Abstract
Cilia are essential for normal organ function and developmental patterning, but their role in injury and regeneration responses is unknown. To probe the role of cilia in injury, we analyzed the function of foxj1, a transcriptional regulator of cilia genes, in response to tissue damage and renal cyst formation. Zebrafish foxj1a, but not foxj1b, was rapidly induced in response to epithelial distension and stretch, kidney cyst formation, acute kidney injury by gentamicin, and crush injury in spinal cord cells. Obstruction-induced up-regulation of foxj1a was not inhibited by cycloheximide, identifying foxj1a as a primary response gene to epithelial injury. Foxj1 was also dramatically up-regulated in murine cystic kidney disease epithelia [jck/jck (nek8) and Ift88Tg737Rpw−/−] as well as in response to kidney ischemia-reperfusion injury. Obstruction of the zebrafish pronephric tubule caused a rapid increase in cilia beat rate that correlated tightly with expanded tubule diameter and epithelial stretch. Zebrafish foxj1a was specifically required for cilia motility. Enhanced foxj1a expression in obstructed tubules induced cilia motility target genes efhc1, tektin-1, and dnahc9. foxj1a-deficient embryos failed to up-regulate efhc1, tektin-1, and dnahc9 and could not maintain enhanced cilia beat rates after obstruction, identifying an essential role for foxj1 in modulating cilia function after injury. These studies reveal that activation of a Foxj1 transcriptional network of ciliogenic genes is an evolutionarily conserved response to multiple forms of tissue damage and highlight enhanced cilia function as a previously uncharacterized component of organ homeostasis.
Keywords: pronephros, cystic kidney disease, neural injury, beat rate, ciliogenesis
Cilia are now known to be central organelles in normal organ function. Primary cilia play a major role in developmental patterning, retinal development, and the pathogenesis of many forms of cystic kidney disease (1–5). Motile cilia are essential for normal left–right asymmetry, fertility, and lung function (6). Mutations causing cilia paralysis underlie the human syndrome primary ciliary dyskinesia (6). Some motile cilia are also sensory, suggesting that cilia function can adapt autonomously to environmental signals (7, 8).
Both cilia length and motility are subject to dynamic regulation by physiologic and pathologic stimuli. Fluid flow-induced cAMP and calcium signaling govern cilia length (9, 10). Cilia structure also varies with the cell cycle (11), and can be modified by estrogen hormones (12) and sphingolipids (13). Increased cilia length following kidney ureteral obstruction (14) or acute tubular necrosis (15), and the appearance of new multiciliated epithelial cells with “9+2” motile cilia structure in kidney disease states (16–19), have suggested that ciliogenesis could be part of a tissue regeneration program. jck/jck (nek8) cystic kidney mutant cells show markedly lengthened cilia (20), and Pkhd1 (fibrocystin) mutant rat kidneys develop multiciliated cells (21). Motile cilia beat frequency is also modulated by physiological signals including cAMP, calcium, and intracellular pH (22). Acute increases in cilia length are correlated with increased rates of anterograde intraflagellar transport (IFT) (9), but how signals generated by injury or by tissue regeneration coordinate changes to cilia function is not known. Given the complexity of cilia structure, consisting of several hundred different proteins (23–25), we hypothesized that modulation of cilia function by injury would involve coordinate gene regulation by ciliogenic transcription factors.
Foxj1 is a winged-helix or forkhead box transcription factor required for motile ciliogenesis (26). Foxj1 null mice lack motile respiratory tract cilia and exhibit randomization of the left-right body axis due to loss of motile cilia in the embryonic node (26). Overexpression of foxj1 in Xenopus or zebrafish embryos causes ectopic motile cilia formation (27, 28), and induces expression of the cilia motility proteins dynein heavy chain 9 (dnahc9), spag6, tektin2, as well as rfx2, another central regulator of ciliogenesis. To investigate the regulation of cilia function in response to injury, we examined foxj1a expression and function in zebrafish and murine models of epithelial injury and cystic kidney disease. Our results show that foxj1a expression is a primary response to tubule injury and is required to maintain enhanced cilia function in injured epithelial cells.
Results
foxj1a Expression Is a Rapid Response to Epithelial Injury and Tubule Distension.
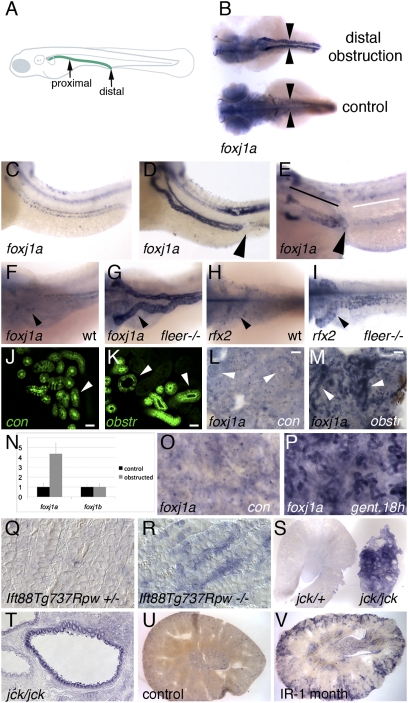
Consistent with previous reports (29), we found that both zebrafish foxj1 paralogs, foxj1a and foxj1b, were highly expressed in ciliated tissues (Fig. S1). In the pronephric kidney, foxj1a was uniformly expressed in all tubule cell types at 24 hpf but down-regulated by 48 hpf in proximal tubule single ciliated cells (Fig. S1D). Both foxj1a and foxj1b were expressed in pronephric multiciliated cells that appear in a characteristic “salt-and-pepper” pattern in the distal pronephric nephron (30) (Fig. S1 D and I). Mechanical obstruction of the pronephros at 50 hpf (31) dramatically up-regulated foxj1a expression throughout the dilated pronephric tubules (Fig. 1B), whereas expression of foxj1b was not affected (Fig. S2). Increased foxj1a expression was observed as early as 3 h postobstruction and increased thereafter. A 4.6-fold increase in foxj1a expression in response to nephron obstruction was detected 6 h after obstruction by quantitative RT-PCR of whole-embryo RNA (Fig. 1N). Up-regulation of foxj1a expression was not affected by pretreatment with the protein synthesis inhibitor cycloheximide (Fig. 1 C and D), indicating that stimulation of foxj1a expression was a primary response to tubule obstruction. In addition, up-regulation of foxj1a mRNA by obstruction was not affected by blocking foxj1a translation (discussed below; Fig. S3), arguing against a feed forward autoregulatory mechanism of induction. To determine whether foxj1a up-regulation was due to mechanical stretch of tubule epithelial cells or to a propagated crush injury, we obstructed the pronephric nephron at an anterior or proximal position (Fig. 1A) where epithelial distension occurs only proximally (32) but crush injury should propagate in both directions from the wound. Significantly, proximal tubule obstruction caused asymmetric up-regulation of foxj1a only proximal to the site of blockage (Fig. 1E), suggesting an epithelial stretch-based mechanism can activate foxj1a expression. Consistent with a stretch-based mechanism of induction, foxj1a was strongly up-regulated in the pronephros of all zebrafish cystic kidney mutants examined including fleer/dyf-1 (Fig. 1 F and G) (33), oval/ift88 (34), double bubble, and tg292a as well as in pkd2- and foxj1a-deficient embryos, which are also cystic (Figs. S3 and S4). In addition, rfx2, another cilia-specific transcription factor, was also up-regulated in the same cystic kidneys (Fig. 1 H and I and Fig. S4). Increased expression of foxj1a was not observed in any mutants before cystic distension (Fig. S4 K and L), indicating that its expression was due to epithelial stretch and not cilia gene mutation.
Fig. 1.
Up-regulation of foxj1a expression in epithelial injury and cystic distension. Embryos in B–I were all imaged at 56 hpf. (A) Schematic diagram illustrating location of the zebrafish pronephric duct (green line) and sites of mechanical proximal and distal obstruction. (B) foxj1a is strongly up-regulated in pronephros of distally obstructed embryos (top), whereas baseline expression is low in paired pronephric ducts (arrowheads) of unobstructed control embryos (bottom). Dorsal views of embryos are shown. Treatment with cycloheximide does not prevent obstruction-mediated increase in foxj1a expression (D) compared with unobstructed embryos (C). (E) Proximal pronephric obstruction (arrowhead) results in up-regulation of foxj1a only in dilated regions proximal to site of obstruction (black line) and not in tubule cells distal to obstruction (white line). (F–I) Both foxj1a and rfx2 are up-regulated in zebrafish cystic kidney mutants. (F) In WT (wt) embryos, foxj1a was expressed at low level in pronephric epithelial cells. (G) foxj1a expression was strongly up-regulated in dilated pronephric tubules of fleer mutant tubules, which develop cysts. Similarly, rfx2 gene expression was up-regulated in dilated kidney tubules of fleer mutants (I) compared with controls (H). (J–M) Obstruction-mediated induction of foxj1a expression in adult zebrafish kidney. Distal mesonephric obstruction in adult zebrafish results in tubule dilation after 12 h (K) compared with control sham-operated kidneys (J) visualized using the Tg(atase1a1a.4:eGFP) transgenic line (30). Whole-mount in situ hybridization of adult zebrafish kidney with foxj1a probe shows low level of expression in sham-operated control (L), whereas distal obstruction (M) induces foxj1a expression in mesonephric tubules after 18 h (arrowheads). (Scale bar: N–Q, 25 μm.) (N) Quantification of foxj1a and foxj1b expression by quantitative RT-PCR in obstructed embryos. (O) foxj1a is expressed at low level in whole-mount adult zebrafish kidney, whereas foxj1a is up-regulated in tubule cells after 18 h of treatment with the nephrotoxin gentamicin (P). (Q) Sections of WT post-embryonic day-7 mouse kidneys stained for Foxj1 mRNA expression show low level of Foxj1 RNA, whereas similar sections of Ift88Tg737Rpw−/− mutant mouse kidneys (R) show strong cortical expression of Foxj1 in subset of tubule cells. (S) jck/+ Kidney slice (left) compared with slice of cystic jck/jck kidney (right), revealed strong up-regulation of Foxj1 in jck/jck cystic kidney. (T) Section of jck/jck kidney showing strong expression in cyst lining epithelial cells. Increased Foxj1 expression was also observed in mice subjected to renal ischemia-reperfusion injury 1 mo postrecovery (V) compared with control kidneys (U).
The zebrafish larval pronephros is later replaced by the mesonephros, which functions as the adult fish kidney. To test whether up-regulation of foxj1a is a general response to tubule injury, we developed a model of mesonephric obstruction in the adult zebrafish. In control, sham-operated adult zebrafish mesonephroi, foxj1a is minimally expressed in kidney tubules (Fig. 1L). Surgical obstruction of the distal collecting duct near the cloaca of adult zebrafish caused mesonephric nephron tubule distension (Fig. 1 J and K) and strongly induced foxj1a gene expression in adult kidney tubules (Fig. 1 L and M). Gentamicin is a nephrotoxin that induces acute tubule injury followed by nephron regeneration in adult fish kidneys (35, 36). foxj1a was strongly up-regulated after 18 h in gentamicin-injured kidney tubules (Fig. 1O) compared with controls (Fig. 1P). In murine cystic kidney mutants or an ischemia reperfusion model of acute injury, Foxj1 was also markedly up-regulated in epithelial cells. Control mouse kidney sections showed very low levels of Foxj1 expression (37) (Fig. 1Q), whereas Foxj1 was induced in mutant Ift88Tg737Rpw−/− cortical tubular epithelial cells (Fig. 1R), even before the development of cysts (38). Foxj1 expression was also strongly up-regulated in the cyst lining epithelium of jck/jck mouse kidneys (Fig. 1 S and T) (39), but not in normal kidneys of heterozygote littermates (Fig. 1S). Finally, increased renal Foxj1 expression was also observed in the cortex of mouse kidneys after 1 mo of recovery from ischemia-reperfusion injury (Fig. 1 U and V). Ependymal cells of the zebrafish spinal cord also generate motile cilia that are required for circulation of cerebrospinal fluid (31). In addition, injury to the spinal cord markedly up-regulated expression of foxj1a, whereas expression of foxj1b remained unchanged (Fig. S2). In this case, foxj1a up-regulation occurred without dilation of the spinal canal and propagated in both directions from the site of injury (Fig. S2), suggesting that injury responses are propagated by a mechanism different from that observed in the kidney. Taken together, the data indicate that Foxj1 induction is a widespread and highly conserved response to injury.
Cilia Beat Rate Is Increased by Tubule Distension.
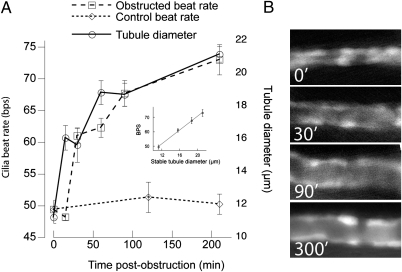
We used the zebrafish tubule obstruction model to determine whether changes in foxj1a expression were associated with changes in cilia function. We correlated tubule diameter and cilia beat rate in the same embryos using high-speed microvideo and the ET11-9 transgenic line that expresses GFP specifically in the pronephros (32). Obstruction caused a marked increase in cilia beat rate as the tubular diameter increased (Fig. 2 A and B). At 48 hpf, control cilia beat at 49.4 ±3.29 beats/s (Fig. 2A and Movie S1), whereas cilia in embryos obstructed for 3.5 h beat at 73 ±7.52 beats/s (Fig. 2A and Movie S2). After long-term obstruction (12–24 h), the cilia beat rate subsided to 58 ± 3.81 beats/s, remaining significantly increased over unobstructed controls which displayed a constant beat rate at all time points examined (Fig. 2A). A 15- to 30-min latency between tubular distension and increased cilia beat rate was consistently observed (Fig. 2A), indicating that increased beat rate was not simply due to relief from tubule luminal wall resistance to cilia movement. Strikingly, after stabilization of tubule lumen diameter in obstructed tubules, cilia beat rate varied linearly with tubule diameter (Fig. 2A, Inset). Cilia length, number, or the proportion of single ciliated vs. multiciliated cells (MCC), were unchanged in acutely stretched epithelia. Thus, obstruction-induced changes in cilia function (beat rate) correlated with induction of foxj1a, suggesting that both events may be linked in a homeostatic response to tubule injury.
Fig. 2.
Obstruction-induced luminal stretch increases cilia beat frequency. (A) Pronephric obstruction at 48 hpf induced an increased cilia beat rate that closely followed tubule luminal stretch with a latency of 15–30 min. (A, Inset) After stabilization of lumen diameter at 0, 30, 90, and 210 min, cilia beat rate was directly proportional to lumen diameter. Error bars represent SEM. (B) Representative images of GFP-positive E11-9 transgenic tubule segment showing distension at time points indicated.
foxj1a and foxj1b Control Cilia Motility.
We clarified the function of foxj1a and foxj1b in cilia function by morpholino knockdown (Fig. S5). Similar to previously published results (27, 28, 40), embryos injected with a mixture of both foxj1a splice donor and ATG morpholino (MO) uniformly developed cilia phenotypes such as ventrally curved body axis, pronephric cysts, hydrocephalus, and randomized right–left body axis (Fig. S5 D–G). foxj1b morphants were normal except for defects in otolith number (Fig. S5M), consistent with specific expression of foxj1b in the otic placode (Fig. S1 G and H). Double knockdown of foxj1a and foxj1b exhibited the combination of phenotypes seen in embryos deficient in either foxj1a or foxj1b alone (Fig. S5 L and M). Cilia motility in foxj1a and foxj1a/b double knockdown embryos was consistently impaired. Although motile olfactory cilia were detected in 14/14 control morpholino-injected embryos (100%) at 56 hpf (Movie S3), only 2/14 (14%) olfactory placodes in foxj1a morphants exhibited any motile cilia, whereas the remainder were completely paralyzed (Movie S4). In the pronephric kidney at 56 hpf, foxj1a knockdown alone did not paralyze multiciliated cell cilia, most likely due to expression of foxj1b in these cells (discussed below). However, only one in 15 double foxj1a/b-deficient pronephric tubules showed any evidence of cilia motility in clearly visualized, dilated tubules (Movie S5), whereas all control morpholino-injected embryos (n = 15) showed vigorous motility.
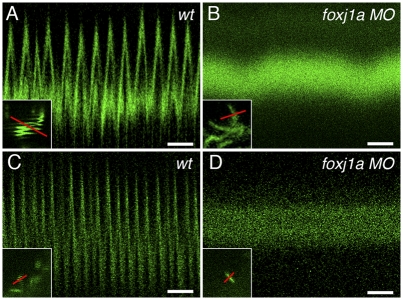
Cilia motility was directly quantified by high-speed confocal line scans of cilia marked by expression of an eGFP-tagged arl13b/scorpion mRNA (sco-eGFP) (41). Motile, GFP-positive cilia appeared as a “squiggled” line image in conventional confocal images as the cilia move back and forth in the direction of the scan (Fig. 3A, Inset). High-speed confocal line scanning accurately resolved individual cilia beat rates and revealed a control morpholino injected embryo ependymal cilia beat rate of 15.02 ± 3.08 beats/s (n = 23; Fig. 3A). In contrast, ependymal cilia in foxj1a morphants were consistently paralyzed (Fig. 3B). Time-lapse movies of sco-eGFP–labeled control (Movie S6) and foxj1a morphant ependymal cilia (Movie S7) further confirmed the cilia motility defect. In control embryo Kupffer's vesicle, the majority of cilia beat at a rate of 27.2 ± 0.17 beats/s (n = 4; Fig. 3C). In contrast, Kupffer's vesicle cilia in foxj1a morphant embryo cilia were immotile (Fig. 3D).
Fig. 3.
foxj1a is required for cilia motility. (A) Control scorpion-eGFP–labeled spinal canal cilia in 24-hpf embryos beat rapidly as seen in high-speed line scanning over time (16.9 beats/s in this example; total time, 768 ms. (Scale bar, 100 ms.) (A, Inset) Single cilia imaged in slow-frame scan appears as squiggled line pattern; red line in Inset indicates line acquired in high-speed line scan. (B) High-speed line scan of foxj1a 24-hpf morphant spinal cord cilia shows no motility (total time, 768 ms). (Scale bar, 100 ms.) (B, Inset) Single cilia imaged in slow frame scan shows no movement; red line in inset indicates line acquired in high-speed line scan. (C) Control Kupffer's vesicle cilia (12-somite stage) beat rapidly, as seen in high-speed line scanning over time (27 beats/s in this example; total time, 768 ms). (Scale bar, 100 ms.) (C, Inset) Single cilia at edge of Kupffer's vesicle imaged in slow frame scan appears as squiggled line pattern; red line in Inset indicates line acquired in high-speed line scan. (D) High-speed line scan of foxj1a morphant Kupffer's cilia (12-somite stage) shows no motility (total time, 768 ms). (Scale bar, 100 ms.) (D, Inset) Single cilia imaged in slow frame scan shows no movement; red line in inset indicates line acquired in high-speed line scan.
foxj1a Regulates Expression of Cilia Motility Genes and Maintains Enhanced Cilia Beat Rates in Obstructed Tubules.
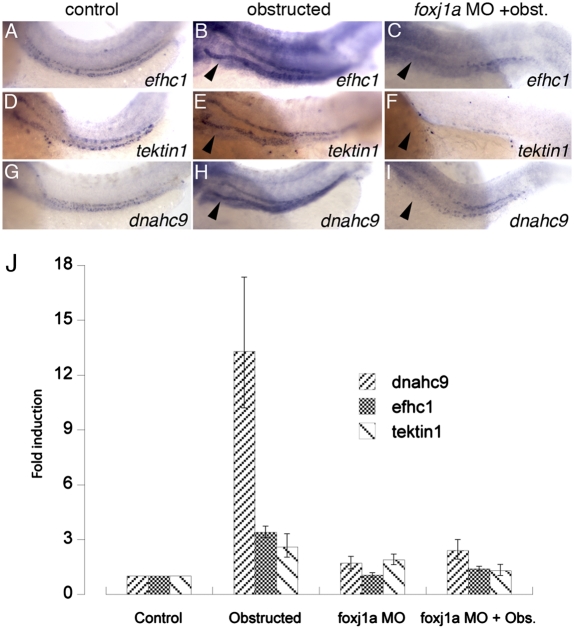
To assess the function of foxj1a/b as a regulator of cilia genes in injured tubules, we first validated foxj1a/b targets by in situ hybridization. Based on foxj1a/b knockdown-induced loss of expression in specific foxj1a and foxj1b-expressing cell types, we validated dynein heavy chain 9 (dnahc9), EF hand-containing protein 1 (efhc1), and tektin1 as foxj1 downstream targets (Fig. S6). We next assayed whether these genes were up-regulated in response to tubule distension and whether this was foxj1a dependent (Fig. 4). In the absence of obstruction, efhc1, tektin1, and dnahc9 were expressed only in a multiciliated cell pattern in control pronephric nephrons at 56 hpf (Fig. 4 A, D, and G), consistent with multiciliated cell-specific expression of foxj1b and the absence of foxj1a expression in other tubule cells at this stage. Dramatic up-regulation of foxj1 target gene expression was observed by quantitative RT-PCR (Fig. 4J) and by in situ hybridization in both single and multiciliated cells of obstructed tubules (Fig. 4 B, E, and H). Target gene expression correlated tightly with distension-induced foxj1a expression (Fig. 1B), and knockdown of foxj1a completely prevented induction of target cilia motility genes in obstructed tubules (Fig. 4 C, F, I, and J), demonstrating a requirement for foxj1a in modulating cilia motility gene expression in response to tubule distension.
Fig. 4.
foxj1a target genes are up-regulated in response to tubule obstruction. Control expression of foxj1a target genes efhc1 (A), tektin-1 (D), and dnahc9 (G) at 56 hpf is up-regulated by distal pronephric obstruction (B, E, and H). foxj1a knockdown prevented up-regulation in response to tubule obstruction (C, F, and I). Quantitative real-time RT-PCR (J) confirmed up-regulation of foxj1a target gene expression in obstructed tubules (dnahc9 13.3-fold; efch1 3.4-fold, and tektin1 2.6-fold) and inhibition of induction in foxj1a-deficient obstructed tubules. Error bars in J represent range of minimum and maximum fold foxj1a induction relative to control embryos (comparative threshold cycle method) (59).
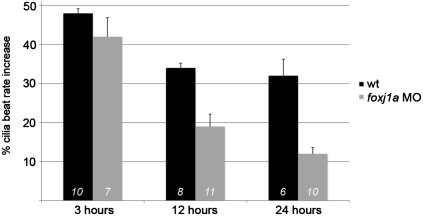
We took advantage of the constitutive expression of foxj1b in pronephric MCC to test whether inducible foxj1a expression was required for initiation or maintenance of enhanced the cilia beat rate observed in obstructed kidney tubules. In single foxj1a knockdown embryos, MCC cilia bundles beat at normal or increased frequency, most likely supported by redundant, MCC-specific expression of foxj1b. Baseline cilia beat rate in control embryos varied between 33 and 42 beats/s at 48 hpf; however, short-term obstruction reproducibly induced a 48% increase in MCC cilia beat rate in control embryos and a 42% increase in foxj1a-deficient embryos over unobstructed tubules (Fig. 5), indicating that acute cilia responses did not require foxj1a expression. However, maintenance of enhanced cilia beat rate over a longer time course of 12–24 h was foxj1a dependent (Fig. 5). The control obstructed tubule cilia beat rate stabilized at a 32% increase over unobstructed controls, whereas in foxj1a-deficient embryos the cilia beat rate decayed to just 12% over control after 24 h of obstruction. The data define an essential role for inducible foxj1a in maintenance of enhanced cilia beat rates in chronically distended epithelial tubules.
Fig. 5.
Induced expression of foxj1a is required to maintain elevated MCC cilia beat rates. Pronephric tubules were obstructed at 48 hpf, and MCC cilia beat rates were measured 3, 12, and 24 h after obstruction. Beat rates were normalized to percent increase in frequency over unobstructed control tubule cilia. Obstruction reproducibly caused a 48% increase in cilia beat rate, which, at 3 h postobstruction, was not significantly affected by foxj1a knockdown. Control cilia beat rate stabilized at a 32% increase in chronically obstructed tubules at 12 and 24 h postobstruction, whereas foxj1a-deficient tubules did not maintain high cilia beat rates. Error bars indicate SEM; numerals in bars indicate number of beat rate measurements made on at least three different embryos.
Discussion
The “cilia hypothesis,” which posits that renal cilia are central to the pathogenesis of most forms of cystic kidney disease, was a major breakthrough in the understanding of these diseases, and has served as a useful framework within which to evaluate newly discovered cystic kidney disease genes. However, much remains to be discovered about the physiologic functions of cilia and changes in cilia structure that occur in response to injury. We report that the ciliogenic transcription factor foxj1a, known to play a major role in the regulation of genes essential for cilia structure and function (26, 28, 42) (i) is rapidly induced by injury, (ii) is required to maintain elevated cilia beat rates in injured tubules, and (iii) coordinates expression of a network of motile cilia-specific genes in response to cystic epithelial distension and epithelial injury. The induction of Foxj1 in kidney injury or cystic distension, observed in both zebrafish and mice, suggests molecular mechanisms by which adaptive alterations in cilia function might be activated in response to tissue injury.
In both fish and mammals, fetal kidney obstruction causes nephron distension and dysplastic organogenesis (43, 44). In zebrafish obstructed tubules, we observed a reproducible increase in cilia beat rate that closely paralleled both the increase in tubule lumen diameter and foxj1a expression. We postulate that the increase in zebrafish cilia beat rate in response to obstruction may be compensatory or adaptive. In a condition of partial obstruction, for instance, increased cilia beating and luminal fluid propulsion could serve to clear blocked tubules of cell debris to overcome obstruction. foxj1a activation of multiple cilia-associated genes may support a greater demand on cilia function due to increased axonemal protein turnover at higher cilia beat rates.
In the mammalian kidney, cilia length significantly increases in response to common types of kidney injury, including acute tubular necrosis and ureteral obstruction (14, 15, 45). Similarly, in the pancreatic duct, increased cilia length has been associated with ductal ligation and inflammation (46, 47). In light of our results and the recent finding that Foxj1 can induce expression of rfx2 (27), a master regulator of ciliogenesis, it is likely that Foxj1 induction plays a broad role as a primary regulator of ciliogenesis in injury. In the case of mammalian kidney cilia, increased cilia length has been postulated to serve as an adaptive response, as longer cilia experience greater shear forces and could generate greater mechanosensory intracellular calcium responses in response to flow (48). Alternatively, induced Foxj1 expression could explain the appearance of multiciliated kidney tubule cells with “9+2” motile cilia structure that are a common but unexplained finding in biopsy samples in multiple pathologic conditions (16–19). Multiciliated cells have also been observed in distended renal tubules of the Pkhd−/− rat, a model of autosomal recessive polycystic kidney disease (21). Injury-induced changes in cilia structure and function may represent a compensatory response to reestablish fluid flow following obstruction or to enhance tubule repair following injury.
The idea that altered cilia length or function could be an adaptive response to injury may also have bearing on recent conditional knockout studies of cilia-associated cystic disease genes. The relatively mild phenotypes induced by postnatal vs. embryonic knockout of the cilia-associated cystic disease genes IFT88, Kif3a, and PKD1 (49–51) have been interpreted to reveal an additional requirement for renal injury in the process of cystogenesis. These findings also suggest that cilia may have functions other than mechanosensors of tubule fluid flow, as nephrons uniformly lacking cilia develop cysts only sporadically and after a significant delay (49). Our findings are consistent with the proposal that mutant animals lacking an ability to modulate cilia structure or function may be sensitized to tubule damage and more susceptible to renal cyst formation.
We found that Foxj1 was induced in several different forms of epithelial injury, suggesting that Foxj1 regulatory elements may respond to several signaling pathways. We focused on zebrafish pronephric obstruction, as it allowed us to control the timing of injury and montior acute responses to injury. Several aspects of foxj1a induction in this context indicate that epithelial stretch may be sufficient for foxj1a expression. Increased foxj1a expression is seen not only in distended tubules but also in zebrafish cystic mutants that do not exhibit cell death or other injury. In addition, obstruction led to increased foxj1a expression only proximal to the site of obstruction, implying a requirement for fluid pressure-driven tubule distension in foxj1a induction. Epithelial stretch is responsible for a wide range of physiologic responses in kidney tubules, including the proliferation of cyst-lining epithelia in polycystic kidney disease (52). Epithelial cell stretch can lead to increased local intracellular calcium concentrations, activation of integrins, focal adhesion kinase, and integrin-linked kinase, signaling an adaptive, strengthening reorganization of the actin cytoskeleton along with thickening of tubular basement membranes (53). Intracellular calcium signaling, as well as cAMP signaling, has also been implicated in acute responses regulating cilia beat rate (22, 54). Further analysis of initial stretch-induced physiological signals regulating cilia beat rate could reveal a role for similar signaling pathways in subsequent foxj1 gene induction. In addition to induction in obstructed kidney tubules, foxj1a was also induced rapidly after spinal cord injury, which may also involve a propagated calcium response (55). foxj1a expression in the spinal cord may act to promote ciliated ependymal cell differentiation and circulation of cerebrospinal fluid or, alternatively, may affect neuronal regenerative responses (56). In either case, the induction of Foxj1 and downstream ciliogenic genes could represent a novel therapeutic target to promote tissue repair in multiple forms of organ injury.
Materials and Methods
Zebrafish Morpholino Injection and Phenotype Analyses.
For foxj1a morpholino knockdowns, WT strain TU-AB zebrafish embryos were injected at the one- to four-cell stage with 4.6 nl of a 0.2-mM solution of an exon 3 splice donor MO (5′-ACCAATGTGAAAATGTGTTACCTGC-3′) plus 0.12 mM of ATG MO (5′-AACTCATGGAGAGCATGGTCCTGAC-3′). Off-target effects were assessed by including 0.1 mM p53 MO (5′-TTGATTTTGCCGACCTCCTCTCCAC-3′). For foxj1b morphants, 4.6 nl solution containing 1 mM exon 2 splice donor MO (5′-ATAAACTGAATTTACCTGCCAGCTC-3′) was injected. Control embryos were injected with equivalent amounts of invert sense morpholinos for foxj1a (5′-CGTCCATTGTGTAAAAGTGTAACCA-3′) and foxj1b (5′-CTCGACCGTCCATTTAAGTCAAATA-3′). pkd2 MO used in this study has been described elsewhere (57). Heart jogging was determined between 28 and 32 hpf.
RT-PCR and Quantitative PCR Analysis.
RNA from single embryos was harvested by TRIzol extraction followed by DNase I treatment. Reverse transcription using SuperScript III (Invitrogen) was carried out on 1 μg RNA using foxj1a or foxj1b cDNA-specific primers and subjected to nested PCR reactions. Mis-splicing was confirmed by sequencing gel-purified RT-PCR products. For quantitative PCR analysis, RNA was extracted from control and 6-h obstructed embryos followed by quantitative PCR (ABI Prism) using 2× SYBR Green Mix (ABgene) and primers against foxj1a and foxj1b or foxj1a target genes dnahc9, efhc1, or tektin1. Gene expression was normalized to GAPDH mRNA expression, and data were analyzed using the 2–DDCt method.
In Situ Hybridization.
In situ hybridization was carried out as described previously (58) and imaged on a Leitz MZ12 microscope with a Spot Image digital camera. For mouse in situ hybridization experiments, kidneys were vibratome sectioned at 80–120 μm and processed as whole mounts. Levimasole was added at a final concentration of 0.2 mM during the color reaction. For histological analysis, vibratome sections were embedded in JB4 (Polysciences) and sectioned at 5–7 μm.
Immunofluorescent Microscopy.
For confocal immunofluorescent analysis, zebrafish embryos were fixed in Dent's fix overnight at 4 °C and stained in wholemount as described previously (33). Primary antibodies used were antiacetylated tubulin 6-11B-1 (1:800; Sigma-Aldrich) and rabbit anti-IFT88 (1:1,000; gift from Brian Perkins, Texas A&M University, College Station, TX). Secondary antibodies were used at 1:1,000 (Alexa Fluor 488 and Alexa Fluor 546 (Molecular Probes). Specimens were dehydrated and cleared with 2:1 benzyl benzoate:benzyl alcohol, and examined with a Zeiss LSM5 Pascal confocal microscope. For visualization of sco-eGFP–labeled cilia, 2.5 ng RNA was injected into one-cell–stage embryos followed by visualization of the Kupffer's vesicle (12-somite–stage) or ependymal cilia (50-hpf) after embedding in 1.5% low-melt agarose. To calculate cilia beat rate, GFP-labeled cilia were analyzed by confocal linescan and xt plots. Beat rate was calculated as (1/t s) • (1/scan lines per cilia beat) with t = s per scan line.
High-Speed Video Microscopy.
Cilia beat frequency was measured in response to obstruction using a high-speed video camera (Point Gray Research) at an acquisition rate of 240 frames/s and converted to 6 frames/s for counting. Ten cilia bundles were counted per time point. Pronephric duct lumen diameter was measured in fluorescence images (E11-9 GFP transgenics) using ImageJ (National Institutes of Health) software.
Kidney Injury Experiments.
Zebrafish pronephric obstruction was carried out as described previously (31). For adult zebrafish kidney obstruction, an incision was made at the level of the distal collecting system of a 12-mo-old zebrafish anesthetized with tricaine. Inox tweezers were used to pinch the distal collecting system for ∼30 s. Fish were allowed to recover in the presence of antibiotics (penicillin and streptomycin) over a period of 12 h overnight, with good survival. Fish were fixed in 4% PFA and the mesonephros was isolated by microdissection. Vibratome sections were processed for in situ hybridization as described above. For cycloheximide experiments, embryos were preincubated for 1 h in cycloheximide (Sigma-Aldrich) at 10 mg/mL, followed by distal obstruction. After 6 h in cycloheximide egg water, embryos were fixed in 4% PFA and processed for in situ hybridization. To elicit spinal cord injury, 48-hpf embryo cords were severed using forceps and fixed for in situ hybridization 8 h later. Ischemia-reperfusion injury in C57BL/6 WT mice was carried out as previously described (51). Kidneys were fixed 1 mo following recovery and processed for in situ hybridization as described above.
Supplementary Material
Acknowledgments
We thank personnel in the zebrafish facility at Massachusetts General Hospital, Randy Peterson for advice on quantitative PCR, Mingyue Lun for harvesting jck/jck mouse tissue, Christopher Ward (Mayo Clinic, Rochester, MN) for sharing Pkhd1−/− rat kidney tissue, and members of the I.A.D. laboratory for critical review and helpful discussions. This work was supported by National Institutes of Health Grants DK053093 (to I.A.D.), DK065655 (to B.K.Y.), DK069528 (to Z.S.), and DK066370 (to D.R.B.), National Institutes of Health Training Grant T32DK007540 (to N.E.H.), and by the UAB Hepato/Renal Fibrocystic Diseases Core Center (P30 DK074038) and the UAB O'Brien Core Center for Acute Kidney Injury Research (P30 DK079337).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005998107/-/DCSupplemental.
References
- 1.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: Disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 4.Lin F, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehman JM, et al. The Oak Ridge Polycystic Kidney mouse: Modeling ciliopathies of mice and men. Dev Dyn. 2008;237:1960–1971. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–450. doi: 10.1146/annurev.physiol.69.040705.141301. [DOI] [PubMed] [Google Scholar]
- 7.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade YN, et al. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168:869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besschetnova TY, et al. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol. 2010;20:182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low SH, et al. Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells. J Biol Chem. 1998;273:3422–3430. doi: 10.1074/jbc.273.6.3422. [DOI] [PubMed] [Google Scholar]
- 11.Santos N, Reiter JF. Building it up and taking it down: The regulation of vertebrate ciliogenesis. Dev Dyn. 2008;237:1972–1981. doi: 10.1002/dvdy.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti CJ, Conner EA, Gimenez-Conti IB, Silverberg SG, Gerschenson LE. Regulation of ciliogenesis and proliferation of uterine epithelium by 20 alpha-hydroxy-pregn-4-en-3-one administration and withdrawal in ovariectomized rabbits. Biol Reprod. 1981;24:903–911. doi: 10.1095/biolreprod24.4.903. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Krishnamurthy K, Bieberich E. Regulation of primary cilia formation by ceramide. J Lipid Res. 2009;50:2103–2110. doi: 10.1194/jlr.M900097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA. Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant. 2008;23:834–841. doi: 10.1093/ndt/gfm743. [DOI] [PubMed] [Google Scholar]
- 15.Verghese E, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol. 2009;20:2147–2153. doi: 10.1681/ASN.2008101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz SM, Morgan JJ. Cilia in the human kidney. Ultrastruct Pathol. 1984;6:285–294. doi: 10.3109/01913128409018587. [DOI] [PubMed] [Google Scholar]
- 17.Duffy JL, Suzuki Y. Ciliated human renal proximal tubular cells. Observations in three cases of hypercalcemia. Am J Pathol. 1968;53:609–616. [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan MO, Subramanyan S. Ciliated renal tubular cells in crescentic glomerulonephritis. Ultrastruct Pathol. 1995;19:201–203. doi: 10.3109/01913129509064222. [DOI] [PubMed] [Google Scholar]
- 19.Ong AC, Wagner B. Detection of proximal tubular motile cilia in a patient with renal sarcoidosis associated with hypercalcemia. Am J Kidney Dis. 2005;45:1096–1099. doi: 10.1053/j.ajkd.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Smith LA, et al. Development of polycystic kidney disease in juvenile cystic kidney mice: Insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol. 2006;17:2821–2831. doi: 10.1681/ASN.2006020136. [DOI] [PubMed] [Google Scholar]
- 21.Woollard JR, et al. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int. 2007;72:328–336. doi: 10.1038/sj.ki.5002294. [DOI] [PubMed] [Google Scholar]
- 22.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 23.Ostrowski LE, et al. A proteomic analysis of human cilia: Identification of novel components. Mol Cell Proteomics. 2002;1:451–465. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 24.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JB, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest. 1998;102:1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubbs JL, Oishi I, Izpisúa Belmonte JC, Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- 29.Aamar E, Dawid IB. Isolation and expression analysis of foxj1 and foxj1.2 in zebrafish embryos. Int J Dev Biol. 2008;52:985–991. doi: 10.1387/ijdb.072477ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 31.Kramer-Zucker AG, et al. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 32.Vasilyev A, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe N, et al. Kidney regeneration through nephron neogenesis in medaka. Dev Growth Differ. 2009;51:135–143. doi: 10.1111/j.1440-169X.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 36.Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med. 2001;51:56–59. [PubMed] [Google Scholar]
- 37.Pelletier GJ, Brody SL, Liapis H, White RA, Hackett BP. A human forkhead/winged-helix transcription factor expressed in developing pulmonary and renal epithelium. Am J Physiol. 1998;274:L351–L359. doi: 10.1152/ajplung.1998.274.3.L351. [DOI] [PubMed] [Google Scholar]
- 38.Yoder BK, et al. Functional correction of renal defects in a mouse model for ARPKD through expression of the cloned wild-type Tg737 cDNA. Kidney Int. 1996;50:1240–1248. doi: 10.1038/ki.1996.433. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, et al. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 40.Tian T, Zhao L, Zhang M, Zhao X, Meng A. Both foxj1a and foxj1b are implicated in left-right asymmetric development in zebrafish embryos. Biochem Biophys Res Commun. 2009;380:537–542. doi: 10.1016/j.bbrc.2009.01.111. [DOI] [PubMed] [Google Scholar]
- 41.Duldulao NA, Lee S, Sun Z. Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development. 2009;136:4033–4042. doi: 10.1242/dev.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomperts BN, Gong-Cooper X, Hackett BP. Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells. J Cell Sci. 2004;117:1329–1337. doi: 10.1242/jcs.00978. [DOI] [PubMed] [Google Scholar]
- 43.Friedmann W, et al. Perinatal differential diagnosis of cystic kidney disease and urinary tract obstruction: Anatomic pathologic, ultrasonographic and genetic findings. Eur J Obstet Gynecol Reprod Biol. 2000;89:127–133. doi: 10.1016/s0301-2115(99)00182-7. [DOI] [PubMed] [Google Scholar]
- 44.Shibata S, Shigeta M, Shu Y, Watanabe T, Nagata M. Initial pathological events in renal dysplasia with urinary tract obstruction in utero. Virchows Arch. 2001;439:560–570. doi: 10.1007/s004280100420. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Weidenfeld R, Verghese E, Ricardo SD, Deane JA. Alterations in renal cilium length during transient complete ureteral obstruction in the mouse. J Anat. 2008;213:79–85. doi: 10.1111/j.1469-7580.2008.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashizawa N, et al. The morphological changes of exocrine pancreas in chronic pancreatitis. Histol Histopathol. 1999;14:539–552. doi: 10.14670/HH-14.539. [DOI] [PubMed] [Google Scholar]
- 47.Hamamoto N, et al. Morphological changes in the rat exocrine pancreas after pancreatic duct ligation. Histol Histopathol. 2002;17:1033–1041. doi: 10.14670/HH-17.1033. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- 49.Davenport JR, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takakura A, Contrino L, Beck AW, Zhou J. Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol. 2008;19:2351–2363. doi: 10.1681/ASN.2007101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel V, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanner GA, McQuillan PF, Maxwell MR, Keck JK, McAteer JA. An in vitro test of the cell stretch-proliferation hypothesis of renal cyst enlargement. J Am Soc Nephrol. 1995;6:1230–1241. doi: 10.1681/ASN.V641230. [DOI] [PubMed] [Google Scholar]
- 53.Quinlan MR, Docherty NG, Watson RW, Fitzpatrick JM. Exploring mechanisms involved in renal tubular sensing of mechanical stretch following ureteric obstruction. Am J Physiol Renal Physiol. 2008;295:F1–F11. doi: 10.1152/ajprenal.00576.2007. [DOI] [PubMed] [Google Scholar]
- 54.Schmid A, et al. Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci. 2006;119:4176–4186. doi: 10.1242/jcs.03181. [DOI] [PubMed] [Google Scholar]
- 55.Mills LR, Velumian AA, Agrawal SK, Theriault E, Fehlings MG. Confocal imaging of changes in glial calcium dynamics and homeostasis after mechanical injury in rat spinal cord white matter. Neuroimage. 2004;21:1069–1082. doi: 10.1016/j.neuroimage.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 56.Jacquet BV, et al. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Obara T, et al. Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol. 2006;17:2706–2718. doi: 10.1681/ASN.2006040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thisse B, et al. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods Cell Biol. 2004;77:505–519. doi: 10.1016/s0091-679x(04)77027-2. [DOI] [PubMed] [Google Scholar]
- 59.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.