Abstract
For nearly 100 million years, the India subcontinent drifted from Gondwana until its collision with Asia some 50 Ma, during which time the landmass presumably evolved a highly endemic biota. Recent excavations of rich outcrops of 50–52-million-year-old amber with diverse inclusions from the Cambay Shale of Gujarat, western India address this issue. Cambay amber occurs in lignitic and muddy sediments concentrated by near-shore chenier systems; its chemistry and the anatomy of associated fossil wood indicates a definitive source of Dipterocarpaceae. The amber is very partially polymerized and readily dissolves in organic solvents, thus allowing extraction of whole insects whose cuticle retains microscopic fidelity. Fourteen orders and more than 55 families and 100 species of arthropod inclusions have been discovered thus far, which have affinities to taxa from the Eocene of northern Europe, to the Recent of Australasia, and the Miocene to Recent of tropical America. Thus, India just prior to or immediately following contact shows little biological insularity. A significant diversity of eusocial insects are fossilized, including corbiculate bees, rhinotermitid termites, and modern subfamilies of ants (Formicidae), groups that apparently radiated during the contemporaneous Early Eocene Climatic Optimum or just prior to it during the Paleocene-Eocene Thermal Maximum. Cambay amber preserves a uniquely diverse and early biota of a modern-type of broad-leaf tropical forest, revealing 50 Ma of stasis and change in biological communities of the dipterocarp primary forests that dominate southeastern Asia today.
Keywords: biogeography, arthropoda, eusociality, tropical forests
The dramatic geological history of the Indian subcontinent has had profound effects on regional climates and biota. The Indian plate separated from Africa along with Madagascar in the Middle Jurassic ca. 160 Ma, then from Madagascar in the mid-Cretaceous ca. 90 Ma, moving at the remarkable rate of 15–25 cm/y before colliding into Asia in the early Cenozoic, uplifting the Himalayas. Dates of initial contacts between India and Asia occurred approximately 50 Ma, with suturing completed by 49 Ma (1, 2), although other critical estimates of initial contact differ from the Paleocene, ca. 55 Ma (3), to as young as the Eocene–Oligocene boundary 35 Ma (4).
The present biota of India, including Sri Lanka, harbors taxa that reflect its geological history, with assorted vertebrates and plants having distributions that also encompass Africa, Madagascar, and often the Seychelles (these islands are a Cretaceous fragment of the India–Madagascar landmass). Such taxa include, for example, boid snakes of the genus Eryx (5); Apocheilus and Pachypanchax killifishes (6); the unusual frog Naiskabatrachus from the western Ghats, which is closely related to the endemic seychellian family Sooglossidae (7); and two sister genera of cichlid fishes, Etroplus in southern India and Paretroplus in Madagascar (8). Plant taxa include two genera of trees in the Moraceae that are closely related to figs (Ficus) [Mesognyne in Africa and Antiaris in India (9)], as well as the family Dipterocarpaceae, which is discussed below. This common, disjunct distribution is usually interpreted as a relict of gondwanan drift, though dispersal has also been invoked for some cases (see, for example, refs. 10–12).
Indeed, phylogenetic relationships further reveal that additional Recent taxa in India are basal to Southeast Asian lineages, suggesting immigration from the docked subcontinent, the so-called “out-of-India” hypothesis. Support for this hypothesis comes from studies of some amphibians, notably ranid frogs (13) and caecilians of the families Uraetyphlidae and Ichthyophiidae (14) as well as some plants (e.g., Crypteroniaceae; ref. 15). An example of the opposite, “out-of-Asia” direction of immigration involves Paracrostoma freshwater snails (16).
As India collided with Asia, there was undoubtedly significant biotic interchange between both landmasses, with India being both biotic “ferry” (17) and biotic sink. The question is how endemic was India’s paleobiota prior to contact, and did it contain numerous African and Malagasy taxa? One-hundred million years of isolation of the subcontinent (partly with Madagascar and the Seychelles) presumably spawned a highly diverse and endemic biota, analogous to the remarkable endemism presently seen in Australia, but which has been isolated for only the past 30 myr since the Oligocene.
Interestingly, the Cretaceous and Early Cenozoic vertebrate fossil record of India thus far reveals little endemism. Fossil mammals from the Early Eocene of western India, for example, have affinities with contemporaneous taxa from Europe (18, 19), and models have been proposed of broad land–bridge connections between drifting India with Africa, Madagascar, and/or island arcs from the Asian landmass (4, 20), which explains the existence of widespread groups in Paleogene India. Abundant evidence is presented here of recently excavated, diverse arthropod fossils preserved in extensive amber deposits from the Cambay Shale [early Ypresian (50–52 Ma)] of western India, which directly addresses conflicting models of the biotic and geological history of India and southern Asia.
Cambay amber was deposited not only during a tectonically critical period, but also at the peak of the Early Eocene Climatic Optimum (EECO), a time when tropical and subtropical biomes spread globally (21, 22). Radiations of disparate, ecological keystone insects that are hypothesized to have taken place during the EECO include highly social lineages, pollinators, parasitoids, and diverse phytophages (23, 24). These specifically are the corbiculate bees, including the eusocial stingless bees and honey bees, which are competitively superior pollinators; ants of the presently dominant subfamilies Formicinae, Dolichoderinae, and Myrmecinae; the “higher” termites (family Termitidae, comprising 80% of all Recent termite species); the highly diverse schizophoran flies and chalcidoid wasps; as well as the phytophagous lineages of neococcoid scale insects and the macrolepidopterans. Although diverse arthropods are preserved as compressions in Eocene shales from western North America and Europe, it is the vast mid-Eocene (Lutetian) Baltic amber deposits that have contributed the most to understanding Early Cenozoic insects (23). Because amber preserves minute, delicate organisms with lifelike, microscopic fidelity, this preservation greatly refines inference on relationships of extinct taxa. Baltic amber and the contemporaneous Rovno amber from the Ukraine, as well as the latest Paleocene-earliest Eocene Oise amber from France, were formed in a warm temperate/“subtropical summerwet” paleoenvironment (25), although the first two of these were formed by conifers and the Oise amber had an angiosperm origin (26, 27). The Cambay Shale amber deposits, in contrast, were formed in a broad-leaved, tropical, everwet paleoenvironment at the paleoequator (vs. 47–52 °N for Baltic and 42–47 °N for Oise amber). Besides the Cambay amber, the only other major deposits of fossiliferous amber produced in a fully tropical paleoenvironment are from the Miocene of Chiapas, Mexico (28) and Hispaniola (29), whose paleobiotas are very similar to Recent ones of Central America and the Caribbean. The Eocene Cambay amber and its diverse arthropod inclusions thus also provide unique data on the early evolution of tropical forest ecosystems.
Results and Discussion
Cambay Amber and Its Geological Context.
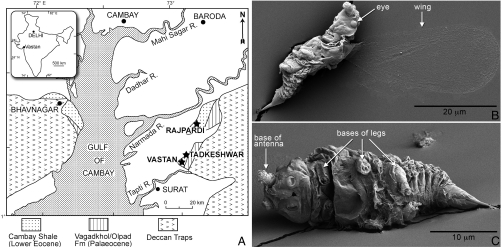
Approximately 150 kg of amber were collected within deep lignitic deposits of the Older Cambay Shale in Gujarat state, western India, from the Vastan (N 21° 25.239, E 073° 07.249) and Tadkeshwar (N 21° 21.400, E 073° 04.532) lignite mines (Fig. 1A). These strata have been assigned an age of mid- to early-Ypresian (50–52 Ma) based on shark teeth (30), the index foraminiferan Nummulites burdigalensis burdigalensis (31), and dinoflagellates (32). Amber was concentrated in near-shore sediments by low energy, brackish water and marine incursions (33) into seams 5–10-cm thick of an “amber conglomerate,” with most pieces ranging in size from sand grains to several centimeters, and scattered pieces up to 20 cm in length (Figs. S1 and S2). Chemical composition is distinctive, being a Class II or dammar-type resin (34, 35), which is a cadinene-based polymer produced mostly by trees in the Dipterocarpaceae (36, 37). Fossil wood from the Vastan mine (Fig. S1 C and D) containing amber and resin canals has an anatomy attributable to that of the Dipterocarpaceae (Fig. S3), thus confirming the botanical source of this amber.
Fig. 1.
(A) Map showing location of mines and distribution of formations yielding Early Eocene Cambay amber from Gujarat, India. (B and C) Scanning electron micrographs of male scale insect (Neococcoidea), fully extracted from Cambay amber using solvents; (B) oblique dorsal view with wing outstretched, (C) ventral view.
Cambay amber is poorly crosslinked and polymerized, as freshly exposed surfaces are soft and often tacky, and pieces dissolve completely in toluene and chloroform. This unique property allows extraction of entire inclusions, although they are extremely fragile and readily fragment. Extracted insects examined with scanning electron microscopy have cuticle with ultrastructural preservation (Fig. 1 B and C), which allows submicron resolution of morphological characters and, therefore, far improved phylogenetic inference.
Alimohammadian et al. earlier reported inclusions in Eocene India amber (38), but from the Vastan mine only, and that report was based on only five arthropod inclusions unidentified to family. Thus far, we have found more than 700 arthropod inclusions, belonging to 14 orders and more than 55 families (Table 1), as well as numerous plant and fungal remains such as spores, mycelia, stems, pollen, portions of leaves, and even several small flowers.
Table 1.
Taxa of arthropods in Cambay Amber
| MYRIAPODA | |
| Diplopoda | |
| Polydesmoidea | |
| ARACHNIDA | |
| Acarina | |
| Families undet. | |
| Araneae | |
| Mimetidae | |
| Pholcidae | |
| Thomisidae | |
| Uloboridae | |
| Scorpiones | |
| Buthidae? | |
| Pseudoscorpiones | |
| HEXAPODA | |
| Collembola | |
| Families undet. | |
| Orthoptera | |
| Family undet. | |
| Dictyoptera | |
| Mantodea | |
| Families undet. | |
| Chaeteessidae | |
| Isoptera | |
| Rhinotermitidae | |
| Blattodea | |
| Families undet. | |
| Psocodea | |
| Lepidopsocidae | |
| Families undet. | |
| Thysanoptera | |
| Families undet. | |
| Hemiptera | |
| Sternorrhyncha | |
| Coccoidea | |
| Auchenorrhyncha | |
| Families undet. | |
| Cicadellidae | |
| Heteroptera | |
| Families undet. | |
| Dipsocoromorpha | |
| Miridae | |
| Leptopodidae: Leptosalda | |
| Coleoptera | |
| Families undet. | |
| Staphylinidae | |
| Chrysomelidae: Alticinae | |
| Hymenoptera | |
| Chalcidoidea | |
| Aphelinidae, nr. Euryischia | |
| Mymaridae | |
| Family undet. | |
| Proctotrupoidea | |
| Diapriidae | |
| Scelionidae | |
| Aculeata | |
| Bethylidae (?) | |
| Formicidae | |
| Dolichoderinae | |
| Formicinae | |
| Myrmecinae | |
| Ponerinae | |
| Pseudomyrmecinae | |
| Apidae | |
| Electrapini | |
| Melikertini | |
| Diptera | |
| Limoniidae | |
| Cecidomyiidae | |
| Chironomidae | |
| Ceratopogonidae | |
| Psychodidae | |
| Phlebotoiella eoindianensis | |
| Sciaroidea | |
| Lygistorrhinidae: Palaeognoriste | |
| Mycetophilidae: Manotinae | |
| Keroplatidae | |
| Brachycera | |
| Rhagionidae | |
| Dolichopodidae | |
| Medeterinae indet. | |
| ?Neurogoninae indet. | |
| Sympycninae indet. | |
| Phoridae | |
| Metopininae indet. | |
| Rhopica sp. | |
| Phorinae indet. | |
| Schizophora | |
| Campichoetidae: Pareuthychaeta sp. | |
| Milichioidea |
Amber Paleobiota and Biogeography.
Some insect taxa in Cambay amber have phylogenetic affinities with taxa from the Eocene of northern Europe, particularly the Baltic amber (younger by some 8–10 myr). These include the following: A genus of small bees near Melikertes (Apidae: Melikertini) (Fig. 2A) and a much larger species of bee in or near the extinct genus Protobombus (Electrapini) (a group also preserved as compressions in the oil shales of Messel/Eckfeld, Germany) (39, 40). In flies (Diptera), Baltic connections include a fungus gnat of the genus Palaeognoriste (family Lygistorrhinidae) (41) (Fig. 2C); the acalyptrate fly genus Pareuthychaeta (Campichoetidae), and a genus of medeterine Dolichopodidae. Affinities specifically to the Eocene of northern Europe are perhaps biased by the fact that Baltic amber is the most prolific and best studied amber Lagerstätte in the world, containing the most diverse insect fauna of all fossil deposits (23).
Fig. 2.
Representative insects in Cambay amber of biogeographic and ecological significance. Taxa have closest known relatives in Baltic amber (A and C), Recent of southeast Asia (B and D), or the Neotropical Region (E and F); some of these taxa are also eusocial (A, B, and G). (A) Bee, near the extinct genus Melikertes (Apidae: Melikertini). (B) Sculptured myrmecine ant (Formicidae), near the Recent southeast Asian genus Lordomyrma. (C) Fungus gnat, Palaeognoriste (Diptera: Lygistorrhinidae). (D) Scuttle fly, Rhopica (Diptera: Phoridae). (E) Leptosalda bug (Leptopodidae: Heteroptera). (F) Mantis nymph of the family Chaeteessidae (Mantodea). (G) Alate termite (Isoptera), family Rhinotermitidae.
Despite the biased comparisons to the Baltic amber paleobiota, phylogenetic affinities of the identified insects in Cambay amber also lie with Recent taxa from southeastern Asia and Australasia. These include a scuttlefly (Phoridae) of the genus Rhopica, known only as eight Recent described species from Australia to southeastern China (Hainan Island) (Fig. 2D) (42), and another medeterine dolichopodid closely related to an undescribed genus from Australasia. In ants, a highly sculptured, distinctive myrmecine (Fig. 2B) is closely related to a small group of Recent southeast Asian and Australian genera, Lordomyrma, Meyriella, and Austromorium. Perhaps the most interesting Australasian ant connection of the Cambay amber concerns the dolichoderine genus Leptomyrmex, known in the Recent of New Caledonia, Australia, and New Guinea and, remarkably, in Miocene amber from the Dominican Republic.
An unexpected biogeographic connection of insects within Cambay amber concerns two rare groups that presently exist in the Neotropical Region and also occur in Miocene amber from Mexico and/or the Dominican Republic. One of these species is a leptosaldine heteropteran (Fig. 2E), known otherwise as one Recent species from Colombia (Saldolepta kistnerorum), and as two species in Miocene amber from nuclear America (Leptosalda chiapensis, in Mexican amber, and an undescribed species of the same genus from the Dominican Republic). The other presently Neotropical group is represented by a late-instar nymph (Fig. 2F) of the distinctive, basal family of mantises, Chaeteessidae, of which there are eight described Recent species throughout the New World tropics and several undescribed species in Dominican amber.
The Cambay amber fauna thus shows widespread, even global, affinities, with only minor indication of isolation or insularity (43, 44), and surprisingly no African or Malagasy connections yet found. This evidence supports the nonexclusive hypotheses that there were at the very least archipelagic connections between precontact India and Asia (4, 20, 31), or that India and Asia collided earlier in the Paleocene (e.g., ref. 3).
The most ecologically significant aspect of the Cambay amber fauna is the diverse social insects. Besides early corbiculate bees, the fauna also includes the earliest definitive Rhinotermitidae termites (Fig. 2G), which is a recently derived, cosmopolitan family of 335 living species whose oldest definitive fossils until now were from the Miocene (24). Cambay amber also preserves one of the earliest diverse paleofaunas of ants (Formicidae), the only other of comparable age is preserved in amber from Oise, France. The oldest ants are from the Albian (Early Cretaceous) (23), and all Cretaceous species are primitive and rare, always comprising much less than 1% of all insect inclusions in any one deposit. Baltic amber contains nearly 150 species of ants in 9 subfamilies (45, 46), a fauna that has accrued after more than a century of study upon hundreds of thousands of inclusions. Ants in Baltic amber comprise 8% of all arthropod inclusions; the 15 species of ants in Cambay amber comprise 6% of all arthropods, including early records for the Recent subfamilies Pseudomyrmicinae and the very diverse Myrmecinae, as well as species in the subfamilies Dolichoderinae, Formicinae, and Ponerinae. Peak diversity of Recent ants occurs in primary, lowland tropical forests, which further reflects the paleoenvironment of the Cambay amber.
This amber preserves a very diverse paleofauna associated with an ancient forest of Dipterocarpaceae, a family that virtually defines forests of tropical southeastern Asia today, and which comprises up to 80% of the canopy-emergent trees in this region (47). Borneo is the center of extant diversity, with some 287 species in 9 genera; India is on the western edge of the Asian distribution. Synthesis of large amounts of resin occurs primarily in five genera in the subfamily Dipterocarpoideae, an Asian monophyletic group of the greatest species diversity (470 spp.). Resin production in these trees can be so voluminous that it is even the source for some Neogene oil deposits from Southeast Asia (48). Despite earlier views that dipterocarp forests of Southeast Asia developed in the Miocene (49, 50)—indeed, the oldest dipterocarp pollen from India is Miocene—the large volume of dammar-type resin and anatomy of the wood in the Cambay Shale reveals an age of Dipterocarpaceae more than twice that commonly held. Molecular phylogeny of the family indicates that subfamilies Pakarimoidea (1 species, South America) and Montoideae (1 species from Colombia, 34 species from Africa and Madagascar) are sister groups to the Dipterocarpoideae (51), although reanalysis of those data indicates that the Malagasy family Sarcolaenaceae is actually most closely related to this last subfamily (52). Either way, a gondwanan origin of Asian dipterocarps is strongly supported (also indicative of a pre-Neogene age of the family), but the Laurasian affinities of arthropods in Cambay amber attest to a disparate biogeographic heritage of this dominant ecosystem.
Molecular estimates of the origins of broad-leaved (angiospermous) tropical forests range from the Early to mid-Cretaceous (53), which conflicts with direct macrofossil evidence such as leaves and wood, that such forests developed in the early Cenozoic, generally the Eocene to Paleocene (54–58), with the earliest records being the Early to Late Paleocene of the Western Hemisphere (55, 58). One reason for the discrepancy is that molecular estimates are typically much older than divergence times based on morphology and fossils (59, 60). Second, molecular estimates and pollen records cannot account for the physiognomy that distinguishes rain forest, such as arborescence, leaf shape, and the presence of epiphytes and lianas (54). Third, many groups existed in the fossil record far before they became ecologically dominant, such as ants and termites (23, 24), so it is likewise unrealistic to assume that the times of estimated taxon divergence or radiation is the same as the spread of a dominant ecosystem. Based on direct fossil evidence, the Cambay Shale Formation provides one of the earliest unequivocal Asian records of a diverse, broad-leaved tropical forest and its diverse arthropod fauna.
Methods
Specimen preparation and photography: Amber was screened for inclusions, and pieces with inclusions were trimmed with a water-fed diamond saw, embedded in epoxy (Buehler) according to the protocols in ref. 61, and ground and polished with a water-fed flat lap wheel using emory papers of 400–4,000 grit sizes. Embedding stabilizes pieces for very close, accurate trimming. Selected inclusions were extracted by dissolving in toluene for several hours, then soaking in 70% ethanol. Photomicrography used a fiber optic flash illumination system (MicrOptics©) coupled to a Nikon digital camera and an Infinity©K-2 lens.
Supplementary Material
Acknowledgments.
The authors thank the authorities of the Vastan and Tadkeshwar Lignite Mines who graciously provided access to the mines, and to the Director of the Birbal Sahni Institute for Palaeobotany, Lucknow, for field work release time for H.S.; D.G. thanks Brian Brown (Natural History Museum of Los Angeles), David Penney (Manchester University), Dan Bickle (Australian Museum), and Lorenzo Prendini [American Museum of Natural History (AMNH)] for identifications of the phorids, spiders, dolichopodids, and the scorpion claw, respectively. Mr. Robert G. Goelet, Chairman Emeritus and Trustee of the AMNH, generously funded P.C.N. and field work. National Science Foundation Grant DEB-0542909 (to M.S.E.) partially supported M.S.E. and J.C.T. Field work of A.S. was partially supported by a National Geographic Society grant. For help in fieldwork, D.G. thanks Keith Luzzi and J.R. thanks A. Bergmann, H. Schmied, and L. Keller, who also screened and prepared samples. Fieldwork of J.R., T.M.C., and F.S. was supported by a Deutsche Forschungsgemeinschaft grant (to J.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007407107/-/DCSupplemental.
References
- 1.Garzanti E, Baud A, Mascle G. Sedimentary record of the northward flight of India and its collision with Eurasia (Ladakh Himalya, India) Geodin Acta. 1987;1:297–312. [Google Scholar]
- 2.Rowley DB. Age of initiation of collision between India and Asia: A review of stratigraphic data. Earth Planet Sci Lett. 1996;145(1–4):1–13. [Google Scholar]
- 3.Beck RA, et al. Stratigraphic evidence for an early collision between northwest India and Asia. Nature. 1995;373:55–58. [Google Scholar]
- 4.Ali JR, Aitchison JC. Gondwana to Asia: Plate tectonics, paleogeography and biological connectivity of the Indian sub-continent from the middle Jurassic through latest Eocene (166–35 Ma) Earth-Sci Rev. 2008;88:145–166. [Google Scholar]
- 5.Noonan BP, Chippendale PT. Dispersal and vicariance: The complex evolutionary history of boid snakes. Mol Phylogenet Evol. 2006;40:347–358. doi: 10.1016/j.ympev.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Murphy WJ, Collier GE. A molecular phylogeny for aplocheiloid fishes (Atherinomorpha, Cyprinidontiformes): the role of vicariance and the origins of annualism. Mol Biol Evol. 1997;14:790–799. doi: 10.1093/oxfordjournals.molbev.a025819. [DOI] [PubMed] [Google Scholar]
- 7.Bijou SD, Bossuyt F. New frog family from India reveals an ancient biogeographical link with the Seychelles. Nature. 2003;425:711–714. doi: 10.1038/nature02019. [DOI] [PubMed] [Google Scholar]
- 8.Sparks JS. Molecular phylogeny and biogeography of the Malagasy and South Asian cichlids (Teleostei: Perciformis: Cichlidae) Mol Phylogenet Evol. 2003;30:599–614. doi: 10.1016/S1055-7903(03)00225-2. [DOI] [PubMed] [Google Scholar]
- 9.Zerega NJC, Clement WL, Datwyler SL, Weiblen GD. Biogeography and divergence times in the mulberry family (Moraceae) Mol Phylogenet Evol. 2005;37:402–416. doi: 10.1016/j.ympev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Murray AM. The fossil record and biogeography of the Cichlidae (Actinopterygii: Labroidei) Biol J Linn Soc. 2001;74:517–532. [Google Scholar]
- 11.Vences M, Freyhof J, Sonnenberg R, Kosuch J, Veith M. Reconciling fossils and molecules: Cenozoic divergence of cichlid fishes and the biogeography of Madagascar. J Biogeogr. 2001;28:1091–1099. [Google Scholar]
- 12.Raxworthy CJ, Forstner MR, Nussbaum RA. Chameleon radiation by oceanic dispersal. Nature. 2002;415:784–787. doi: 10.1038/415784a. [DOI] [PubMed] [Google Scholar]
- 13.Bossuyt F, Milinkovitch MC. Amphibians as indicators of Early Tertiary “out-of-India” dispersal of vertebrates. Science. 2001;292:93–95. doi: 10.1126/science.1058875. [DOI] [PubMed] [Google Scholar]
- 14.Gower DJ, et al. A molecular phylogeny of ichthyophiid caecilians (Amphibia: Gymnophiona: Ichthyophiidae): Out of India or out of South East Asia? Proc Roy Soc B–Biol Sci. 2002;269:1563–1569. doi: 10.1098/rspb.2002.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti E, Eriksson T, Schönenberger J, Sytsma KJ, Baum DA. Early Tertiary out-of-India dispersal of Crypteroniaceae: Evidence from phylogeny and molecular dating. Evolution. 2002;56:1931–1942. doi: 10.1554/0014-3820(2002)056[1931:ETOOID]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Köhler F, Glaubrecht M. Out of Asia and into India: On the molecular phylogeny and biogeography of the endemic freshwater gastropod Paracrostoma Cossmann, 1900 (Caenogastropoda: Pachychilidae) Biol J Linn Soc. 2007;91:627–651. [Google Scholar]
- 17.McKenna MC. In: Implications of Continental Drift to the Earth Sciences. Tarling DH, Runcorn SK, editors. London: Academic; 1973. pp. 291–304. [Google Scholar]
- 18.Rose KD, et al. Early Eocene primates from Gujarat, India. J Hum Evol. 2009;56:366–404. doi: 10.1016/j.jhevol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Smith T, et al. High bat (Chiroptera) diversity in the Early Eocene of India. Naturwissenschaften. 2007;94:1003–1009. doi: 10.1007/s00114-007-0280-9. [DOI] [PubMed] [Google Scholar]
- 20.Briggs JC. The biogeographic and tectonic history of India. J Biogeogr. 2003;30:381–388. [Google Scholar]
- 21.Pearson PN, et al. Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature. 2001;413:481–470. doi: 10.1038/35097000. [DOI] [PubMed] [Google Scholar]
- 22.Jahren AH. The Arctic forest of the middle Eocene. Annu Rev Earth Planet Sci. 2007;35:509–540. [Google Scholar]
- 23.Grimaldi DA, Engel MS. Evolution of the Insects. Cambridge: Cambridge Univ Press; 2005. [Google Scholar]
- 24.Engel MS, Grimaldi D, Krishna K. Termites: Their phylogeny, classification, and rise to ecological dominance. Am Mus Novit. 2009;3650:1–27. [Google Scholar]
- 25.Willis KJ, McElwain JC. The Evolution of Plants. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 26.Perkovsky EE, Rasnitsyn AP, Vlaskin AP, Taraschuk MV. A comparative analysis of the Baltic and Rovno amber arthropod faunas: Representative samples. Afr Invertebr. 2007;48:229–245. [Google Scholar]
- 27.Nel A, De Ploëg G, Menier J-J, Waller A. The French ambers: A general conspectus and the Lowermost Eocene amber deposit of Le Quesnoy in the Paris Basin. Geol Acta. 2004;2:3–8. [Google Scholar]
- 28.Solórzano-Kraemer MM. Systematics, palaeoecology, and palaeobiogeography of the insect fauna from the Mexican amber. Palaeontogr Abt A. 2007;282(3/4):1–133. [Google Scholar]
- 29.Grimaldi DA. In: Amber, Resinite, and Fossil Resins. Anderson KB, Crelling JC, editors. Washington, DC: Am Chemical Society; 1995. pp. 203–217. [Google Scholar]
- 30.Rana RS, Kumar K, Singh H. Vertebrate fauna from the subsurface Cambay Shale (Lower Eocene), Vastan lignite mine, Gujarat, India. Curr Sci India. 2004;87:1726–1733. [Google Scholar]
- 31.Sahni A, et al. Temporal constraints and depositional palaeoenvironments of the Vastan lignite sequence, Gujarat: Analogy for the Cambay Shale hydrocarbon source rock. Indian J Petrol Geol. 2006;15:1–20. [Google Scholar]
- 32.Garg RK-A, et al. Age-diagnostic dinoflagellate cysts from the lignite-bearing sediments of the Vastan lignite mine, Surat District, Gujarat, western India. J Palaeont Soc India. 2008;53:99–105. [Google Scholar]
- 33.McCann T. Chenier plain sedimentation in the Palaeogene of western India. Z Dtsch Ges Geowiss. 2010 in press. [Google Scholar]
- 34.Mallick M, Dutta S, Greenwood PF, Bertram N. Pyrolytic and spectroscopic studies of Eocene resin from Vastan Lignite Mine, Cambay Basin, western India. J Geol Soc India. 2009;74:16–22. [Google Scholar]
- 35.Dutta S, Mallick M, Bertram N, Greenwood PF, Matthews RP. Terpenoid composition and class of Tertiary resins from India. Int J Coal Geol. 2009;80:44–55. [Google Scholar]
- 36.Van Aarssen BGK, DeLeeuw JW, Collinson M, Boon JJ, Goth K. Occurrence of polycadinene in fossil and recent resins. Geochim Cosmochim Acta. 1994;58:223–229. [Google Scholar]
- 37.Anderson KB, Muntean JV. The nature and fate of natural resins in the geosphere. Part X. Structural characteristics of the macromolecular constituents of modern Dammar resin and Class II ambers. Geochem Trans. 2000;7:2. [Google Scholar]
- 38.Alimohammadian H, Sahni A, Patnaik R, Rana RS, Singh H. First record of an exceptionally diverse and well preserved amber-embedded biota from Lower Eocene (ca. 52 Ma) lignites, Vastan, Gujarat. Curr Sci India. 2005;89:1328–1330. [Google Scholar]
- 39.Engel MS. A monograph of the Baltic amber bees and evolution of the Apoidea (Hymenoptera) Bull Am Mus Nat Hist. 2001;259:1–192. [Google Scholar]
- 40.Wappler T, Engel MS. The middle Eocene bee faunas of Eckfeld and Messel, Germany (Hymenoptera: Apoidea) J Paleontol. 2003;77:908–921. [Google Scholar]
- 41.Grimaldi DA, Blagoderov V. A new genus of Lygistorrhinidae from Vietnam (Diptera: Sciaroidea), and phylogenetic relationships in the family. Stud Dipterol. 2001;8:43–57. [Google Scholar]
- 42.Disney RHL. The scuttlefly genus Rhopica Schmitz (Diptera, Phoridae) Ann Zool. 2002;52:285–291. [Google Scholar]
- 43.Solórzano-Kraemer MM, Evenhuis NL. The first keroplatid (Diptera: Keroplatidae) species from the Lower Eocene amber of Vastan, Gujarat, India. Zootaxa. 2008;1816:57–60. [Google Scholar]
- 44.Solórzano-Kraemer MM, Wagner R. The first psychodid (Diptera: Psychodidae: Phlebotominae) species from the Lower Eocene amber of Vastan, Gujarat, India. Zootaxa. 2009;2152:63–68. [Google Scholar]
- 45.Dlussky GM. Genera of ants (Hymenoptera: Formicidae) from Baltic amber. Paleontol J. 1997;31:616–627. [Google Scholar]
- 46.Dlussky GM, Rasnitsyn AP. Ants (Insecta: Vespida: Formicidae) in the Upper Eocene amber of central and eastern Europe. Paleontol J. 2009;43:1024–1042. [Google Scholar]
- 47.Whitmore TC. An Introduction to Tropical Rain Forests. Oxford: Oxford Univ Press; 1990. [Google Scholar]
- 48.Stout SA. In: Amber, Resinite, and Fossil Resins. Anderson KB, Crelling JC, editors. Washington, DC: Am Chemical Society; 1995. pp. 43–75. [Google Scholar]
- 49.Ashton P, Gunatilleke CVS. New light on the plant geography of Ceylon. 1. Historical plant geography. J Biogeogr. 1987;14:249–285. [Google Scholar]
- 50.Morley RJ. Origin and Evolution of Tropical Rain Forests. New York: Wiley; 2000. [Google Scholar]
- 51.Dayanandan S, Ashton PS, Williams SM, Primack SB. Phylogeny of the tropical tree family Dipterocarpaceae based on nucleotide sequences of the chloroplast rbCL gene. Am J Bot. 1999;86:1182–1190. [PubMed] [Google Scholar]
- 52.Ducousso M, et al. The last common ancestor of Sarcolaenaceae and Asian dipterocarp trees was ectomycorrhizal before the India-Madagascar separation, about 88 million years ago. Mol Ecol. 2004;13:231–236. doi: 10.1046/j.1365-294x.2003.02032.x. [DOI] [PubMed] [Google Scholar]
- 53.Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ. Explosive radiation of Malphighiales supports a mid-Cretaceous origin of modern tropical rain forests. Am Nat. 2005;165:36–65. doi: 10.1086/428296. [DOI] [PubMed] [Google Scholar]
- 54.Burnham RJ, Johnson KR. South American palaeobotany and the origins of neotropical rainforests. Philos Trans Roy Soc B. 2004;359:1595–1610. doi: 10.1098/rstb.2004.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson KR, Ellis B. A tropical rainforest in Colorado 1.4 million years after the Cretaceous-Tertiary boundary. Science. 2002;296:2379–2383. doi: 10.1126/science.1072102. [DOI] [PubMed] [Google Scholar]
- 56.Wheeler EA, Baas P. A survey of the fossil record for dicotyledonous wood and its significance for evolutionary and ecological wood anatomy. IAWA Bull. 1991;12:275–332. [Google Scholar]
- 57.Wing SL, Boucher LD. Ecological aspects of the Cretaceous flowering plant radiation. Ann Rev Earth Planet Sci. 1998;26:379–421. [Google Scholar]
- 58.Wing SL, et al. Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest. Proc Natl Acad Sci USA. 2009;106:18627–18632. doi: 10.1073/pnas.0905130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benton MJ, Ayala FJ. Dating the tree of life. Science. 2003;300:1698–1700. doi: 10.1126/science.1077795. [DOI] [PubMed] [Google Scholar]
- 60.Nixon KC. Paleobotany, evidence, and molecular dating: an example from the Nymphaeales. Ann Miss Bot Gard. 2008;95:43–50. [Google Scholar]
- 61.Nascimbene PC, Silverstein H. In: Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Grimaldi DA, editor. Leiden, The Netherlands: Backhuys; 2000. pp. 93–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.