Abstract
Foxp3 is a key transcription factor for differentiation and function of regulatory T (Treg) cells that is critical for maintaining immunological self-tolerance. Therefore, increasing Treg function by Foxp3 transduction to regulate an inflammatory immune response is an important goal for the treatment of autoimmune and allergic diseases. Here we have generated a cell-permeable Foxp3 protein by fusion with the unique human HHph-1-PTD (protein transduction domain), examined its regulatory function in T cells, and characterized its therapeutic effect in autoimmune and allergic disease models. HHph-1-Foxp3 was rapidly and effectively transduced into cells within 30 min and conferred suppressor function to CD4+CD25− T cells as well as directly inhibiting T-cell activation and proliferation. Systemic delivery of HHph-1 Foxp3 remarkably inhibited the autoimmune symptoms of scurfy mice and the development of colitis induced by scurfy or wild-type CD4 T cells. Moreover, intranasal delivery of HHph-1-Foxp3 strongly suppressed ovalbumin-induced allergic airway inflammation. These results demonstrate the clinical potential of the cell-permeable recombinant HHph-1-Foxp3 protein in autoimmune and hypersensitive allergic diseases.
Keywords: protein transduction domain, immunotherapy, autoimmunity, allergy
Autoimmune disease is a consequence of insufficient control of autoreactive cells and regulatory T (Treg) cells that have a central role in maintaining immune tolerance. They are characterized by the expression of CD25 and Foxp3 and have the capacity to suppress activation of effector T cells (1, 2). The importance of regulatory T cells and the role of Foxp3 in their development and function is highlighted by mutations in Foxp3 that result in fatal autoimmune lymphoproliferative disease (3, 4). The spontaneous scurfy mutation in mice has been shown to be a loss-of-function mutation in the Foxp3 gene, which results in complete loss of Treg cells and death of mice at 3–5 wk of age (5, 6). Similarly, humans with IPEX (immunodysregulation, polyendocrinopathy and enteropathy, X-linked syndrome) also have mutations in Foxp3 and have a range of symptoms that is consistent with a lack of functional Treg cells (7, 8). The fact that the loss of a single gene can have such a profound consequence is a clear indication of the central importance of Foxp3. Foxp3 is also a crucial molecule for regulating the anergic state of Treg with weak proliferation and cytokine production following stimulation in vitro (9, 10). Foxp3 has been shown to act as a transcriptional repressor of inflammatory cytokines including IL-2 and interacts with transcription factors such as NFAT (11) and NF-κB (11, 12).
The role of Treg or Foxp3 has been studied in various autoimmune disease models such as inflammatory bowel disease (IBD) (13, 14), experimental allergic encephalomyelitis (15, 16), and arthritis (17). Transfer of CD4+CD25+ Tregs completely inhibits development of these autoimmune diseases, and in vitro expansion methods of Tregs have been developed for use in clinical trials (18–20). Many recent studies have also examined the role of Foxp3 and CD4+CD25+ Treg cells in allergic asthma and atopic patients (21). Peripheral blood mononuclear cells from allergic rhinitis and asthma patients have significantly fewer Foxp3+ lymphocytes and decreased Foxp3 mRNA expression (22, 23). Tregs from atopic patients have less suppressive activity compared with the ones from nonatopic groups (24), suggesting that Foxp3 and Treg have an important role in regulating hypersensitive immune responses in human patients. Therefore, Foxp3 is a very important target for development of an immunosuppressive agent for both autoimmune and allergic diseases. Although the importance of Foxp3 in immune tolerance has been extensively studied, the clinical use of Treg or Foxp3 gene transfer for these diseases still has been limited due to the difficulty of isolating human Treg, low transfection efficiency, and high risk of virus-mediated gene delivery.
“Protein transduction domain” (PTD) refers to a class of peptides that can cross biological membranes and deliver various cargo molecules, including protein, RNA, and DNA, into all cells. Several PTDs, such as TAT, VP22, Antp, R7, or R9, have been developed as potential drug delivery systems to be used for clinical applications (25–29). In this study, a cell-permeable form of Foxp3 protein was generated using a unique peptide of human origin, HHph-1-PTD, to examine its potential to induce immune tolerance by regulation of T-cell function in vitro and in vivo. HHph-1-Foxp3, the recombinant fusion protein between Foxp3 and two tandem sequences of Hph-1-PTD, was rapidly delivered into CD4+ T cells and inhibited cytokine production and proliferation. Moreover, it converted CD4+CD25− T cells to Treg-like CD25 and CTLA-4 high suppressor cells in vitro. The life span of scurfy mice was substantially increased, and tissue infiltration of inflammatory cells and T-cell activation was remarkably reduced by injection of HHph-1-Foxp3. It also strongly inhibited IBD caused by transfer of scurfy CD4 T cells or wild-type CD4+CD25−CD45RBhigh T cells. More interestingly, intranasal delivery of HHph-1-Foxp3 prevented ovalbumin (OVA)-induced allergic airway inflammation by regulation of T-cell function. These results demonstrate a unique approach to regulating immune responses by Foxp3 and providing preclinical support for the therapeutic use of HHph-1-Foxp3 as an immunosuppressive agent for autoimmune and allergic diseases.
Results
Generation of Cell-Permeable Foxp3 Proteins.
To investigate the anti-inflammatory effect of Foxp3 in autoimmune or allergic diseases, we generated a cell-permeable form of Foxp3 recombinant protein. Mouse Foxp3 DNA was inserted into the pRSET plasmid vector with or without the HHph-1-PTD (Fig. 1A). The proteins were expressed in Escherichia coli, purified under denaturing conditions, separated by SDS gel electrophoresis, and characterized by Western blot with anti-HA or anti-Foxp3 monoclonal antibody (Fig. 1B). Next, the transduction efficiency of HHph-1-Foxp3 was tested in CD4+CD25− T cells by intracellular staining. After 1 h of incubation, HHph-1-Foxp3 was detected in the cells (Fig. 1C), and the intracellular level of HHph-1-Foxp3 reached a maximal level by 1 h and then started to decrease (Fig. 1D). The transduction efficiency of mutant Foxp3 containing HHph-1 and Foxp3 lacking HHph-1 was compared as a control (Fig. 1E). Within 30 min, HHph-1-Foxp3 was detected in the nucleus as well as in the cytoplasm (Fig. 1F), suggesting that this cell-permeable Foxp3 protein is efficiently delivered to cells and is localized in the nucleus and cytoplasm within a short time.
Fig. 1.
Generation of cell-permeable Foxp3 protein. (A) Mouse Foxp3 and the mutant that lacks the C-terminal FKH domain (Foxp3Δ) was combined with HA, His tags, and HHph-1 sequences as diagrammed. (B) Proteins were purified in bacterial cultures under denaturing conditions. Purified proteins were characterized by Western blot with anti-HA and anti-Foxp3 antibody. (C) Transduction efficiency of HHph-1-Foxp3 was confirmed in mouse CD4+CD25− T cells showing concentration-dependent expression. Thirty minutes after HHph-1-Foxp3 was incubated with the cells, intracellular levels of Foxp3 were measured by intracellular staining with anti-HA antibody. (D) Transduction kinetics was characterized using 1 μM of HHph-1-Foxp3. (E) Transduction efficiency of mutant Foxp3 with HHph-1 and Foxp3 without HHph-1 was compared as a control. (F) HHph-1-Foxp3 is expressed in the nucleus as well as in the cytoplasm. HeLa cells were incubated with HHph-1-Foxp3 or Foxp3 for 10–30 min, intracellular levels of Foxp3 were detected with FITC-labeled anti-HA antibody, and the images were captured by confocal microscopy.
HHph-1-Foxp3 Directly Inhibits T-Cell Activation and Converts CD4+CD25− T Cells to Suppressor T Cells.
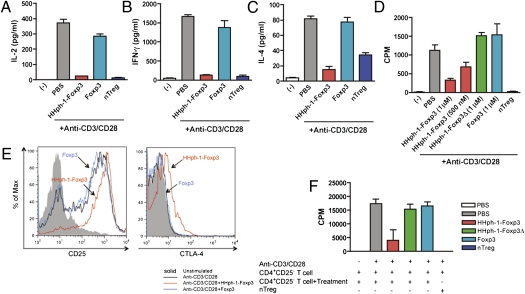
To examine the function of cell-permeable Foxp3 in CD4+ T cells, CD4+CD25− T cells were isolated from mouse splenocytes by flow cytometry and were incubated with HHph-1-Foxp3 or Foxp3 for 1 h. Then they were stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibody for 24 h. Intracellular delivery of Foxp3 into CD4+CD25− T cells strongly inhibited their IL-2 production compared with non-PTD Foxp3 treatment and secretion of Th1 (IFN-γ) or Th2 (IL-4) cytokines was also suppressed by Foxp3 transduction (Fig. 2 A–C). When the cells were stimulated for 3 d to assess their proliferation, HHph-1-Foxp3–treated cells were less proliferative than Foxp3-treated cells, and the inhibitory effect on T-cell proliferation was concentration dependent (Fig. 2D). CD4+CD25+ natural Treg cells were used as a positive control. These results indicate that intracellular delivery of Foxp3 directly inhibits T-cell activation and results in hyporesponsiveness following T-cell receptor (TcR) stimulation in CD4+ T cells similar to natural Treg cells. Because Foxp3 gene transfer induces regulatory T cells in vitro, we determined whether HHph-1-Foxp3 protein transfer converts conventional CD4+CD25− T cells to suppressor T cells. The expression of Treg markers CD25 and CTLA-4 on CD4+CD25− T cells after HHph-1-Foxp3 transduction in the presence of TcR stimulation was increased compared with treatment with a non-cell-permeable Foxp3 (Fig. 2E). The surface expression of CD25 was significantly increased, and a trend toward increased CTLA-4 was also observed (Fig. S1). To assay the suppressive function of HHph-1-Foxp3–transduced T cells, responder CD4+CD25− T cells were cultured with HHph-1-Foxp3–treated CD4+CD25− T cells for 3 d, and proliferation of cells was analyzed by thymidine incorporation (Fig. 2F). HHph-1-Foxp3 treated CD4+CD25− T cells could inhibit the proliferation of conventional CD4 T cells strongly at a 2:1 ratio (suppressor:responder). However, the mutant form of Foxp3 lacking the forkhead (FKH) domain (HHph-1-Foxp3Δ) could not convert conventional CD4+CD25− T cells to suppressor cells or inhibit their proliferation. This activity of suppression depends on the ratio between suppressor cells and responder cells (Fig. S2A). Adding IL-2 restored the proliferation of activated T cells with HHph-1-Foxp3, suggesting that the effect of HHph-1-Foxp3 on proliferation is not simply due to toxicity (Fig. S2B). The control protein of HHph-1-EGFP did not affect the proliferation of T cells (Fig. S2C). These results demonstrate that cell-permeable Foxp3 protein can induce the suppressive function of CD4 T cells as well as directly inhibit T-cell activation.
Fig. 2.
HHph-1-Foxp3 inhibits T-cell activation and converts conventional T cells to suppressor cells. (A–C) HHph-1-Foxp3 suppresses cytokine secretion from activated T cells. After CD4+CD25− T cells were treated with 1 μM HHph-1-Foxp3 or Foxp3 for 30 min, cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibody for 24 h. (A) IL-2, (B) IL-4, and (C) IFN-γ expression level in the same culture media was analyzed by ELISA. (D) HHph-1-Foxp3–treated CD4+CD25− T cells are hypo-proliferative. CD4+CD25− T cells treated with HHph-1-Foxp3, Foxp3, or HHph-1-Foxp3Δ were stimulated for 3 d as described above, and proliferation of the cells was analyzed by thymidine incorporation. (E) HHph-1-Foxp3 treatment enhances CD25 and CTLA-4 expression on CD4+CD25− T cells. After CD4+CD25− T cells with HHph-1-Foxp3 or Foxp3 were stimulated for 24 h as described above, CD25 and CTLA-4 expression levels were analyzed by flow cytometry. (F) HHph-1-Foxp3 converts CD4+CD25− T cells to suppressor cells. A total of 5 × 104 responder cells (CD4+CD25− T cells) were stimulated with plate-bound anti-CD3 and soluble anti-CD28 antibody for 3 d with 105 suppressor cells (HHph-1-Foxp3–, Foxp3–, or HHph-1-Foxp3Δ–treated CD4+CD25− T cells). Proliferation of all cells was analyzed by thymidine incorporation. In these experiments, nTreg (CD4+CD25+ natural Treg) was used as a positive control.
Inhibition of Systemic Autoimmune Disease in Scurfy Mice by HHph-1-Foxp3.
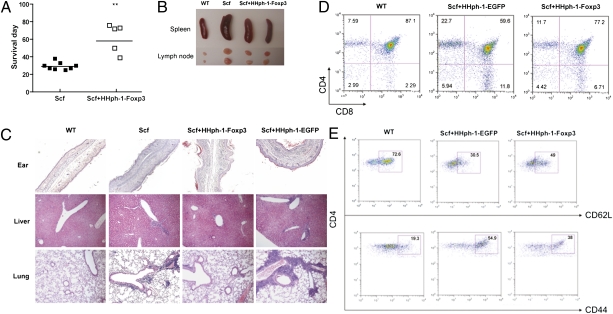
The scurfy (sf) mutation results in severe autoimmune consequences, including a complete loss of Foxp3+ Treg cells, systemic inflammation in many organs, and death of the mice at 3–4 wk of age (6). We examined the in vivo reconstitution effect of HHph-1-Foxp3 in the autoimmune scurfy mouse that lacks Foxp3 to study its clinical potential to treat autoimmune disease. Beginning at day 3 after birth, mice were injected with HHph-1-Foxp3 every other day, which resulted in a prolonged life span of scurfy mice (Fig. 3A) and reduced splenomegaly and lymphadenopathy (Fig. 3B). On day 21 after birth, scurfy mice treated with HHph-1-Foxp3 or HHph-1-EGFP (a control protein) were killed, and ear, liver, and lung tissues were stained with H&E to examine tissue inflammation (Fig. 3C). In scurfy mice treated with HHph-1-EGFP, ears were swollen and contained severe cellular infiltrates within the dermis. Moderate levels of cellular infiltrate were observed in the portal triad regions in the livers of HHph-1-EGFP–treated scurfy mice. By contrast, the cellular infiltrate was minimal in the livers of scurfy mice that received HHph-1-Foxp3. Marked levels of perivascular cellular infiltrate were observed in the lungs of scurfy mice treated with HHph-1-EGFP. Importantly, this inflammation was inhibited in scurfy mice treated with HHph-1-Foxp3, suggesting the potential use of this protein in airway inflammatory disease. Scurfy mice have increased percentages of mature CD4 and CD8 single positive thymocytes compared with normal C57BL/6 mice. Treatment with HHph-1-Foxp3 significantly reduced the percentages of single positive cells compared with HHph-1-EGFP treatment (Fig. 3D). There was also a partial reduction of activated T cells with high expression of CD44 and low expression of CD62L (Fig. 3E). No significant effect on normal mice with HHph-1-Foxp3 injection was observed, suggesting that the inhibitory effect on systemic inflammation is not due to toxic effects on the cells (Fig. S3). These results are consistent with others showing inhibition of disease in scurfy mice by injection of natural Treg (nTreg) (4) or induced Treg (iTreg) (30). These results indicate that systemic administration of HHph-1-Foxp3 protein inhibits systemic inflammation of scurfy mice and suggest that it may have therapeutic potential for the treatment of autoimmune disease.
Fig. 3.
Reduction of scurfy mouse disease by HHph-1-Foxp3. Scurfy mice were injected i.p. with HHph-1-Foxp3 every other day beginning 3 d after birth. (A) Administration of HHph-1-Foxp3 prolongs the life span of scurfy mice. (B) HHph-1-Foxp3 treatment reduces the size of the spleen and lymph nodes in mice treated for 21 d. (C) Reduced infiltration of inflammatory cells in tissues including ear, liver, and lung by HHph-1-Foxp3. The H&E stained each tissue, which is shown at 20× magnification. (D) Inhibition of differentiation of CD4 or CD8 single positive cells by HHph-1-Foxp3 in the thymus. Cells isolated from the thymus were stained with APC-anti-CD4 and PerCP-anti-CD8 antibody and analyzed by flow cytometry. (E) Inhibition of T-cell activation by HHph-1-Foxp3 in the lymph node. FITC-anti-CD62L and PE-anti-CD44 antibody were used to detect activated cells with flow cytometry. In these experiments, HHph-1-EGFP was used as a negative control.
Prevention of Autoimmune Colitis by HHph-1-Foxp3.
To demonstrate a therapeutic effect of HHph-1-Foxp3 on autoimmune disease, we used two colitis models by transfer of scurfy CD4 T cells or wild-type CD4+CD25−CD45RBhigh T cells into immunodeficient mice. Scurfy mice were injected with HHph-1-Foxp3 or HHph-1-EGFP every other day beginning 3 d after birth. Then 1 million CD4 T cells were transferred into RAG-2 KO mice followed by HHph-1-Foxp3, HHph-1-EGFP, or PBS treatment. Transfer of scurfy CD4 T cells into RAG-2 KO mice results in development of severe autoimmune inflammation in the intestine. The mutation in the Foxp3 gene in scurfy mice removes Treg from this strain. Injection of HHph-1-Foxp3 inhibited weight loss of RAG-2 KO mice after scurfy CD4 T-cell transfer (Fig. 4A), and the size of the spleen and mesenteric lymph nodes was much smaller than the in the positive control group (Fig. 4B). Infiltration of cells into the colon and tissue destruction, including the crypt cells, also was reduced by HHph-1-Foxp3 (Fig. 4C). By contrast, similar injection of HHph-1-EGFP or PBS did not inhibit the inflammation.
Fig. 4.
Prevention of autoimmune colitis by HHph-1-Foxp3. (A–C) HHph-1-Foxp3 injection inhibits colitis induced by scurfy CD4 T cells. Scurfy mice were injected with HHph-1-Foxp3 or HHph-1-EGFP every other day beginning 3 d after birth until day 21, and 106 CD4 T cells from scurfy mouse were transferred into RAG-2 KO mice following HHph-1-Foxp3 or HHph-1-EGFP injection or without treatment. (A) Weight was monitored every week for 5 wk after the cell transfer and labeled as percentage of weight change. (B) The size of lymph node, spleen, and the shape of large intestine. (C) Reduction of infiltration of inflammatory cells in intestine by HHph-1-Foxp3. Colon tissue of each mouse was stained by H&E, and the image is magnified 10×. (D–F) HHph-1-Foxp3 administration inhibits colitis induced by CD4+CD25−CD45RBhigh T cells. A total of 5 × 105 wild-type (WT) CD4+CD25−CD45RBhigh T cells were transferred into RAG-2 KO mice, and HHph-1-Foxp3 or HHph-1-EGFP was injected with 50 μg every other day until the day of sacrifice. (D) Weight was monitored every week for 5 wk after the cell transfer. (E) The size of lymph node, spleen, and the shape of large intestine. (F) Infiltration of inflammatory cells in intestine is reduced by HHph-1-Foxp3 treatment. Colon tissue of each mouse shown was stained by H&E and is shown at 10× magnification. Four to nine mice from each group were used for the analysis.
Transfer of wild-type CD4+CD25−CD45RBhigh T cells into RAG-2 KO mice followed by injection of HHph-1-Foxp3 every other day blocked autoimmune disease and prevented weight loss (Fig. 4D). The treatment suppressed enlargement of the intestine and the size of the spleen and lymph nodes (Fig. 4E), and the infiltration of inflammatory cells and destruction of crypt structure was dramatically inhibited by HHph-1-Foxp3 but not by HHph-1-EGFP (Fig. 4F). These preventive effects of HHph-1-Foxp3 in this experimental colitis model support the in vitro results of HHph-1-Foxp3’s regulatory effect on T-cell function and its therapeutic potential in the treatment of autoimmune diseases such as IBD.
Prevention of Allergic Airway Inflammation by Intranasal Delivery of HHph-1-Foxp3.
In the previous experiments characterizing the effects of HHph-1-Foxp3 in scurfy mice, the lung inflammation was substantially reduced compared with effects in the skin. Therefore, we determined whether intranasal administration of HHph-1-Foxp3 could prevent airway inflammation. BALB/c mice were sensitized with OVA and alum on day 0 and day 14 and then challenged with OVA on days 25, 26, and 27. HHph-1-Foxp3 was delivered by intranasally 2 h before challenge, and on day 28 mice were killed and all of the tissue samples and bronchoalveolar lavage (BAL) samples were prepared for further analysis. Eosinophils, lymphocytes, and total cell numbers in the BAL were counted, and HHph-1-Foxp3–treated mice showed a dramatic reduction of cell numbers compared with HHph-1-EGFP–treated mice (Fig. 5 A–C). Intranasal administration of HHph-1-Foxp3 reduced Th2 cytokine expression, including that of IL-4 and IL-13. By contrast, HHph-1-EGFP–treated mice have significant levels of these cytokines in BAL fluid (Fig. 5 D and E). The IFN-γ (Th1 cytokine) levels were not different among all samples (Fig. 5F), and the serum IgE level was strongly repressed by HHph-1-Foxp3 treatment but not by HHph-1-EGFP treatment (Fig. 5G). Treatment with HHph-1-Foxp3 prevented the infiltration of inflammatory cells into the peribronchial and perivascular areas, and very few goblet cells were detected around the bronchial airway (Fig. 5H). However, a high level of inflammatory cell infiltration and many periodic acid–Schiff (PAS)-positive and mucus-containing goblet cells around the bronchial airways were detected in the PBS- or HHph-1-EGFP–treated mice. These results clearly demonstrate the potential of HHph-1-Foxp3 to inhibit allergic airway inflammatory disease by intranasal administration.
Fig. 5.
Prevention of allergic airway inflammation by HHph-1-Foxp3. Mice were sensitized with alum and OVA for 3 wk and challenged with nebulized OVA. HHph-1-Foxp3 or HHph-1-EGFP was administered intranasally 2 h before the OVA challenge. Recruitment of (A) eosinophils, (B) lymphocytes, and (C) total cell number in BAL fluid was reduced by HHph-1-Foxp3. Th2 inflammatory cytokines, including (D) IL-4 and (E) IL-13 in BAL fluid, was reduced by HHph-1-Foxp3, and (F) the Th1 cytokine level of IFN-γ was measured as a control. (G) The level of serum IgE in the blood of the mice was reduced by HHph-1-Foxp3. (H) Reduction of infiltration of inflammatory cells in peri-bronchial and peri-vascular regions in the lung by HHph-1-Foxp3. Lung tissue of each mouse was stained by PAS and shown at 20× magnification. Five to nine mice from each group were used for the analysis.
Discussion
For development of an immunosuppressive agent to treat autoimmune or allergic diseases, regulation of T-cell activation and Treg function is an important target. Foxp3 has important roles not only as a key molecule in the development of regulatory T cells, but also as an inhibitor of T-cell activation and a repressor of transcription factors (31–33). Regulatory T cells have been widely used in experimental models for treating autoimmune diseases including IBD (13), arthritis (17), diabetes (18, 34), and atherosclerosis (35), allergic diseases including asthma (36) and dermatitis (37), and in preventing transplantation rejection (38, 39).
The most widely investigated methods to transfer the Foxp3 gene for generation of regulatory T cells involve retroviral (39, 40) and lentiviral (34, 41) vectors. However, they have a limited transfection efficiency, require extensive cell culture to derive, and are of questionable safety for human trials. We have generated Hph-1-EGFP fusion proteins with multiple copies of Hph-1-PTD at the N-terminal position of EGFP and compared their transduction efficiency in mouse splenocytes. Two repeats of Hph-1-PTD (HHph-1) resulted in optimal intracellular transduction (Fig. S4A). As with other PTD-containing proteins, HHph-1-Foxp3 can be rapidly delivered into most of the cells, and their retention time in the cells is less than 12 h both in vitro and in vivo (Fig. S4 B–D).
In this study, intracellular delivery of Foxp3 protein inhibits T-cell activation following TcR stimulation and confers suppressive function to CD4+CD25− T cells. HHph-1-Foxp3 treatment also inhibits FKH promoter activity when the FKH-Luc plasmid DNA is transfected into 293T cells containing HHph-1-Foxp3, suggesting that HHph-1-Foxp3 binds directly to DNA to regulate transcriptional activity (Fig. S5). The mutant form of HHph-1-Foxp3ΔC that lacks the C-terminal DNA-binding region did not generate suppressive T cells, suggesting that the C-terminal FKH domain is critical for Foxp3 to regulate transcription, which is consistent with previous work (33).
In our in vivo results, the functional activity of HHph-1-Foxp3 has been demonstrated in scurfy mice, which have no Foxp3-positive Treg cells. The autoimmune phenotype of scurfy mice is partially reversed by administration of HHph-1-Foxp3. The mice treated with HHph-1-Foxp3 live longer and have less tissue inflammation with fewer activated T cells compared with control groups. Consistent with our results, transfer of nTreg into scurfy mice prevented the development of disease (42), and transfer of antigen-specific Foxp3-transduced Treg effectively prevented autoimmune disease in animal models (43). More recently, scurfy mice treated with TGF-β to generate iTreg also did not show any signs of disease because the CD4 T cells retained the expression of Foxp3 for long periods (30). Migration of iTreg to all of the tissues suggests that regulatory T-cell transfer or Foxp3 transfer could be a potential therapy to inhibit inflammation in autoimmune or allergic diseases.
The HHph-1-Foxp3 protein studied here showed remarkable therapeutic effects on autoimmune colitis and an allergic experimental asthma model by systemic and local treatment, respectively. Treatment with HHph-1-Foxp3 did not affect the function of macrophages, dendritic cells, and B cells (Fig. S6), suggesting that the therapeutic effect of Foxp3 transfer might be specific for T-cell functions. The mechanism results in an inhibition of T-cell activation as judged by reduced inflammatory cytokine secretion and numbers of activated T cells and a trend toward induction of CD25 high T cells in both disease models (Fig. S7 A and B). Splenocytes from the mice treated with HHph-1-Foxp3 in the OVA-induced asthma model do not respond to OVA as much as control mice, but they are still responsive to polyclonal activation, implying in vivo generation of Treg (Fig. S7 C and D). The response against keyhole limpet hemocyanin in this model is still normal, suggesting that the response is antigen specific and that the effect is not due to a systemic toxic effect (Fig. S7E). Interestingly, the mice treated with HHph-1-Foxp3 still show reduced immunopathology 4 d following termination of treatment in the scurfy CD4 T-cell–mediated colitis model (Fig. S8A). However, when cells are transferred directly from scurfy mice treated with HHph-1-Foxp3 but the recipient mice are no longer treated, there is no apparent protection (Fig S8B). Treatment of these recipients with HHph-1-Foxp3 does inhibit development of colitis. These results suggest that the duration of the functional effects are somewhat transient.
From our results, the HHph-1-Foxp3–mediated approach might have the combined advantage of generating suppressor cells and inhibiting T-cell activation. As previously reported, a possible mechanism of HHph-1-Foxp3–mediated immunosuppression of induced anergic T cells might be related to the inhibition of a transcriptional factor such as NFAT and NF-κB (11, 12). This report demonstrates that both systemic and local administration of a cell-permeable Foxp3 protein can be used for the treatment of autoimmune and allergic diseases.
Materials and Methods
Construction and Purification of HHph-1-Foxp3 Protein.
Mouse Foxp3, EGFP, and PTD (HHph-1) were combined and cloned as outlined in Fig. 1 into a pRSET-B (Invitrogen) or pET28a (Novagen) plasmid, and each DNA was transformed into BL-21 (DE3) star E. coli (Novagen). The protein was expressed following induction of protein expression using 1 mM isopropyl thiogalactoside. After 5 h of induction at 37 °C and an additional overnight culture at 25 °C, cells were harvested and sonicated in 6 M guanidine buffer containing 10 mM Tris·HCl and 100 mM NaH2PO4 (pH 6.3). Lysates were clarified by centrifugation and loaded (10 mL/500 mL culture) on HisTrap chelating columns (Qiagen). Bound proteins were washed with 6 M urea buffer (pH 6.0), and the target proteins were eluted with 6 M urea buffer (pH 4.5). The eluted proteins were desalted using PD-10 Sephadex G-25 columns (GE Healthcare) with PBS (10% glycerol), and aliquots were stored at −80 °C. The purity of proteins was characterized by limulus amoebocyte lysate (LAL) (Lonza) and Picogreen assays (Invitrogen). The endotoxin level was 27.0 EU/mg, and the bacterial DNA level was 8.6 ng/mg of protein in four independent preparations (Table S1).
Statistics.
Statistical analysis of group differences was examined using the nonparametric Mann–Whitney U test or unpaired two-tailed Student's t tests. *P < 0.05 and **P < 0.01 were considered to be significant.
Supplementary Material
Acknowledgments
We thank Dr. Shimon Sakaguchi (Kyoto University) for kindly providing the mouse Foxp3 cDNA. This work was supported by National Institutes of Health P01 Grant AI036529 (to A.L.M.B.) and Grant RO1-HL084225 (to C.-G.L.); National Research Foundation of Korea Grant NRF-2010-0000733, NRF-R11-2007-040-02004-0, and by Seoul Development Institute Grant 11112 (to S.-K.L.); Korea Research Foundation Grant KRF-2010-0000733, NRF-R11-2007-040-02004-0, and by Seoul Development Institute Grant 11112 (to S.-K.L.); Korea Research Foundation Grant KRF-2006-E00010, Basic Science Research Program through National Research Foundation of Korea Grant 2010-0025389, and Hanyang University Grant HY-2010-00000000219 (to J.-M.C); and Czech Ministry of Education Grant MSM 00211620812 (to Z.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000400107/-/DCSupplemental.
References
- 1.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Clark LB, et al. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 6.Chang X, Zheng P, Liu Y. Homeostatic proliferation in the mice with germline FoxP3 mutation and its contribution to fatal autoimmunity. J Immunol. 2008;181:2399–2406. doi: 10.4049/jimmunol.181.4.2399. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 8.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 9.Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol. 2003;171:1216–1223. doi: 10.4049/jimmunol.171.3.1216. [DOI] [PubMed] [Google Scholar]
- 10.Carson BD, Ziegler SF. Impaired T cell receptor signaling in Foxp3+ CD4 T cells. Ann N Y Acad Sci. 2007;1103:167–178. doi: 10.1196/annals.1394.022. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 14.Maul J, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol. 2008;180:2830–2838. doi: 10.4049/jimmunol.180.5.2830. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 17.Morgan ME, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 18.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss L, et al. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 20.Bluestone JA. Regulatory T-cell therapy: Is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 21.Stock P, DeKruyff RH, Umetsu DT. Inhibition of the allergic response by regulatory T cells. Curr Opin Allergy Clin Immunol. 2006;6:12–16. doi: 10.1097/01.all.0000200502.69672.44. [DOI] [PubMed] [Google Scholar]
- 22.Hartl D, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, et al. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling EM, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 25.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 26.Gratton JP, et al. Cell-permeable peptides improve cellular uptake and therapeutic gene delivery of replication-deficient viruses in cells and in vivo. Nat Med. 2003;9:357–362. doi: 10.1038/nm835. [DOI] [PubMed] [Google Scholar]
- 27.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 28.Bennett RP, Dalby B, Guy PM. Protein delivery using VP22. Nat Biotechnol. 2002;20:20. doi: 10.1038/nbt0102-20. [DOI] [PubMed] [Google Scholar]
- 29.Rothbard JB, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 30.Huter EN, et al. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 32.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 33.Lopes JE, et al. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol. 2006;177:3133–3142. doi: 10.4049/jimmunol.177.5.3133. [DOI] [PubMed] [Google Scholar]
- 34.Wang R, et al. Foxp3-expressing CD4(+)T cells under the control of INF-gamma promoter prevent diabetes in NOD mice. Mol Ther. 2007;15:1551–1557. doi: 10.1038/sj.mt.6300208. [DOI] [PubMed] [Google Scholar]
- 35.Ait-Oufella H, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 36.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122:617–624.e6. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loser K, et al. In vitro-generated regulatory T cells induced by Foxp3-retrovirus infection control murine contact allergy and systemic autoimmunity. Gene Ther. 2005;12:1294–1304. doi: 10.1038/sj.gt.3302567. [DOI] [PubMed] [Google Scholar]
- 38.Joffre O, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chai JG, et al. Regulatory T cells, derived from naïve CD4+CD25- T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplantation. 2005;79:1310–1316. doi: 10.1097/01.tp.0000159147.56408.9c. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 41.Allan SE, et al. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 42.Sharma R, et al. Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice. J Autoimmun. 2007;29:10–19. doi: 10.1016/j.jaut.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.