Abstract
Lon is the major protease in the mitochondrial matrix in eukaryotes, and is well conserved among species. Although a role for Lon in mitochondrial biogenesis has been proposed, the mechanistic basis is unclear. Here, we demonstrate a role for Lon in mtDNA metabolism. An RNA interference (RNAi) construct was designed that reduces Lon to less than 10% of its normal level in Drosophila Schneider cells. RNAi knockdown of Lon results in increased abundance of mitochondrial transcription factor A (TFAM) and mtDNA copy number. In a corollary manner, overexpression of Lon reduces TFAM levels and mtDNA copy number. Notably, induction of mtDNA depletion in Lon knockdown cells does not result in degradation of TFAM, thereby causing a dramatic increase in the TFAM∶mtDNA ratio. The increased TFAM∶mtDNA ratio in turn causes inhibition of mitochondrial transcription. We conclude that Lon regulates mitochondrial transcription by stabilizing the mitochondrial TFAM∶mtDNA ratio via selective degradation of TFAM.
Keywords: AAA+ protease, mtDNA maintenance, quality control
Lon is the major protease in the mitochondrial matrix and is well conserved among species (1–4). Lon is a member of the super family of ATPases associated with diverse cellular activities (AAA+ ATPases)1, and forms a homooligomeric, ring-shaped structure (5, 6). Lon contributes to protein quality control surveillance in mitochondria by degrading preferentially oxidatively-modified or misfolded proteins before they aggregate (7–9). In bacteria, in addition to proteolysis of damaged proteins, Lon also plays a key role in turnover of specific unstable proteins involved in a variety of biological processes (3, 4). Similarly, the steroidogenic acute regulatory protein StAR, several subunits of cytochrome c oxidase, and oxidized mitochondrial aconitase are known to be Lon substrates in animal mitochondria (10–14).
In addition to its proteolytic function, mitochondrial Lon has the ability to bind DNA in vitro (15–17), and has been shown to interact with mtDNA in human cultured cells (18). However, the physiological role of DNA binding by Lon is not clear. In yeast, loss of PIM1, which is the ortholog of animal Lon protease, causes mtDNA deletion, impairs mitochondrial gene expression and results in respiratory deficiency (19, 20). A role for Lon has been postulated in mtDNA replication, transcription, and/or maintenance, but this remains to be validated. Lon was demonstrated to be a component of mitochondrial nucleoids, which are protein: DNA complexes formed to package mtDNA (21, 22). The major protein component of mtDNA nucleoids is mitochondrial transcription factor A (TFAM or mtTFA) (23, 24). TFAM contains two high mobility group (HMG) amino acid sequence boxes; it binds to mtDNA both specifically and nonspecifically (25). TFAM is essential for mtDNA transcription and for mtDNA packaging in mtDNA maintenance (26–30). Interestingly, mtDNA and TFAM levels are interdependent, such that knockdown of TFAM results in mtDNA depletion, and reduction of mtDNA copy number causes reduction of TFAM levels (26, 29, 31).
In this study, we investigated the role of Lon protease in regulating mtDNA maintenance and transcription, and the protein components of mitochondrial nucleoids in cultured cells. Our results argue strongly that Lon modulates mtDNA biogenesis by the selective degradation of TFAM.
Results
Overexpression of Lon Reduces TFAM Levels and mtDNA Copy Number.
Mitochondrial localization of the Drosophila Lon gene product (CG8798) was confirmed in Schneider cells by fluorescence microscopy (Fig. S1). Next, Drosophila Lon was subcloned into the inducible expression vector pMt/Hy under the control of the metallothionein promoter. The resulting expression vector, pMt/Lon/Hy, was introduced into Schneider cells, and stable cell lines harboring this plasmid were cultured in media with or without 0.2 mM CuSO4. After 10 d of incubation in the presence of copper, immunoblot analysis indicated a fivefold increase in Lon relative to that in the uninduced control cells (Fig. 1A). In contrast, expression of β-tubulin, used as a control protein, was unchanged. Levels of protein components of the mitochondrial nucleoid were measured by immunoblotting of cells carrying no plasmid, pMt/Hy, or pMt/Lon/Hy.
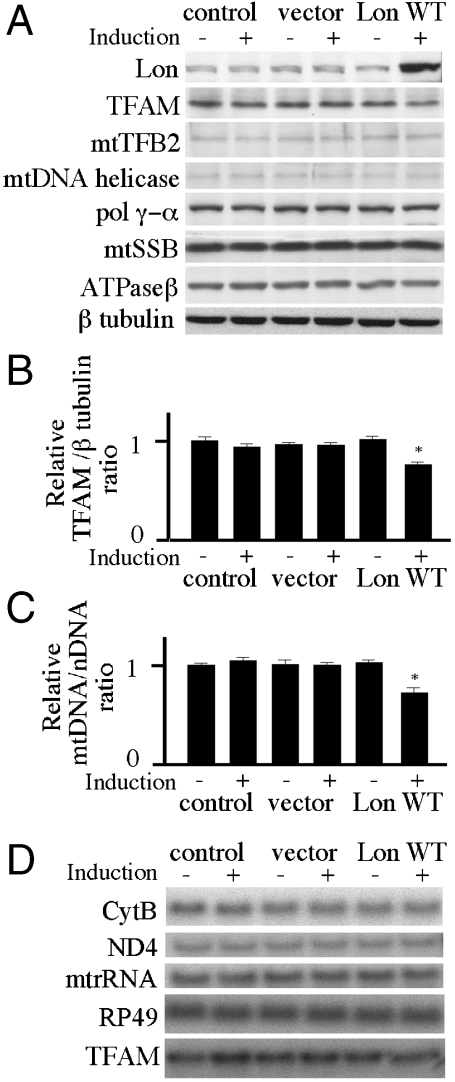
Fig. 1.
Expression of Drosophila Lon protease in Schneider cells. Schneider cells with no plasmid (control) or carrying pMt/Hy (vector) or pMt/Lon/Hy (Lon WT) were cultured for 10 d in the presence or absence of 0.2 mM CuSO4. (A) Protein extracts (20 μg) were fractionated by 7.5%, 10.5%, or 13.5% SDS-PAGE, transferred to nitrocellulose filters and probed with antibodies against Lon protease, TFAM, mtTFB2, mtDNA helicase, pol γ-α, mtSSB, ATPase β, or β tubulin as indicated. (B) The TFAM/ β tubulin ratio was quantitated by normalizing TFAM protein levels to β tubulin protein levels as described under Materials and Methods. Error bars indicate means ± standard error of three independent experiments. The asterisk indicates P < 0.05 in comparison to control. (C) Total DNA (10 μg) was extracted from Schneider cells or Schneider cells carrying pMt/Hy or pMt/Lon/Hy that were cultured for 10 d in the presence of 0.2 mM CuSO4. DNA was digested with XhoI, fractionated in a 0.7% agarose/ TBE gel, and then blotted to a nylon membrane. The membrane was hybridized with a radiolabeled probe for CytB, and then stripped and rehybridized with radiolabeled probe for the histone gene cluster as a control. The relative mtDNA copy number was quantitated as described under Materials and Methods. Error bars indicate means ± standard error of three independent experiments. The asterisk indicates P < 0.05 in comparison to control. (D) Total RNA (10 μg) was extracted from Schneider cells or Schneider cells carrying pMt/Hy or pMt/Lon/Hy after 10 d of culture in the presence or absence of 0.2 mM CuSO4. RNA was fractionated in a 1.2% agarose/formaldehyde gel, blotted to nylon membrane, and hybridized with radiolabeled probes for the mitochondrial transcripts 12S rRNA, ND4, and Cytb, the nuclear transcript RP49, and TFAM.
Overexpression of Lon reduced the level of TFAM to 75% of that in the control cells (Fig. 1 A, B). Levels of other proteins localized in mitochondrial nucleoids, including mtTFB2, mtDNA helicase, pol γ-α, and mtSSB, were not changed. We used Southern blots to quantify relative mtDNA copy number in the Lon overexpression cells. We found that the relative mtDNA copy number in the overexpression cells was ∼0.7-fold of that in the control cells (Fig. 1C). Northern blots were used to quantify the relative expression of the Cytb, ND4, and 12S rRNA genes in cells grown for 10 d in the presence or absence of copper. Overexpression of Lon did not show any significant changes on these mitochondrial transcript levels as compared to the control cells (Fig. 1D). Furthermore, TFAM mRNA levels were unchanged by Lon overexpression, indicating that the reduction in TFAM protein levels in the knockdown cells does not result from the reduction of TFAM mRNA.
RNAi-Dependent Knockdown of Lon Increases TFAM, Mitochondrial DNA Copy Number, and Mitochondrial Transcription.
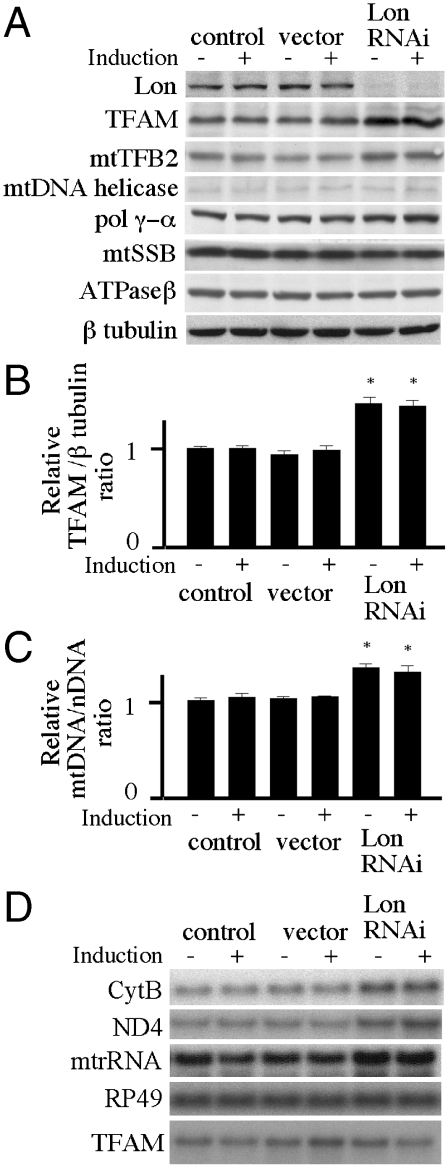
We reduced the abundance of Lon by expressing a metallothionein-inducible Lon-targeted RNAi species from the plasmid pMt/invLon/Hy. The RNA species produced a form of dsRNA hairpin homologous to Lon. Schneider cells stably expressing pMt/invLon/Hy showed the accumulation of oxidized proteins in mitochondria (Fig. S2), but the knockdown cells could be maintained for at least 6 mo under normal culture conditions. Schneider cells stably expressing pMt/invLon/Hy were cultured for 10 d in the absence or presence of 0.2 mM CuSO4. Immunoblot analysis of copper-treated cells showed that cells carrying pMt/invLon/Hy expressed > 10-fold less Lon than cells carrying the control vector (Fig. 2A). Even in the uninduced condition, the cells carrying pMt/invLon/Hy suppressed expression of Lon by > 10-fold, most likely due to leaky expression (32–35). In contrast, expression of β-tubulin was unchanged. Again levels of mitochondrial nucleoid proteins were measured by immunoblotting of cells carrying no plasmid, pMt/Hy, or pMt/invLon/Hy. Depletion of Lon increased the protein levels of TFAM and mtTFB2 ∼1.4-fold relative to their levels in the control cells (Fig. 2 A, B). At the same time, the levels of other mitochondrial nucleoid proteins were not changed significantly. Next, relative mtDNA copy number was measured in the knockdown cells in the presence or absence of copper and found to be ∼1.3-fold higher than in the control cells (Fig. 2C). These results suggest that the increase in mtDNA copy number results from the increased TFAM levels.
Fig. 2.
Expression of Drosophila Lon-targeted RNAi in Schneider cells. Schneider cells with no plasmid (control) or carrying pMt/Hy (vector) or pMt/invLon/Hy (Lon RNAi) were cultured for 10 d in the presence or absence of 0.2 mM CuSO4. (A) Immunoblot analysis was carried out as described in the legend to Fig. 1A. (B) The TFAM/β tubulin ratio was quantitated by normalizing TFAM protein levels to β tubulin protein levels as described under Materials and Methods. Error bars indicate means ± standard error of three independent experiments. The asterisk indicates P < 0.05 in comparison to control. (C) Relative mtDNA copy number was determined as described in the figure legend to Fig. 1C. Error bars indicate means ± standard error of three independent experiments. The asterisk indicates P < 0.05 in comparison to control. (D) Northern blot analysis using 12S rRNA, ND4, Cytb, RP49, and TFAM was carried out as described in the legend to Fig. 1D.
Northern blots were used to quantitate relative expression of the Cytb, ND4, and 12S rRNA genes in cells grown for 10 d in the presence or absence of copper. Basal expression of Lon-targeted RNAi increased the transcript levels of Cytb, ND4, and 12S rRNA to ∼1.4-fold that of the control cells (Fig. 2D). This increase in the mitochondrial transcripts may result from either the increase in mtTFB2 or mtDNA copy number, or both. In contrast, the level of transcripts from the nuclear gene RP49 was unchanged by Lon knockdown. Similar to that observed upon the overexpression of Lon, TFAM mRNA levels were unchanged in the Lon knockdown cells (Fig. 2D), indicating that the increase in TFAM protein in the knockdown cells does not result from an increase in the steady-state level of TFAM transcripts.
Lon Regulates mtDNA-Dependent TFAM Degradation in Schneider Cells.
Our data show that Lon regulates TFAM levels and mtDNA copy number. Because of the interdependent relationship of TFAM and mtDNA, we asked whether or not Lon degrades TFAM directly. To do so, we cultured the cell lines in the presence of ethidium bromide (EtBr), an inhibitor of mtDNA replication. In the control cells, EtBr treatment results in a rapid reduction of mtDNA copy number, and TFAM protein levels were also reduced, albeit more slowly than that of mtDNA (Fig. 3 A, B). At the same time, TFAM mRNA levels were unchanged (Fig. S3). Because the levels of other mitochondrial nucleoid proteins were not affected by mtDNA depletion with EtBr, we conclude that TFAM is depleted selectively following mtDNA reduction. In the Lon overexpression cells, EtBr treatment also resulted in the reduction of mtDNA (Fig. 3B). Interestingly, here the reduction of TFAM was faster than in the control cells (Fig. 3A). EtBr treatment also caused mtDNA depletion in the Lon knockdown cells. However in this case, the relative level of TFAM protein was increased 1.2-fold (Fig. 3A), while again TFAM mRNA levels were unchanged (Fig. S3). Similar to the control cells, the levels of other mitochondrial nucleoid proteins were unchanged in the EtBr treated Lon knockdown cells.
Fig. 3.
Dynamics of mitochondrial nucleoid proteins during mtDNA depletion in Lon overexpressing or knockdown Schneider cells. Schneider cells with no plasmid (control) or carrying pMt/Lon/Hy (Lon WT) or pMt/invLon/Hy (Lon RNAi) were cultured for 6 d in the presence of 200 ng/mL EtBr. The cells were harvested prior to EtBr treatment (0 d) and after 2, 4, and 6 days of EtBr treatment. (A) Immunoblot analysis was carried out as described in the legend to Fig. 1A. (B) Relative mtDNA copy number was determined as described in the legend to Fig. 1C. Error bars indicate means ± standard error of two independent experiments. (C) The TFAM/ mtDNA ratio was quantitated by normalizing TFAM levels to relative mtDNA copy number. Error bars indicate means ± standard error of two independent experiments.
To demonstrate conclusively the proteolytic role of Lon in mtDNA-dependent TFAM degradation, we established a cell line expressing a Lon mutant carrying a S880A amino acid substitution, in which the conserved serine in the proteolytic active site was replaced by alanine. The cell line expressing Lon S880A showed severe retardation of TFAM degradation following mtDNA depletion, likely because overexpression of Lon S880A results in a dominant negative phenotype that is caused by the formation of mixed oligomeric forms (Fig. S4). Taken together, our data show clearly that Lon is responsible for specific degradation of TFAM.
What Is the Physiological Role of Lon Degradation of TFAM?
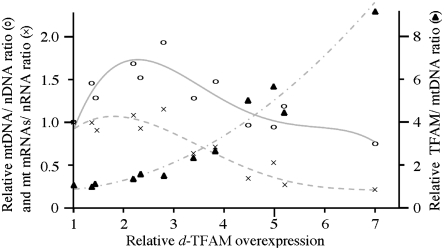
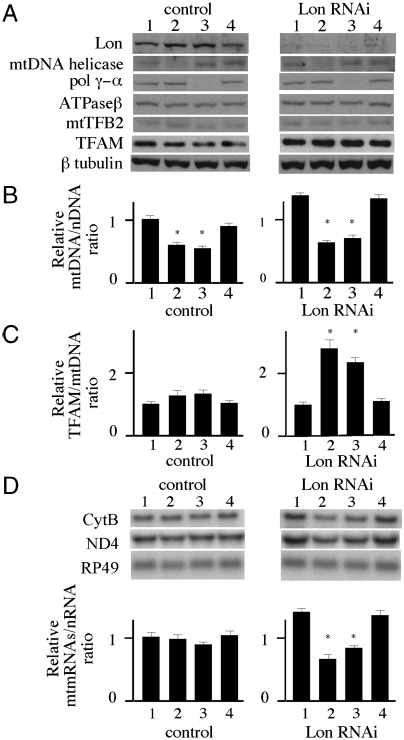
We sought to investigate the functional significance of the specific degradation of TFAM in mtDNA-depleted cells. In control cells treated with EtBr, the TFAM∶mtDNA ratio was raised transiently, and then reverted to normal levels within 6 d (Fig. 3C). However, Lon knockdown cells did not recover a normal TFAM∶mtDNA ratio. In mammalian cells, overexpression of TFAM causes suppression of mitochondrial transcription (36). We confirmed this phenomenon in Schneider cells overexpressing TFAM under the control of the metallothionein promoter (Fig. S5), and find that suppression occurs in cells containing a TFAM∶mtDNA ratio > 2 (Fig. 4). Interestingly, the highest overexpression level showed depletion of mtDNA copy number in addition to transcriptional suppression. We thus hypothesized that Lon regulates mtDNA transcription by stabilizing the cellular TFAM∶mtDNA ratio. To document this hypothesis, we measured mitochondrial transcript levels in mtDNA-depleted cells. Because EtBr also inhibits mtDNA transcription, we instead induced mtDNA depletion by knockdown of mtDNA replication factors and in particular, the catalytic subunit of mitochondrial DNA polymerase and the mtDNA helicase. Control and Lon knockdown cells were cultured for 10 d with dsRNA targeted against these proteins or GFP as a control, and the relevant protein levels were then evaluated by immunoblotting (Fig. 5A). The cells cultured with GFP dsRNA showed no change in these protein levels, whereas in the cell lines cultured with the mtDNA helicase or mtDNA polymerase dsRNAs, mtDNA copy number was reduced to ∼60% of that in the control cells (Fig. 5B). After the dsRNA treatments, the TFAM levels decreased to 75% in control cells, but increased 1.2-fold in the Lon knockdown cells (Fig. 5A). After the dsRNA treatments, the TFAM∶mtDNA ratio in the control cells was increased ∼1.3-fold relative to the control cells alone, and the ratio in the Lon RNAi cells was increased ∼2.5-fold (Fig. 5C). Mitochondrial transcripts in the control cells were unchanged with or without dsRNA treatment (Fig. 5D). However, the transcript levels in mtDNA-depleted Lon RNAi cells were reduced to 47%–60% of those in the mtDNA-depleted cells that showed transcript levels equivalent to 66%–84% of the control cells. Interestingly, the reduced mitochondrial transcript levels were lower than that of control cells. Together, these results indicate that Lon regulates mitochondrial transcription by controlling the TFAM∶mtDNA ratio.
Fig. 4.
Expression of TFAM in Schneider cells. Relative ratio of mtDNA copy number (open circles, solid line), mt mRNAs/nRNA (crosses, dotted line), and TFAM/mtDNA (filled triangles, dashed line) were measured at different overexpression levels of TFAM in Schneider cells as indicated. Relative mtDNA copy number was determined as described in the legend to Fig. 1C. The mt mRNAs/nRNA ratio was quantitated by normalizing mitochondrial transcript abundance (ND4 and Cyt b) to that of nuclear Rp49. TFAM/mtDNA ratio was quantitated by normalizing TFAM protein levels to relative mtDNA copy number.
Fig. 5.
Effects of Lon knockdown on mitochondrial transcript levels after mtDNA depletion in Schneider cells. Schneider cells carrying no plasmid (control) and or pMt/invLon/Hy (Lon RNAi) were cultured for 10 d in the presence or absence of dsRNA of mtDNA helicase, pol γ-α, or GFP as control. (A) Immunoblot analysis was carried out as described in the legend to Fig. 1A. (B) Relative mtDNA copy number was determined as described in the legend to Fig. 1C. Error bars indicate means ± standard error of two independent experiments. The asterisk indicates P < 0.05 in comparison to the control. (C) The ratio of TFAM/mtDNA was determined as described in the legend to Fig. 3C. Error bars indicate means ± standard error of two independent experiments. The asterisk indicates P < 0.05 in comparison to each cell that was cultured in the absence of dsRNA. (D) Northern blot analysis using ND4, Cytb, and RP49 was carried out as described in the legend to Fig. 1D. Relative mitochondrial mRNA levels were quantitated by normalizing ND4 and Cytb abundance to that of RP49. Error bars indicate means ± SE of two independent experiments. The asterisk indicates P < 0.05 in comparison to the control.
Discussion
We have established that Lon knockdown cell lines express < 10% of endogenous Lon protein levels, yet these cells grow for at least 6 mo. In human fibroblast cells, depletion of Lon over 4 d resulted in apoptotic cell death (37, 38), whereas Lon knockdown in human colon carcinoma cells allows survival for at least 15 d (18). Thus, the effects of Lon depletion may be species- or cell type- specific. As in a recent report with human rhabdomyosarcoma cells (39), depletion of Lon protease in Drosophila Schneider cells results in an increase in the levels of oxidized proteins in mitochondria, indicating that Lon is responsible for degradation of oxidized mitochondrial proteins, and suggesting that variations in cell viability as a result of Lon depletion may reflect varying cellular tolerances for oxidative damage to mitochondrial proteins.
It is known that TFAM protein levels are reduced coincident with mtDNA depletion in animal cells, but the mechanism for this phenomenon is unclear (31). Here, we show that Lon is responsible for degradation of TFAM upon mtDNA depletion. Moreover, Lon may be also responsible for TFAM degradation under normal conditions, because the cellular level of TFAM varies in concert with Lon levels. These findings imply that TFAM turnover is strongly dependent on Lon protease function. In addition to TFAM, we found that the mtTFB2 level is increased in Lon knockdown cells, so it may also be a specific substrate for the Lon protease. Other mitochondrial biogenesis proteins tested were unchanged appreciably in either Lon knockdown or overexpression cells. In Escherichia coli, some substrates for Lon overlap those of other AAA+ proteases such as the ClpP protease; notably, the ClpP ortholog in animal cells comprises another major protease in the mitochondrial matrix space (2, 3, 40). Therefore, the depletion of mitochondrial Lon may be compensated partially by mitochondrial ClpP protease. Alternatively, the residual Lon in knockdown cells may be sufficient to degrade its protein targets.
We found that mitochondrial transcripts in Lon knockdown cells are increased moderately in association with an increase of TFAM and mtTFB2 levels. Previous studies showed that the relative levels of mtDNA and TFAM are not critical to observe stimulation of mitochondrial transcription in Drosophila Kc167, and Schneider cells (29, 35). We suggest that in contrast, increase of mtTFB2, which is essential for mitochondrial transcription, may be responsible for up-regulation of mitochondrial transcription.
We show that degradation of TFAM by Lon protease is facilitated by mtDNA depletion. Interestingly, a similar phenomenon was reported in Bacillus subtilis. B. subtilis LonA, which is the ortholog of Lon, is involved in degradation of the structural maintenance of chromosomes protein (SMC), and the degradation of SMC is facilitated by DNAase treatment (41). Although the mechanisms by which Lon recognizes its target proteins are not well understood, a recent report showed that E. coli Lon recognizes specific aromatic residue-rich sequences that are hidden in the hydrophobic cores of native structures, but are accessible in unfolded structures (42). Interestingly, the HMG boxes in TFAM contain four conserved aromatic residues within a hydrophobic core, and these residues may be masked when TFAM binds DNA (25, 27, 29). Another possible explanation is that TFAM not bound to mtDNA becomes exposed to oxidative stress, whereas TFAM bound to mtDNA comprises part of the core of the mitochondrial nucleoids, and is thus surrounded by other proteins (43). Mitochondrial Lon has the ability to bind DNA and localizes in mitochondrial nucleoids (15–18). Our current hypothesis is that excess, free TFAM is degraded by Lon before the TFAM binds DNA. Alternatively, it seems possible, albeit more complicated, that excessive DNA compaction resulting from binding of high TFAM levels signals the degradation of TFAM by DNA-bound Lon. Further experiments are warranted to address these and other possibilities, and to clarify the link between the DNA-binding activity of Lon and TFAM turnover.
Suzuki and colleagues showed that mtDNA copy number was unchanged after 15 d of Lon knockdown in human colon carcinoma cells (18). This model differs from ours, in which we observe an increase in mtDNA copy number. We established our Lon knockdown cells over a period of 8 wks and because of leaky expression from the inducible promoter in the RNAi vector, Lon depletion was already ongoing prior to the 10 d induction period. Thus, one possible explanation for the difference we observe is that TFAM accumulation may be slower in their model and consequently, increased mtDNA copy number might not have been apparent. Another possible explanation is that compensation by other proteases such as ClpP (2, 40) might effect TFAM degradation in the Lon-depleted human colon carcinoma cells.
We found that upon EtBr treatment, the TFAM∶mtDNA ratio is nearly restored within 6 d in both control and Lon overexpressing cells, whereas restoration did not occur in Lon knockdown cells. Moreover, TFAM turnover resulting from mtDNA depletion by EtBr treatment is strongly reduced in cells overexpressing a protease-deficient Lon variant, which shows a dominant negative effect. Similar results involving restoration of normal TFAM/ mtDNA ratios were produced in the case of knockdowns of mtDNA helicase and mtDNA polymerase, though there the reduction of mtDNA is lower as compared to that upon EtBr treatment. Until stabilization occurs, excess overexpression of TFAM results in reduction of mitochondrial transcript levels; notably, in TFAM overexpressing cells, ratios of TFAM∶mtDNA > 2 show inhibitory effects on mitochondrial transcription. In the case of knockdown of mtDNA helicase or mtDNA polymerase, mitochondrial transcription levels are unchanged in control cells upon mtDNA depletion, whereas Lon knockdown cells showed a similar reduction in mitochondrial transcripts. Upon knockdown of mtDNA helicase or mtDNA polymerase, the TFAM∶mtDNA is ∼1.3 in control cells and > 2 in Lon knockdown cells, consistent with the observations in TFAM overexpression cells. Because there are environmental conditions under which mtDNA copy number is known to vary, such as during development and upon drug treatment (44, 45), transient Lon degradation of excess TFAM would be important to maintain normal mitochondrial transcription levels. We conclude that the TFAM∶mtDNA ratio is crucial for both mtDNA biogenesis and homeostasis, and that Lon stabilizes the TFAM∶mtDNA ratio by degradation of excess TFAM. With the example of TFAM, we provide direct evidence of the physiological role of Lon protease activity in mtDNA maintenance in animal cells, warranting future study of its potential involvement in the surveillance and turnover of other proteins involved in mtDNA replication, transcription, and translation.
Materials and Methods
Preparation of Lon Antibody.
A recombinant protein corresponding to amino acids Asp613 to Ser838 of Drosophila Lon (CG8798) was used to immunize rabbits to obtain polyclonal antibody.
Preparation of Inducible Plasmids Expressing Lon, TFAM, and Lon-Targeted RNAi.
The plasmid pMt/Lon/Hy, in which Lon is regulated by the metallothionein promoter and the plasmid pMt/invLon/Hy, which carries an inverted repeat of a nucleotide sequence from Lon cDNA that is transcribed from the metallothionein promoter were constructed as described in the SI Text. Plasmid pMt/TFAM/Hy is as described previously (35).
Generation and Induction of Stable Cell Lines.
Drosophila Schneider S2 cells were cultured at 25 °C in Drosophila Schneider Medium (Invitrogen) supplemented with 10% FBS. Cells were subcultured to 3 × 106 cells/mL every fifth day. Cells were transfected using Effecten (QIAGEN). Hygromycin-resistant cells were selected with 200 μg/mL hygromycin. Cells were passaged for 8 wks in hygromycin-containing medium and then cultured in standard medium. The cell lines were grown to a density of 3 × 106/mL and then treated with 0.2 mM CuSO4 to induce expression from the metallothionein promoter.
Immunoblotting.
Total cellular protein (20 μg per lane) was fractionated by 13.5%, 10.5%, or 7.5% SDS-PAGE and transferred to nitrocellulose filters. Immunoblotting was performed as described previously (46). Protein bands were visualized using ECL Western blotting reagents (Amersham). ECL luminescence was quantified on a Kodak Image Station 4000R. Antibodies against Drosophila mtSSB (47), TFAM (29), Pol γ-α (48), mtDNA helicase (32), ATPase β (32), mtTFB2 (35), and β tubulin (E7) (Developmental Studies Hybridoma Bank) were prepared and used as described. The ratio of the signals for TFAM and β tubulin was used to estimate the relative protein levels of TFAM. The TFAM immunoblotting experiments shown in Figs. 1, 2, 3, and 5 were performed two or thee times with each of the two or three independent cell lines carrying each plasmid construct, including control (no plasmid). The data presented represent one such experiment, and the quantitation is provided for the duplicate or triplicate experiments from one of two or thee cell lines.
Northern and Southern Blotting.
Northern blotting was performed as described previously (46), and the data were analyzed using a PhosphoImager (Molecular Dynamics). The signal for RP49 was used to normalize mitochondrial transcripts.
Southern blotting was performed as described previously (32), and the data were analyzed using a PhosphoImager (Molecular Dynamics). Blots were probed with radiolabeled DNAs for the mitochondrial gene Cytb and the nuclear histone gene cluster. The ratio of the signals for these two genes was used to estimate the relative copy number of mtDNA. The Northern and Southern blot experiments shown in Figs. 1, 2, 3, 4, and 5 were performed two or thee times with each of the two or three independent cell lines carrying each plasmid construct, including control (no plasmid). The data presented represent one such experiment, and the quantitation is provided for the duplicate or triplicate experiments from one of two or thee cell lines.
RNA Interference.
To generate double-stranded RNA (dsRNA) for RNAi, sequences directed against the protein to be silenced were amplified by PCR from each cDNA. Each primer used in the PCR contained a T7 RNA promoter followed by sequences specific for the targeted genes. The following primer sets were used for each protein: mtDNA helicase for GTAATACGACTCACTATAGGGCATGGAAAATGAGACGCGC and GTAATACGACTCACTATAGGGGATTGCTGACTAGAACCGC; DNA polymerase γ-α for TAATACGACTCACTATAGGGTGCCTACGCCTGCGGTGAGC and TAATACGACTCACTATAGGGCTCCAATGCTCGACTAAGAC; GFP for GTAATACGACTCACTATAGGGGGAGAAGAACTTTTCACTGG and GTAATACGACTCACTATAGGGTCTGCTAGTTGAACGCTTC. PCR products were used as templates for in vitro transcription using the T7 Megascript RNAi kit (Ambion). 3 × 107 S2 Drosophila tissue culture cells were plated into a T25 flask in 5 mL of medium without FBS. 100 μg of dsRNA was added and mixed by swirling. After 30 min, 5 mL of media containing 20% FBS was added. The cells were collected after 5 d culture and repeat dsRNA treatment. After 5 d after second dsRNA treatment, the cells were harvested for the analysis.
Supplementary Material
Acknowledgments.
We thank Marcos Oliveira for critical comments on the manuscript. This work was supported by National Institutes of Health (NIH) Grant GM45295 (to L.S.K.), and by a research grant (21A-6) for Nervous and Mental Disorders, a Health and Labour Science Research Grant on Intractable Diseases, and a grant of the Comprehensive Research Project on Health Sciences Focusing on Drug Innovation from the Ministry of Health, Labour, and Welfare of Japan (to Y.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008924107/-/DCSupplemental.
References
- 1.Lee I, Suzuki CK. Functional mechanics of the ATP-dependent Lon protease- lessons from endogenous protein and synthetic peptide substrates. Biochim Biophys Acta. 2008;1784:727–735. doi: 10.1016/j.bbapap.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koppen M, Langer T. Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol. 2007;42:221–242. doi: 10.1080/10409230701380452. [DOI] [PubMed] [Google Scholar]
- 3.Tsilibaris V, Maenhaut-Michel G, Van Melderen L. Biological roles of the Lon ATP-dependent protease. Res Microbiol. 2006;157:701–713. doi: 10.1016/j.resmic.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Chandu D, Nandi D. Comparative genomics and functional roles of the ATP-dependent proteases Lon and Clp during cytosolic protein degradation. Res Microbiol. 2004;155:710–719. doi: 10.1016/j.resmic.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Park SC, et al. Oligomeric structure of the ATP-dependent protease La (Lon) of Escherichia coli. Mol Cells. 2006;21:129–134. [PubMed] [Google Scholar]
- 6.Stahlberg H, et al. Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proc Natl Acad Sci USA. 1999;96:6787–6790. doi: 10.1073/pnas.96.12.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voos W. Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol. 2009;160:718–725. doi: 10.1016/j.resmic.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Van Melderen L, Aertsen A. Regulation and quality control by Lon-dependent proteolysis. Res Microbiol. 2009;160:645–651. doi: 10.1016/j.resmic.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Friguet B, Bulteau AL, Petropoulos I. Mitochondrial protein quality control: implications in ageing. Biotechnology Journal. 2008;3:757–764. doi: 10.1002/biot.200800041. [DOI] [PubMed] [Google Scholar]
- 10.Granot Z, et al. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol Endocrinol. 2007;21:2164–2177. doi: 10.1210/me.2005-0458. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda R, et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Ondrovicova G, et al. Cleavage site selection within a folded substrate by the ATP-dependent Lon protease. J Biol Chem. 2005;280:25103–25110. doi: 10.1074/jbc.M502796200. [DOI] [PubMed] [Google Scholar]
- 13.Hori O, et al. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 15.Lu B, et al. The ATP-dependent Lon protease of Mus musculus is a DNA-binding protein that is functionally conserved between yeast and mammals. Gene. 2003;306:45–55. doi: 10.1016/s0378-1119(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 16.Fu GK, Markovitz DM. The human LON protease binds to mitochondrial promoters in a single-stranded, site-specific, strand-specific manner. Biochemistry. 1998;37:1905–1909. doi: 10.1021/bi970928c. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, et al. DNA and RNA binding by the mitochondrial Lon protease is regulated by nucleotide and protein substrate. J Biol Chem. 2004;279:13902–13910. doi: 10.1074/jbc.M309642200. [DOI] [PubMed] [Google Scholar]
- 18.Lu B, et al. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J Biol Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- 19.Van Dyck L, Pearce DA, Sherman F. PIM1 encodes a mitochondrial ATP-dependent protease that is required for mitochondrial function in the yeast Saccharomyces cerevisiae. J Biol Chem. 1994;269:238–242. [PubMed] [Google Scholar]
- 20.Suzuki CK, Suda K, Wang N, Schatz G. Requirement for the yeast gene LON in intramitochondrial proteolysis and maintenance of respiration. Science. 1994;264:273–276. doi: 10.1126/science.8146662. [DOI] [PubMed] [Google Scholar]
- 21.Kucej M, Butow RA. Evolutionary tinkering with mitochondrial nucleoids. Trends Cell Biol. 2007;17:586–592. doi: 10.1016/j.tcb.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Cheng X, et al. PDIP38 associates with proteins constituting the mitochondrial DNA nucleoid. J Biochem. 2005;138:673–678. doi: 10.1093/jb/mvi169. [DOI] [PubMed] [Google Scholar]
- 23.Garrido N, et al. Composition and dynamics of human mitochondrial nucleoids. Mol Biol Cell. 2003;14:1583–1596. doi: 10.1091/mbc.E02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam TI, et al. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 2003;31:1640–1645. doi: 10.1093/nar/gkg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangelhoff TA, Mungalachetty PS, Nix JC, Churchill ME. Structural analysis and DNA binding of the HMG domains of the human mitochondrial transcription factor A. Nucleic Acids Res. 2009;37:3153–3164. doi: 10.1093/nar/gkp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanki T, et al. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol. 2004;24:9823–9834. doi: 10.1128/MCB.24.22.9823-9834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushima Y, et al. Functional domains of chicken mitochondrial transcription factor A for the maintenance of mitochondrial DNA copy number in lymphoma cell line DT40. J Biol Chem. 2003;278:31149–31158. doi: 10.1074/jbc.M303842200. [DOI] [PubMed] [Google Scholar]
- 28.Falkenberg M, et al. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat Genet. 2002;31:289–294. doi: 10.1038/ng909. [DOI] [PubMed] [Google Scholar]
- 29.Goto A, Matsushima Y, Kadowaki T, Kitagawa Y. Drosophila mitochondrial transcription factor A (d-TFAM) is dispensable for the transcription of mitochondrial DNA in Kc167 cells. Biochem J. 2001;354:243–248. doi: 10.1042/0264-6021:3540243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson NG, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 31.Seidel-Rogol BL, Shadel GS. Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res. 2002;30:1929–1934. doi: 10.1093/nar/30.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushima Y, Kaguni LS. Differential phenotypes of active site and human autosomal dominant progressive external ophthalmoplegia mutations in Drosophila mitochondrial DNA helicase expressed in Schneider cells. J Biol Chem. 2007;282:9436–9444. doi: 10.1074/jbc.M610550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushima Y, Adan C, Garesse R, Kaguni LS. Functional analysis by inducible RNA interference in Drosophila melanogaster. Methods in Molecular Biology. 2007;372:207–217. doi: 10.1007/978-1-59745-365-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsushima Y, Adan C, Garesse R, Kaguni LS. Drosophila mitochondrial transcription factor B1 modulates mitochondrial translation but not transcription or DNA copy number in Schneider cells. J Biol Chem. 2005;280:16815–16820. doi: 10.1074/jbc.M500569200. [DOI] [PubMed] [Google Scholar]
- 35.Matsushima Y, Garesse R, Kaguni LS. Drosophila mitochondrial transcription factor B2 regulates mitochondrial DNA copy number and transcription in schneider cells. J Biol Chem. 2004;279:26900–26905. doi: 10.1074/jbc.M401643200. [DOI] [PubMed] [Google Scholar]
- 36.Pohjoismaki JL, et al. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 2006;34:5815–5828. doi: 10.1093/nar/gkl703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngo JK, Davies KJ. Importance of the Lon protease in mitochondrial maintenance and the significance of declining Lon in aging. Ann N Y Acad Sci. 2007;1119:78–87. doi: 10.1196/annals.1404.015. [DOI] [PubMed] [Google Scholar]
- 38.Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radical Biol Med. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radical Biol Med. 2009;46:1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu AY, Houry WA. ClpP: a distinctive family of cylindrical energy-dependent serine proteases. FEBS Lett. 2007;581:3749–3757. doi: 10.1016/j.febslet.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 41.Mascarenhas J, et al. Dynamic assembly, localization, and proteolysis of the Bacillus subtilis SMC complex. BMC Cell Biol. 2005;6:28. doi: 10.1186/1471-2121-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gur E, Sauer RT. Degrons in protein substrates program the speed and operating efficiency of the AAA+ Lon proteolytic machine. Proc Natl Acad Sci USA. 2009;106:18503–18508. doi: 10.1073/pnas.0910392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogenhagen DF, Rousseau D, Burke S. The layered structure of human mitochondrial DNA nucleoids. J Biol Chem. 2008;283:3665–3675. doi: 10.1074/jbc.M708444200. [DOI] [PubMed] [Google Scholar]
- 44.Foster C, Lyall H. HIV and mitochondrial toxicity in children. J Antimicrob Chemother. 2008;61:8–12. doi: 10.1093/jac/dkm411. [DOI] [PubMed] [Google Scholar]
- 45.Moraes CT. What regulates mitochondrial DNA copy number in animal cells? Trends Genet. 2001;17:199–205. doi: 10.1016/s0168-9525(01)02238-7. [DOI] [PubMed] [Google Scholar]
- 46.Matsushima Y, Farr CL, Fan L, Kaguni LS. Physiological and biochemical defects in carboxyl-terminal mutants of mitochondrial DNA helicase. J Biol Chem. 2008;283:23964–23971. doi: 10.1074/jbc.M803674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farr CL, Wang Y, Kaguni LS. Functional interactions of mitochondrial DNA polymerase and single-stranded DNA-binding protein. Template-primer DNA binding and initiation and elongation of DNA strand synthesis. J Biol Chem. 1999;274:14779–14785. doi: 10.1074/jbc.274.21.14779. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Farr CL, Kaguni LS. Accessory subunit of mitochondrial DNA polymerase from Drosophila embryos. Cloning, molecular analysis, and association in the native enzyme. J Biol Chem. 1997;272:13640–13646. doi: 10.1074/jbc.272.21.13640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.