
- 31.Stouffer RJ. Time scales of climate response. J Climate. 2004;17:209–214. [Google Scholar]
- 32.Levitus S, et al. Anthropogenic warming of Earth’s climate system. Science. 2001;292:267–270. doi: 10.1126/science.1058154. [DOI] [PubMed] [Google Scholar]
- 33.Hansen J, et al. Earth’s energy imbalance: Confirmation and implications. Science. 2005;308:1431–1435. doi: 10.1126/science.1110252. [DOI] [PubMed] [Google Scholar]
- 34.Murphy DM, et al. An observationally based energy balance for the Earth since 1950. J Geophys Res. 114:D17107. [Google Scholar]
- 35.Gregory JM, Forster PM. Transient climate response estimated from radiative forcing and observed temperature change. J Geophys Res. 2008;113:D23105. [Google Scholar]
- 36.Friedlingstein P, et al. Climate-carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J Climate. 2006;19:3337–3353. [Google Scholar]
- 37.Kintisch E. New push focuses on quick ways to curb global warming. Science. 2009;324:323. doi: 10.1126/science.324.5925.323a. [DOI] [PubMed] [Google Scholar]
- 38.Joos F, Plattner G-K, Stocker TF, Marchal O, Schmittner A. Global warming and marine carbon cycle feedbacks on future atmospheric CO2. Science. 1999;284:464–467. doi: 10.1126/science.284.5413.464. [DOI] [PubMed] [Google Scholar]
- 39.Knutti R, Stocker TF. Limited predictability of the future thermohaline circulation close to an instability threshold. J Climate. 2002;15:179–186. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.