Abstract
T-cell homeostasis is essential for normal functioning of the immune system. IL-7 receptor (IL-7R) and T-cell receptor (TCR) signaling are pivotal for T-cell homeostatic regulation. The detailed mechanisms regulating T-cell homeostasis and how IL-7R and TCR signaling are coordinated are largely unknown. Here we demonstrate that T cell-specific deletion of cell-division cycle 42 (Cdc42) GTPase causes a profound loss of mature T cells. Deletion of Cdc42 leads to a markedly increased expression of growth factor independence-1 (Gfi-1) and represses expression of IL-7Rα. In the absence of Cdc42, aberrant ERK1/2 MAP kinase activity results in enhanced, TCR-mediated T-cell proliferation. In vivo reconstitution of effector-binding–defective Cdc42 mutants and the effector p21 protein-activated kinase 1 (PAK1) into Cdc42-deficient T cells showed that PAK1 is both necessary and sufficient for Cdc42-regulated T-cell homeostasis. Thus, T-cell homeostasis is maintained through a concerted regulation of Gfi-1–IL-7R–controlled cytokine responsiveness and ERK-mediated TCR signaling strength by the Cdc42-PAK1 signaling axis.
T-cell homeostasis is critical for both protective immunity and limitation of autoimmunity (1). T cells mature in the thymus and then enter the periphery, where they are maintained as resting naive cells. The mechanisms by which T-cell homeostasis is maintained are not fully defined. On one hand, the availability of several soluble cytokine “survival factors” regulates the overall size of the T-cell compartment (2). One of these factors, IL-7, is critical for T-cell survival and increases expression of the antiapoptotic molecule B-cell leukemia/lymphoma 2 (Bcl-2) (2, 3). On the other hand, MHC/T-cell receptor (TCR) signals also contribute to T-cell homeostasis (4). MHC/TCR interactions promote actin cytoskeletal rearrangement and immunological synapse formation that are followed by activation of proximal TCR signaling molecules, including ζ-chain (TCR)-associated protein kinase 70 kDa (ZAP70) and linker for activation of T cells (LAT), and distal TCR signaling molecules, such as MAP kinases (5). Low-level activation of the TCR signaling pathways has been shown to be critical for maintaining T-cell homeostasis (6). However, how the TCR- and cytokine-mediated signals are integrated in T cells to control their homeostasis remains unclear.
Cell-division cycle 42 (Cdc42) of the Rho GTPase family is an intracellular signal transducer that cycles between an inactive GDP-bound form and an active GTP-bound form under tight regulation (7, 8). Upon activation, Cdc42 can engage multiple effector molecules directly to elicit regulatory functions in cell actin cytoskeleton reorganization, adhesion, migration, proliferation, and survival (9, 10). To date, more than 10 putative Cdc42 effectors have been identified, mostly by direct protein pulldown or yeast two-hybrid approaches (10, 11). These findings may indicate that Cdc42 utilizes an individual effector or a combination of effectors to mediate a defined cell function. Many of these effectors contain the Cdc42/Ras-related C3 botulinum toxin substrate (Rac)-interactive binding (CRIB) motif or a Rac/Cdc42 (p21) binding domain (PBD) domain. Among them, a class of serine/threonine kinases, group A p21-activated kinases (PAK), are activated upon binding to Cdc42, resulting in activation of PI3K, JNK, and p38 pathways, inhibition of proapoptotic effects of BCL2-associated agonist of cell death (Bad), and actin reorganization (12–14). The Wiskott–Aldrich syndrome protein (WASP) and its homologous molecule, N-WASP, were shown to couple Cdc42 GTPase to the actin-related protein 2/3 (Arp2/3) complex in actin nucleation and polymerization (15–17). Partitioning-defective protein 6 (Par6) has been suggested to regulate Cdc42-mediated cell polarity through interaction with glycogen synthase kinase 3β (GSK3β) and adenomatous polyposis coli (APC) (18). The multidomain adaptor molecules IQ motif containing GTPase-activating protein 1 (IQGAP1) and IQGAP2 may be important for connecting Cdc42 signaling to actin and microtubule cytoskeleton structures (19). Recent studies also revealed that IQGAP1 can interact directly with APC for cell polarization and migration (20). Structurally, Cdc42 recognizes its effectors through the GTP-binding–sensitive switch I/II regions and additional distal sites that have been pinpointed recently by site-directed mutagenesis studies (21–24). These studies have identified a panel of effector-binding–defective mutants of Cdc42, among which Cdc42D38A is defective in binding to PAK, Cdc42D63H is defective in binding to IQGAP, and Cdc42I173AL174A is defective in binding to WASP and Par6. The availability of such specific effector-recognition–defective mutants allows a fine definition of the physiologic role of individual effectors among the Cdc42-regulated pathways.
Previous studies , using the overexpression of dominant active or negative Cdc42 mutants, suggest that Cdc42 is involved in T-cell cytoskeletal polarization (25, 26). However, the role and mechanism of signal transduction of Cdc42 in T-cell homeostatic regulation remains unclear. In the present studies, we generated a mouse strain with T cell-specific deletion of Cdc42 by cross-breeding the lymphocyte-specific protein tyrosine kinase (Lck)-cause recombination (Cre) transgenic mice with Cdc42flox/flox mice. By characterizing this mouse model, we demonstrate that Cdc42, through activation of its effector PAK1, positively regulates T-cell homeostasis by coordinating growth factor independence-1 (Gfi-1)/IL-7 receptor (IL-7R)-mediated survival signaling and ERK kinase-mediated TCR signaling. Our study proposes forward a regulatory mechanism for T-cell homeostasis that may have broad implications in T-cell biology.
Results
Cdc42 Deficiency Causes a Defect in T-Cell Homeostasis.
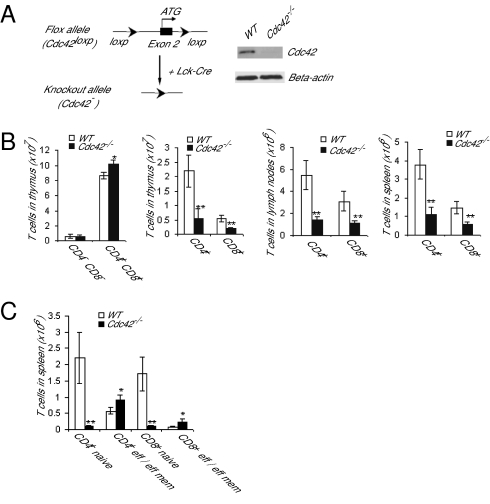
To determine the physiological function of Cdc42 in T cells, we generated a mouse strain bearing a conditional deletion of Cdc42 in the T-cell lineage by cross-breeding Lck-Cre transgenic mice with previously described Cdc42flox/flox mice (27) (Fig. 1A). The effective removal of Cdc42 protein was confirmed by Western blot of thymocytes from Cdc42flox/flox; Lck-Cre mice (Fig. 1A). Ablation of Cdc42 led to a significant reduction in mature CD4+ and CD8+ T cells in a variety of tissues, including thymus, lymph nodes, and spleen (Fig. 1B). Analysis of naive and effector/memory T cells in Cdc42−/− mice revealed a marked decrease in naive T cells (CD4+/CD44low/CD62Lhigh and CD8+/CD44low/CD62Lhigh) and a modest increase in effector/effector memory T cells (CD4+/CD44high/CD62Llow and CD8+/CD44high/CD62Llow) (Fig. 1C). These results demonstrate that Cdc42 plays a critical role in maintaining T-cell homeostasis.
Fig. 1.
Deletion of Cdc42 causes a defect in T-cell homeostasis. (A) Generation of Cdc42−/− T cells. The loxP/Cre-mediated gene-targeting strategy was used to generate the Cdc42 gene-deleted allele (Cdc42−) in T cells. Expression of Cdc42 in thymocytes was analyzed by anti-Cdc42 Western blotting. The level of β-actin was blotted in parallel as a loading control. (B) Cdc42 deficiency causes a loss of mature T cells. Single-cell suspensions from thymus, lymph nodes, and spleen were immunostained with anti-CD4, anti-CD8, and/or anti-TCRβ antibodies. Numbers of T cells are shown. (C) Cdc42 deficiency leads to a loss of naive T cells. The splenocytes were stained for naive and effector (eff) or effector memory (eff mem) T cells with a combination of anti-CD4, anti-CD8, anti-CD44, and anti-CD62L antibodies. The numbers of naive and effector or effector memory T cells are shown. Data are shown as means ± SD; n = 5. In B and C, **P < 0.01; *P < 0.05.
Deletion of Cdc42 Results in a Survival Defect.
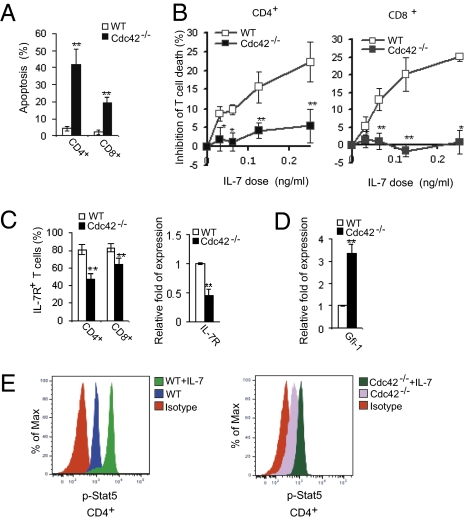
Survival regulation is pivotal for T-cell homeostasis. Annexin V-staining analysis revealed that Cdc42-deficient CD4+ and CD8+ T cells were more susceptible to apoptosis than their WT counterparts (Fig. 2A). Consistent with these data, we found that Cdc42−/− T cells were largely unresponsive to IL-7–induced survival, unlike WT T cells (Fig. 2B). These results suggest that one way Cdc42 maintains T-cell homeostasis is by promoting T-cell survival.
Fig. 2.
Cdc42-deficient T cells display impaired cell survival. (A) Cdc42 deficiency causes increased apoptosis. Freshly isolated splenocytes were stained with anti-CD4, anti-CD8, anti-CD62L and anti-CD44 antibodies followed by staining with Annexin V. Naive T cells were gated and analyzed for Annexin V staining by flow cytometry. Data are represented as means ± SD; n = 7. **P < 0.01. (B) Cdc42-deficient T cells gain resistance to IL-7–mediated T-cell survival. Splenocytes were cultured with different doses of IL-7 (0, 0.0313, 0.0625, 0.125, and 0.25 ng/mL) for 36 h and stained with anti-CD4, anti-CD8, and anti-TCRβ antibodies followed by staining with Annexin-V, and analyzed by flow cytometry. The results are shown as % inhibition of death by IL-7. Data are shown as means ± SD; n = 5. *P < 0.05; **P < 0.01. (C) Cdc42 deficiency leads to down-regulation of IL-7R expression. Splenocytes were stained with anti-CD4, anti-CD8, anti-CD62L, anti-CD44, and anti-IL-7R antibodies. Naive T cells were gated and analyzed for IL-7R expression by flow cytometry (Left). Data are shown as means ± SD; n = 5. **P < 0.01. Alternatively, naive T cells were purified from splenocytes and subjected to quantitative RT-PCR analysis of IL-7R mRNA. The expression of the GAPDH gene was used to normalize samples, and the relative fold expression is shown (Right). Data are shown as means ± SD; n = 3. **P < 0.01. (D) Cdc42 deficiency causes up-regulation of Gfi-1 expression. Naive T cells were purified from splenocytes and subjected to quantitative RT-PCR analysis of Gfi-1 mRNA. The expression of the GAPDH gene was used to normalize samples, and the relative fold expression is shown. Data are shown as means ± SD; n = 3. **P < 0.01. (E) Cdc42 deficiency results in defective Stat5 activation. Splenocytes were left unstimulated or were stimulated with IL-7 for 30 min, stained with anti-CD4, anti-CD62L, and anti-CD44 antibodies, fixed, permeabilized, and stained with anti-phosphorylated Stat5 (anti-pStat) antibody. The naive cells were gated and analyzed for pStat5 by flow cytometry. Representative results from five mice are shown.
The impaired IL-7–mediated survival of mutant T cells was associated with significantly reduced expression of IL-7Rα mRNA and protein (Fig. 2C). A major negative regulator of IL-7Rα expression in T cells is Gfi-1 (28, 29). Previously we observed a significant increase of Gfi-1 expression in Cdc42−/− hematopoietic progenitor cells (27), leading us to investigate whether increased Gfi-1 expression might explain decreased IL-7Rα expression in Cdc42−/− T cells. Significantly, we found that Cdc42−/− T cells had a roughly threefold increase in Gfi-1 mRNA (Fig. 2D).
Further analysis of IL-7R signaling revealed that IL-7–induced Stat5 activation was compromised in Cdc42−/− naive T cells (Fig. 2E). These results indicate that Cdc42 serves as a positive regulator of both IL-7Rα expression and IL-7Rα signaling. To determine whether IL-7 could stimulate Cdc42 activity directly, we added IL-7 to isolated WT T cells and assessed Cdc42 activation. Surprisingly Cdc42 activity was not detectably altered in response to IL-7 stimulation at various time points, whereas Cdc42 was activated upon TCR engagement (used as a positive control) (Fig. S1). Thus, our data suggest a regulatory circuit in which Cdc42 promotes T-cell survival by repressing Gfi-1 expression, and this repression, in turn promotes appropriate IL-7Rα expression. Nonetheless, Cdc42 is not an immediate transducer of IL-7 signaling per se.
Cdc42 Deficiency Leads to T-Cell Hyperproliferation and Activation.
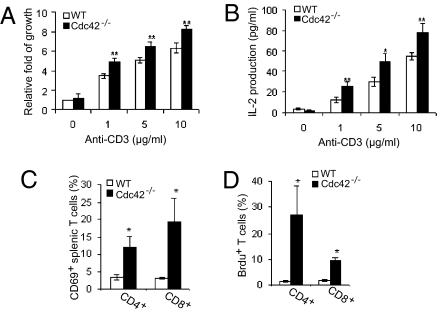
Because effector/memory T-cell numbers were increased in Cdc42−/− mice, and previous studies have suggested that Cdc42 has a role in TCR-mediated signal transduction, we next examined TCR signaling in Cdc42-deficient T cells. Compared with WT controls, Cdc42−/− naive T cells displayed higher TCR-induced proliferation activity (Fig. 3A) and had increased production of IL-2 (Fig. 3B). Accordingly, we found that the T-cell activation marker CD69 was significantly up-regulated in Cdc42−/− T cells (Fig. 3C). Moreover, in vivo BrdU labeling studies revealed increased cell-cycle progression into S phase in Cdc42−/− naive T cells (Fig. 3D). Therefore, depletion of Cdc42 induces T-cell activation. Consistent with the literature that T-cell activation induces cell death (1), Cdc42 deficiency caused more naive T-cell death upon TCR restimulation (Fig.S2). Taken together, these data suggest that Cdc42 plays an inhibitory role in naive T-cell proliferation and activation and thus controls the magnitude of TCR signal transduction.
Fig. 3.
Cdc42 deletion leads to T-cell hyperproliferation and hyperactivation. (A and B) Depletion of Cdc42 causes hyperproliferation in vitro. Purified splenic naive T cells were cultured with or without the indicated dosage of plate-bound anti-CD3 antibody together with 2 μg/mL anti-CD28 antibody. Culture supernatant was collected for analysis of IL-2 by ELISA at day 1 (B). The cells were cultured for another 48 h and assessed for cell growth rate as described in the text. The data were normalized to that of WT T cells in the absence of anti-CD3 and anti-CD28 antibodies (A). (C) Cdc42 depletion up-regulates the T-cell activation marker CD69. Splenocytes were stained with anti-CD4, anti-CD8, anti-TCRβ, and anti-CD69 antibodies. CD69 expression in T cells was analyzed by flow cytometry. (D) Cdc42 deficiency results in accelerated cell proliferation in vivo. Cdc42-deficient and WT Mice were injected i.p. with BrdU. Splenocytes were isolated 12 h later and analyzed for BrdU incorporation in CD4+ and CD8+ naive T cells. Data are shown as means ± SD; n = 5. *P < 0.05; **P < 0.01.
It is possible that the lymphopenic environment in Cdc42−/− mice contributes to the augmented proliferative renewal of Cdc42−/− T cells. We tested this possibility by adoptive transfer of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled Cdc42−/− and WT T cells into immunodeficient recombination activating gene 2 double-knockout (Rag2−/−) mice, followed by tracking of T-cell division in the recipient mice (Fig. S3): Thirty-seven percent of WT CD4+ T cells and 56% of WT CD8+ T cells underwent more than four and more than five divisions, respectively; in contrast, 69% of Cdc42−/− CD4+ T cells and 82% of Cdc42−/− CD8+ T cells divided more than four and more than five times, respectively. Thus, Cdc42−/− T cells undergo homeostatic proliferation. Together with the observed elevation of CD69 expression that is not induced by homeostatic proliferation (30), these results suggest that the increased cell-cycle S-phase progression in Cdc42−/− naive T cells is attributable to both cell-intrinsic hyperactivation and homeostatic proliferation.
Naive T-cell hyperactivation in Cdc42−/− mice probably is a causal factor for the increased number of effector/memory T cells in mutant mice. To demonstrate functional significance of these findings, we inoculated Cdc42−/− and control mice with lymphocytic choriomeningitis virus (LCMV) and assessed memory-cell proliferative potential upon in vitro restimulation with LCMV epitopic peptides. We found that LCMV-specific Cdc42-deficient memory T cells proliferated faster than WT memory T cells (Fig. S4), suggesting that Cdc42 negatively regulates memory T-cell proliferation.
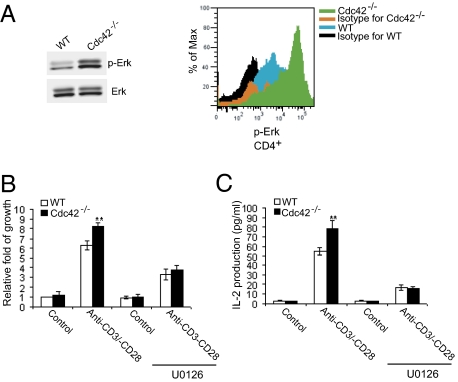
ERK Activation Is Necessary for Cdc42 Deficiency-Induced T-Cell Hyperactivation.
T-cell activation phenotypes, including proliferation, IL-2 production, and CD69 expression, are known to be regulated by p44/p42 ERK MAP kinase (31, 32). Indeed, immunoblotting of phosphorylated ERK found that ERK was constitutively activated in Cdc42−/− T cells (Fig. 4A). However, the augmented activity of ERK in Cdc42−/− T cells may be a result of increased numbers of effector/memory T cells in mutant mice and thus may not reflect the ERK activation status in Cdc42−/− naive T cells. To this end, we carried out flow cytometry analysis of ERK phosphorylation in naive T-cell compartments and were able to show that ERK activity was significantly higher in Cdc42−/− naive T cells (Fig. 4A). More importantly, culture of Cdc42−/− naive T cells in vitro with ERK inhibitor U0126 reversed both hyperproliferation and overproduction of IL-2 (Fig. 4 B and C), indicating that elevated ERK activity is required for the hyperactivation of Cdc42−/− T cells. In an investigation of the molecular mechanisms underlying ERK activation in Cdc42−/− T cells, we did not detect changes in activities of c-Raf and MAP kinase-ERK kinase (MEK) that act sequentially as activators of ERK (Fig. S5). Nonetheless, we found that ERK activation induced by TCR engagement was sustained in the absence of Cdc42 (Fig. S6). Moreover, in a measurement of JNK activity in Cdc42−/− T cells, we found that although JNK activation was maintained in WT T cells at 50 min of TCR stimulation, it was diminished in Cdc42−/− T cells, inversely correlating with prolonged activation of ERK. Given that JNK may negatively regulate ERK activation (33), our data raise the possibility that Cdc42 suppresses ERK activity by promoting JNK activation.
Fig. 4.
(A) Cdc42 deficiency leads to ERK activation. Pan T cells were purified from spleen and subjected to anti-phosphorylated ERK (anti-pERK) Western blotting. Total ERK also was blotted as loading control. Data shown are representative of three independent experiments (Left). Alternatively, splenocytes were stained with anti-CD4, anti-CD62L, and anti-CD44 antibodies, fixed, permeabilized, and stained with anti-pERK. The naive cells were gated and analyzed for pERK by flow cytometry (Right). Data shown are representative of five mice. (B and C) ERK activation is required for loss of Cdc42-induced T-cell hyperproliferation. Naive T cells were purified and cultured with or without the ERK inhibitor U0126 in the presence or absence of 10 μg/mL plate-bound anti-CD3 and 2 μg/mL anti-CD28 antibodies. The cell growth rate (B) and IL-2 production (C) were assayed. Data are shown as means ± SD; n = 5. **P < 0.01; *P < 0.05.
PAK1, but Not WASP, IQGAP, or Par6, Is both Required and Sufficient for Cdc42-Mediated T-Cell Homeostasis.
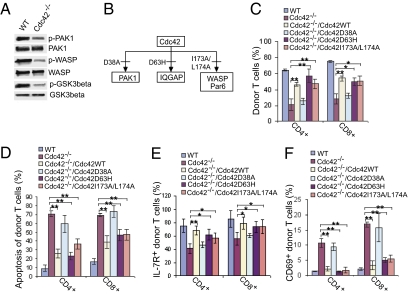
Cdc42 regulates cellular functions through direct binding and activation of its immediate effectors such as PAK1, IQGAP, WASP, and Par6 (10). We found that activation of PAK1, WASP, and GSK3β was significantly reduced in Cdc42−/− T cells (Fig. 5A), suggesting that PAK1, WASP, and/or Par6 (a regulator of GSK3β) could be involved in Cdc42-maintained T-cell homeostasis. To test this possibility, we used Cdc42 effector-binding mutants that are defective for binding to PAK (Cdc42D38A), or WASP and Par6 (Cdc42I173A/L174A). We also included the mutant Cdc42D63H that is defective for binding to IQGAP (21) (Fig. 5B and Fig. S7A). WT Cdc42 (Cdc42WT) or Cdc42 effector-binding mutants were introduced into Cdc42−/− T cells by adaptive bone marrow transplantation following retrovirus-mediated transduction into the Cdc42flox/flox; Lck-Cre bone marrow cells. Expression of exogenous HA-tagged Cdc42 mutants and Cdc42WT and deletion of endogenous Cdc42 were confirmed by immunoblotting the thymocytes isolated from the transplant-recipient mice (Fig. S7B). Flow cytometry analysis of splenic T cells from these chimeric mice revealed that reconstitution of Cdc42−/− T cells with Cdc42WT, Cdc42D63H, or Cdc42I173A/L174A could rescue T-cell homeostasis (Fig. 5C), survival (Fig. 5D), IL-7R expression (Fig. 5E), and normal activation (Fig. 5F). In contrast, Cdc42D38A was ineffective in rescuing these T-cell defects (Fig. 5 C–F). These data suggest that the PAK1 pathway, but not IQGAP, Par6, or WASP, is necessary for Cdc42-mediated T-cell homeostasis, survival, and activation.
Fig. 5.
The Cdc42 mutant defective in binding to PAK1 cannot rescue T-cell homeostasis. (A) Cdc42 deficiency causes an impaired activation of Cdc42 effectors. Pan T cells were purified from splenocytes of WT and Cdc42−/− mice and Western blotted with antibodies against phosphorylated (p) and total PAK1, WASP, and GSK3β. (B) Cdc42 mutants cannot bind to certain effectors. Cdc42D38A is defective in binding to PAK1, Cdc42D63H is defective in binding to IQGAP, and Cdc42I173AL174A is defective in binding to WASP and Par6. (C–F) Reconstitution of Cdc42D38A into Cdc42−/− T cells cannot rescue T-cell homeostasis. Bone marrow cells from WT and Cdc42-deficient mice were transduced with the indicated constructs. The transduced bone marrow cells were transplanted into lethally irradiated BoyJ mice. Recipient mice were killed 2 mo later. Splenocytes were prepared and analyzed for frequency (C), apoptosis (D), IL-7R expression (E), and activation status (F) of CD45.2+ donor naive T cells. Data are shown as means ± SD; n = 4. **P < 0.01; *P < 0.05.
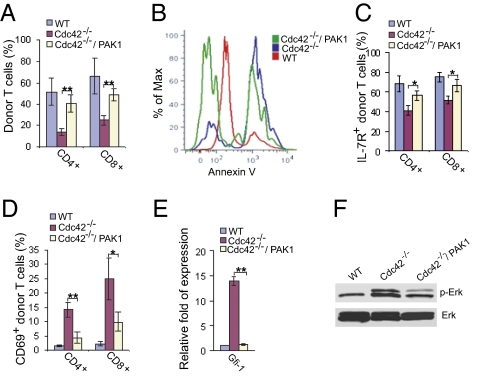
To confirm a sufficient role of PAK1 activity in Cdc42-mediated T-cell homeostatic phenotypes, we reconstituted Cdc42−/− T cells with constitutively active PAK1 mutant. We found that T-cell homeostasis, survival, IL-7R expression, and normal T-cell activation were mostly restored in the PAK1 mutant-reconstituted Cdc42−/− T cells (Fig. 6 A–D). Moreover, PAK1 restored normal Gfi-1 expression (Fig. 6E) and ERK activity (Fig. 6F). Taken together, these results demonstrate that PAK1 plays a central role in the regulation of Cdc42-mediated T-cell homeostatic phenotypes through its action on Gfi-1 and ERK.
Fig. 6.
PAK1 is essential for Cdc42-mediated T-cell homeostasis. (A–D) Reconstitution of constitutively active PAK1 into Cdc42−/− T cells restores T-cell homeostasis. Bone marrow chimeric mice expressing constitutively active PAK1 were generated much as described in Fig. 5. Two months after transplantation, splenocytes were isolated and analyzed for frequency (A), apoptosis (B), IL-7R expression (C), and activation status (D) of CD42+ donor naive T cells. (D) PAK1 restores normal Gfi-1 expression. Donor pan T cells were purified from spleens of chimeric mice and subjected to quantitative RT-PCR analysis of mRNA of Gfi-1. The expression of the GAPDH gene was used to normalize samples, and the relative fold expression is shown. Data are shown as means ± SD; n = 3. *P < 0.05; **P < 0.01. (E) PAK1 restores normal ERK activation. Donor pan T cells were purified from spleens of chimeric mice and subjected to anti-pERK Western blotting. Total ERK also was blotted as loading control.
Discussion
Mostly by overexpression of the constitutively active or dominant negative mutants in clonal cell lines, Cdc42 has been shown to regulate positively cytoskeleton reorganization, cell migration, proliferation, and survival (9, 10). This approach is hampered by its nonspecific nature (34). Indeed, distinct cell functions of Cdc42 have been observed in studies of Cdc42 knockout mouse models. For example, contrary to the prevailing view that Cdc42 promotes cell growth and survival, hematopoietic stem cells (HSCs) and HSC-derived myeloid cells deficient in Cdc42 exhibit hyperproliferative properties, and Cdc42-deficient HSCs do not display survival defects, whereas Cdc42-deficient myeloid cells show enhanced survival (27, 35). Further, a study of Cdc42-deficient fibroblastoid cells suggests that Cdc42 is not required for filopodia formation, directed migration, or mitosis (36). In lymphocytes, we found that Cdc42 deficiency did not affect migration of B cells (37). Thus, the physiologic role of Cdc42 in T lymphocytes may be revealed in the context of T cell-specific signaling only by a genetic means.
In this study, we examined the role of Cdc42 in T-cell homeostasis by targeting Cdc42 in the T-cell lineage. We show that disruption of Cdc42 causes a significant loss of T cells, particularly naive T cells. The impaired T-cell homeostasis in Cdc42−/− mice is caused by dampened T-cell survival and enhanced T-cell activation and proliferation. These phenotypes are the specific effects of Cdc42 deletion, because they are fully rescued by reconstitution of Cdc42. Our in vivo analyses reveal that Cdc42 maintains T-cell homeostasis by promoting IL-7R signaling and by controlling the strength of TCR signaling through its effector PAK1. As such, our results suggest a model in which IL-7R–mediated T-cell survival and TCR signaling, two of the major mechanisms by which T-cell homeostasis is maintained, are integrated through the physiological level of Cdc42 activity. The role of Cdc42 in T-cell homeostasis is reminiscent of that of Rac1 and Rac2, two closely related Rho family members, because deletion of Rac1/Rac2 also leads to a marked reduction in T-cell numbers (38). Further, because Cdc42 deficiency mimics Rac1/Rac2 deficiency, resulting in increased T-cell apoptosis (38), it appears that Cdc42 plays a role similar to that of Rac1 and Rac2 in T-cell survival. However, the role of Cdc42 in T-cell activation is clearly distinct from and in contrast with that of Rac1/Rac2, because T-cell proliferation is essentially impaired in Rac1/Rac2-deficient T cells (38).
IL-7 is a central mediator of T-cell survival and homeostatic maintenance of peripheral T cells (2, 3). We found that Cdc42 plays an important role in T-cell responsiveness to IL-7 through promoting IL-7R expression and IL-7–mediated Stat5 activation. Because IL-2 suppresses IL-7Rα expression (39), the increase of TCR-stimulated and ERK-mediated IL-2 production might explain the decreased expression of IL-7R in Cdc42-deficient T cells. With the appreciation that Gfi-1 represses IL-7R expression and regulates IL-2 signaling (28, 29), our data showing an increased expression of Gfi-1 in Cdc42−/− T cells suggest a regulatory circuit in which Cdc42 negatively regulates ERK activation and thus IL-2 production in the course of TCR signaling transduction, which in turn suppresses Gfi-1 expression and hence maintains a steady level of IL-7R expression. However, given that IL-7 could down-regulate IL-7R through Gfi-1 (29), the decreased expression of IL-7R in Cdc42−/− T cells may be an effect of elevated availability of IL-7 resulting from lymphopenia (40) caused by Cdc42 deficiency. Cdc42 deficiency causes a drastic increase in T-cell apoptosis but only a modest decrease in IL-7R expression, suggesting that the induction of IL-7R expression is an important but perhaps not exclusive pathway underlying Cdc42-mediated T-cell survival. Given that TCR restimulation-induced apoptosis is increased in Cdc42−/− T cells and that the Fas/Fas ligand (FasL) pathway plays a major role in TCR restimulation-induced apoptosis (1), Cdc42 also might regulate T-cell survival through control of Fas/FasL pathway.
Cdc42 deficiency leads to a hyperresponsiveness of T cells to TCR ligation manifested by enhanced T-cell proliferation. We attribute this hyperresponsiveness to an increased activation of ERK: Inhibition of the hyperactive ERK activity by a pharmacologic inhibitor readily reverses the hyperproliferative phenotype in Cdc42−/− T cells. The augmented activation of ERK in Cdc42−/− T cells is contradictory to observations in other cell types, suggesting that Cdc42 is a positive regulator of ERK activity (41, 42). Our data suggest that Cdc42 negatively regulates ERK activation in T cells, possibly by stimulating JNK activity. Although Raf and MEK1 have been shown to mediate ERK activation by Cdc42 (43, 44), we did not find significant alterations in Raf and MEK1 activities in Cdc42−/− T cells, suggesting that Cdc42 inhibition of ERK activation is independent of Raf and MEK1. In addition to the impact on JNK, Cdc42 also may suppress ERK activation through other mechanisms: (i) by up-regulation of negative regulators of T-cell activation such as cytotoxic T lymphocyte-associated protein 4 (CTLA-4), IκB, and suppressor of cytokine signaling 1 (Socs1) (45) and/or (ii) by activating LAT adaptor, removal of which recently was shown to cause a lymphoproliferative phenotype (46).
Our data show that Cdc42-deficient T cells undergo both intrinsic hyperproliferation and homeostatic proliferation. Because homeostatic proliferation gives rise to effector/effector memory T cells without up-regulation of T-cell activation marker CD69 (30), a combined effect of homeostatic proliferation and T-cell intrinsic hyperproliferation/hyperactivation may account for the observed increase of effector/effector memory T cells in Cdc42 knockout mice. Intriguingly, LCMV-specific Cdc42−/− memory T cells are hyperproliferative to LCMV epitopic peptide restimulation, suggesting an antagonistic role of Cdc42 in memory cell-mediated immune responses.
PAK1, WASP, and Par6, three immediate effectors of Cdc42, have been shown to play a role in immunological synapse formation and/or T-cell activation (47–49). We found that the activities of these three effectors were impaired in Cdc42-deficient T cells, suggesting that they may be involved in Cdc42-regulated T-cell functions. However, by reconstituting effector-binding–defective mutants of Cdc42 and active PAK1 mutants into Cdc42−/− T cells, we found that only PAK1 is necessary and sufficient for the Cdc42-mediated T-cell homeostatic phenotypes. Although WASP−/− mice also are lymphopenic (49), our data suggest that Cdc42 maintains T-cell homeostasis through a WASP-independent mechanism. It is possible that N-WASP compensates for WASP in Cdc42-maintained T-cell homeostasis, given that WASP and N-WASP play redundant roles in T-cell development (50). In view of the published literature on WASP and Par6, our study suggests that WASP and Par6 may regulate T-cell functions independently of Cdc42. In support of this notion, it has been shown that Cdc42 activation is not required for WASP recruitment to the T-cell–antigen-presenting cell contact site (51).
In summary, we identified Cdc42 as an essential coordinator of TCR signaling and cytokine signaling in the maintenance of T-cell homeostasis. Loss of Cdc42 in naive T cells leads to T-cell hyperactivation by promoting ERK activation and down-regulates IL-7R expression by up-regulating Gfi-1 expression. Further, we dissected in vivo Cdc42 effector pathways in T-cell homeostasis by using Cdc42 effector-binding–defective mutants and show that PAK1, but not WASP, IQGAP, or Par6, is necessary and sufficient to maintain T-cell homeostasis through promoting T-cell survival and repressing T-cell hyperactivation downstream of Cdc42. Further understanding of the role of Cdc42 in T-cell biology could provide important insights into the mechanisms of immunodeficiency and lymphoproliferative disorders.
Materials and Methods
Phenotypic Characterization of T Cells by Cell-Surface Staining.
Single-cell suspensions were prepared from thymus, spleen, and lymph nodes. Cells were incubated for 20 min at room temperature with various combinations of the following cell-surface marker antibodies: anti-CD4, anti-CD8, anti-CD69, anti-TCRβ (H57-597), anti-CD44, anti-CD62L, and anti-IL-7R (BD Biosciences). Immunolabeled cells were analyzed by flow cytometry on a FACSCanto system using FACSDiVa software (BD Biosciences).
Cell Apoptosis Analysis.
Freshly isolated splenocytes or splenocytes cultured at 37 °C with IL-7 (R&D Systems) were incubated with anti-CD4, anti-CD8, anti-CD44, anti-CD62L, and/or anti-TCRβ antibodies for 20 min. Cells were washed, incubated with Annexin V (BD Biosciences) for 20 min, and then analyzed by flow cytometry.
Gene-Expression Analysis.
RNA was isolated from purified splenic T cells using the RNeasy Micro Kit (Qiagen) and converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (ABI). Real-time PCR was performed with a Taqman system on a 7900HT Real-Time machine (ABI). The primer sequences for amplification of IL-7Rα cDNA were AGCAACTGGACGCATGTATCTTTAT (forward primer) and GGGAATGGATCGGACTTTGATTTCA (reverse primer). The Taqman primer/probe sets for Gfi1 were Mm00515853_m1. Data were analyzed using SDS 2.3 software (ABI) and were normalized to GAPDH.
Flow Cytometry Analysis of STAT5 and ERK Activation.
Isolated splenocytes were stimulated (or not) with 80 ng/mL IL-7 at 37 °C for 30 min. Cells were immunolabeled with anti-CD4, anti-CD8, anti-CD44, and anti-CD62L antibodies and then were fixed, permeabilized with methanol, stained with anti-phospho Stat5 or anti-phospho ERK antibody (BD Biosciences), and analyzed by flow cytometry.
In Vitro Proliferation and IL-2 Production.
Naive T cells from spleen were purified by FACS of TCRβ+/CD62Lhigh/CD44low cells. Naive T cells (5 × 105) were added to culture plates coated (or not) with various doses of anti-CD3 antibody (BD Biosciences) and 2 μg/mL anti-CD28 in the presence or absence of the ERK inhibitor U0126 (Sigma) and were cultured for 24 h. Supernatant was collected for assay of IL-2 using an ELISA kit (eBiosciences), and fresh medium was added. Cells were incubated for another 48 h and then analyzed for cell growth rate by a Non-Radioactive Cell Proliferation Assay kit (Promega).
In Vivo BrdU Incorporation.
Mice were injected i.p. with 500 μg BrdU. Twelve hours after injection, splenocytes were isolated, immunolabeled with antibodies against CD4, CD8, CD44, and CD62L, and then fixed, permeabilized, and incubated with anti-BrdU antibody according to the manufacturer's protocol (BD Biosciences). Immunolabeled cells then were analyzed by flow cytometry.
Immunoblotting.
Whole-cell lysates were prepared and separated by 10% SDS-PAGE. Expression or activation (phosphorylation) of ERK, PAK1, WASP, GSK3β, and Cdc42 was probed using the corresponding antibodies against total or phosphorylated ERK, PAK1, and GSK3β (Cell Signaling Technology); anti-phospho-WASP antibody (Bethyl Laboratories); anti-WASP antibody (Santa Cruz Biotechnology); and anti-Cdc42 antibody (Millipore).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010249107/-/DCSupplemental.
References
- 1.Sun H, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–426. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Wojciechowski S, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seddon B, Zamoyska R. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J Immunol. 2002;169:3752–3759. doi: 10.4049/jimmunol.169.7.3752. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–1016. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. doi: 10.1016/s0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 9.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 10.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions in vivo. Bioessays. 2007;29:356–370. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson JW, Cerione RA. Multiple roles for Cdc42 in cell regulation. Curr Opin Cell Biol. 2001;13:153–157. doi: 10.1016/s0955-0674(00)00192-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhao ZS, et al. A conserved negative regulatory region in alphaPAK: Inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 14.Bagrodia S, Dérijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 15.Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 16.Rohatgi R, et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 17.Symons M, et al. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 18.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda S, et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, et al. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Li R, et al. Localization of the PAK1-, WASP-, and IQGAP1-specifying regions of Cdc42. J Biol Chem. 1999;274:29648–29654. doi: 10.1074/jbc.274.42.29648. [DOI] [PubMed] [Google Scholar]
- 22.Abdul-Manan N, et al. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich syndrome’ protein. Nature. 1999;399:379–383. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- 23.Mott HR, et al. Structure of the small G protein Cdc42 bound to the GTPase-binding domain of ACK. Nature. 1999;399:384–388. doi: 10.1038/20732. [DOI] [PubMed] [Google Scholar]
- 24.Morreale A, et al. Structure of Cdc42 bound to the GTPase binding domain of PAK. Nat Struct Biol. 2000;7:384–388. doi: 10.1038/75158. [DOI] [PubMed] [Google Scholar]
- 25.Pernis AB. Rho GTPase-mediated pathways in mature CD4+ T cells. Autoimmun Rev. 2009;8:199–203. doi: 10.1016/j.autrev.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 26.Tskvitaria-Fuller I, et al. Specific patterns of Cdc42 activity are related to distinct elements of T cell polarization. J Immunol. 2006;177:1708–1720. doi: 10.4049/jimmunol.177.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, et al. Cdc42 critically regulates the balance between myelopoiesis and erythropoiesis. Blood. 2007;110:3853–3861. doi: 10.1182/blood-2007-03-079582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doan LL, et al. Growth factor independence-1B expression leads to defects in T cell activation, IL-7 receptor alpha expression, and T cell lineage commitment. J Immunol. 2003;170:2356–2366. doi: 10.4049/jimmunol.170.5.2356. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: A novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Murali-Krishna K, Ahmed R. Cutting edge: Naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto N, Tezuka K, Kato M, Abe R, Tsuji T. PI3-kinase and MAP-kinase signaling cascades in AILIM/ICOS- and CD28-costimulated T-cells have distinct functions between cell proliferation and IL-10 production. Biochem Biophys Res Commun. 2003;310:691–702. doi: 10.1016/j.bbrc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 32.Dumont FJ, Staruch MJ, Fischer P, DaSilva C, Camacho R. Inhibition of T cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production. J Immunol. 1998;160:2579–2589. [PubMed] [Google Scholar]
- 33.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. doi: 10.1016/j.tcb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, et al. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc Natl Acad Sci USA. 2007;104:5091–5096. doi: 10.1073/pnas.0610819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czuchra A, et al. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol Biol Cell. 2005;16:4473–4484. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo F, Velu CS, Grimes HL, Zheng Y. Rho GTPase Cdc42 is essential for B-lymphocyte development and activation. Blood. 2009;114:2909–2916. doi: 10.1182/blood-2009-04-214676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo F, Cancelas JA, Hildeman D, Williams DA, Zheng Y. Rac GTPase isoforms Rac1 and Rac2 play a redundant and crucial role in T-cell development. Blood. 2008;112:1767–1775. doi: 10.1182/blood-2008-01-132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue HH, et al. IL-2 negatively regulates IL-7 receptor alpha chain expression in activated T lymphocytes. Proc Natl Acad Sci USA. 2002;99:13759–13764. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosco N, Agenès F, Ceredig R. Effects of increasing IL-7 availability on lymphocytes during and after lymphopenia-induced proliferation. J Immunol. 2005;175:162–170. doi: 10.4049/jimmunol.175.1.162. [DOI] [PubMed] [Google Scholar]
- 41.Szczur K, Xu H, Atkinson S, Zheng Y, Filippi MD. Rho GTPase CDC42 regulates directionality and random movement via distinct MAPK pathways in neutrophils. Blood. 2006;108:4205–4213. doi: 10.1182/blood-2006-03-013789. [DOI] [PubMed] [Google Scholar]
- 42.Cheng TL, Symons M, Jou TS. Regulation of anoikis by Cdc42 and Rac1. Exp Cell Res. 2004;295:497–511. doi: 10.1016/j.yexcr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Chong H, Guan KL. Function of the Rho family GTPases in Ras-stimulated Raf activation. J Biol Chem. 2001;276:34728–34737. doi: 10.1074/jbc.M103496200. [DOI] [PubMed] [Google Scholar]
- 45.Saito T, Yamasaki S. Negative feedback of T cell activation through inhibitory adapters and costimulatory receptors. Immunol Rev. 2003;192:143–160. doi: 10.1034/j.1600-065x.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 46.Mingueneau M, et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity. 2009;31:197–208. doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Phee H, Abraham RT, Weiss A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat Immunol. 2005;6:608–617. doi: 10.1038/ni1199. [DOI] [PubMed] [Google Scholar]
- 48.Sasahara Y, et al. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell. 2002;10:1269–1281. doi: 10.1016/s1097-2765(02)00728-1. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cotta-de-Almeida VL, et al. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proc Natl Acad Sci USA. 2007;104:15424–15429. doi: 10.1073/pnas.0706881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cannon JL, et al. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity. 2001;15:249–259. doi: 10.1016/s1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.