Abstract
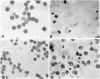
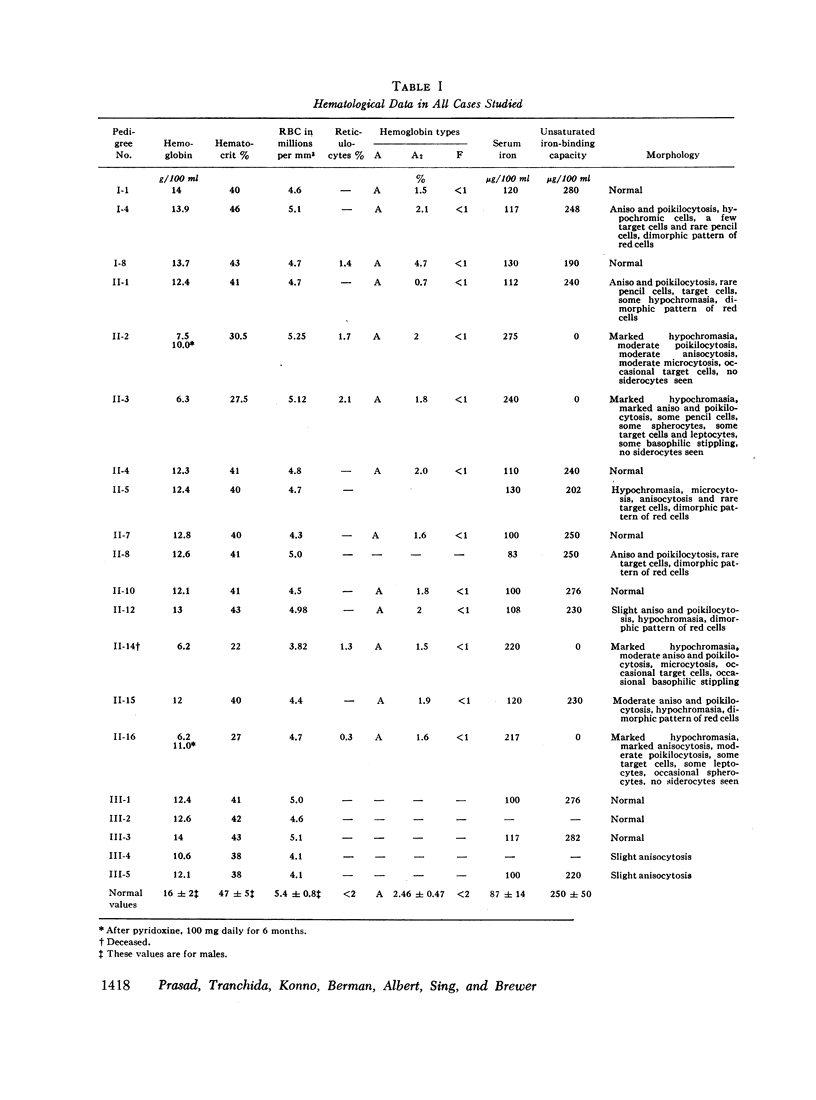
Detailed clinical and genetic studies have been performed in a Negro family, which segregated for sex-linked sideroblastic anemia and glucose-6-phosphate dehydrogenase (G-6-DP) deficiency. This is the first such pedigree reported. Males affected with sideroblastic anemia had growth retardation, hypochromic microcytic anemia, elevated serum iron, decreased unsaturated iron-binding capacity, increased 59Fe clearance, low 59Fe incorporation into erythrocytes, normal erythrocyte survival (51Cr), normal hemoglobin electrophoretic pattern, erythroblastic hyperplasia of marrow with increased iron, and marked increase in marrow sideroblasts, particularly ringed sideroblasts. Perinuclear deposition of ferric aggregates was demonstrated to be intramitochondrial by electron microscopy. Female carriers of the sideroblastic gene were normal but exhibited a dimorphic population of erythrocytes including normocytic and microcytic cells. The bone marrow studies in the female (mother) showed ringed marrow sideroblasts.
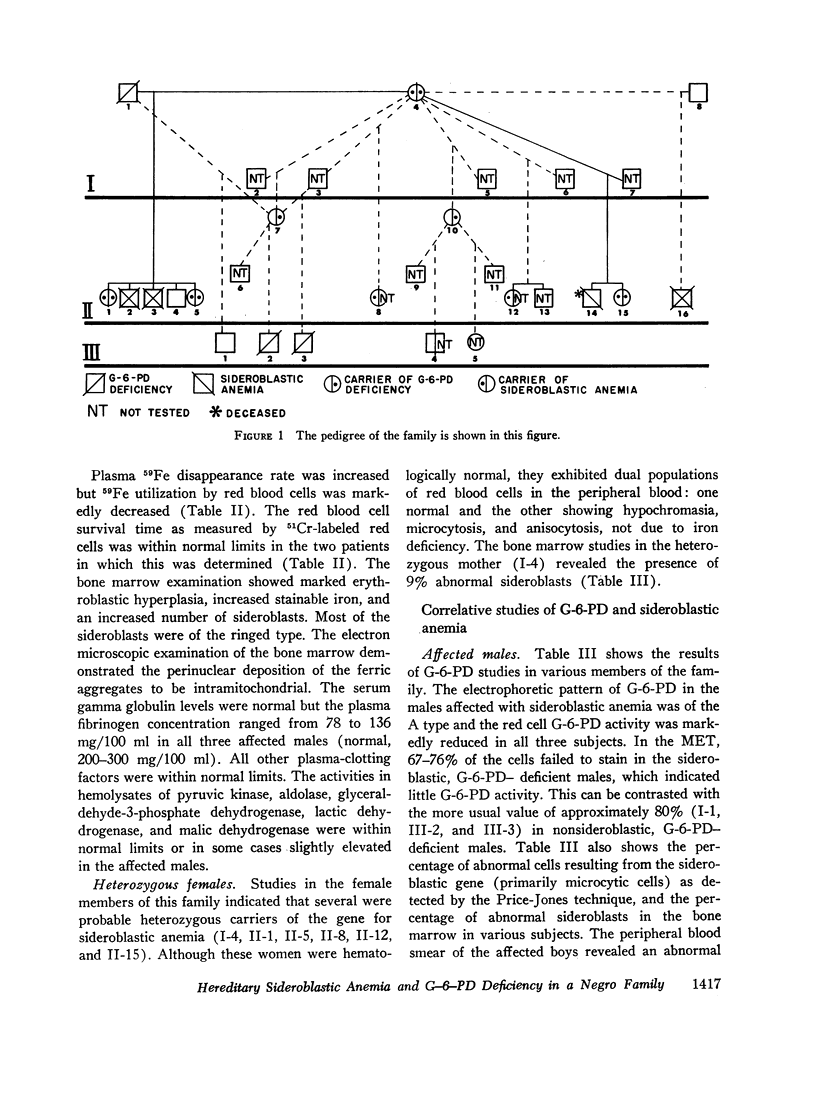
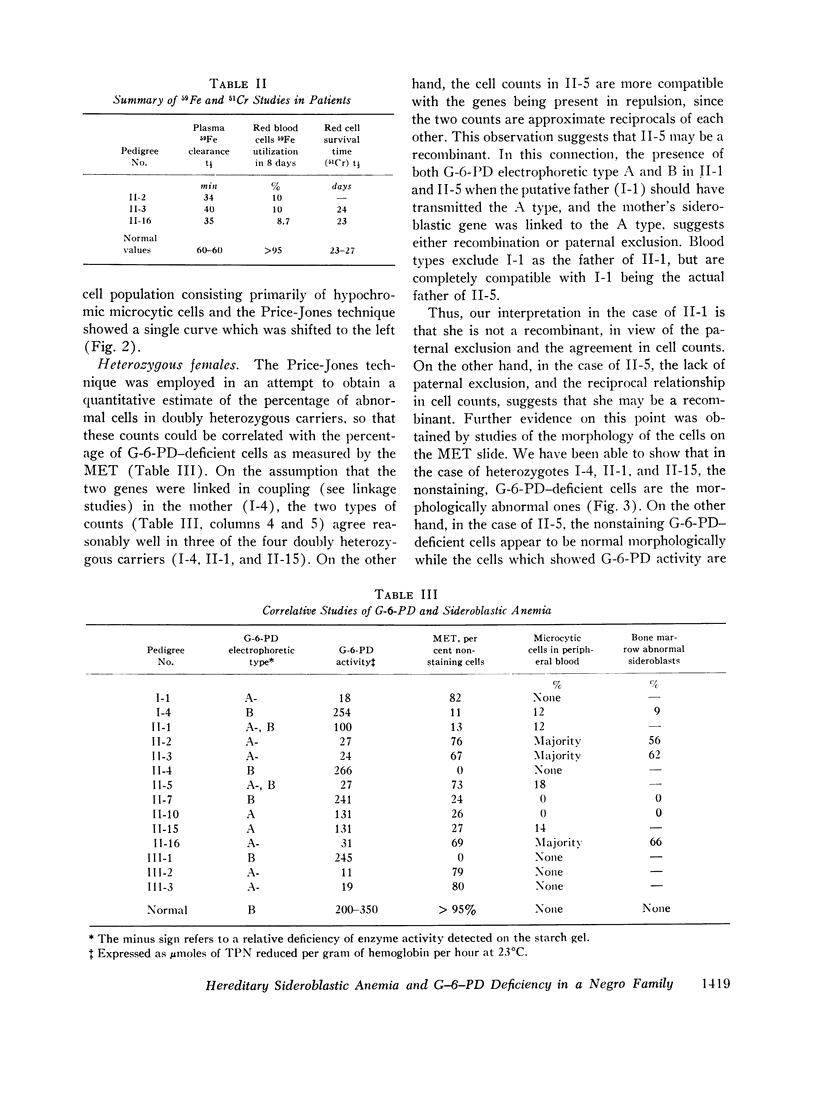
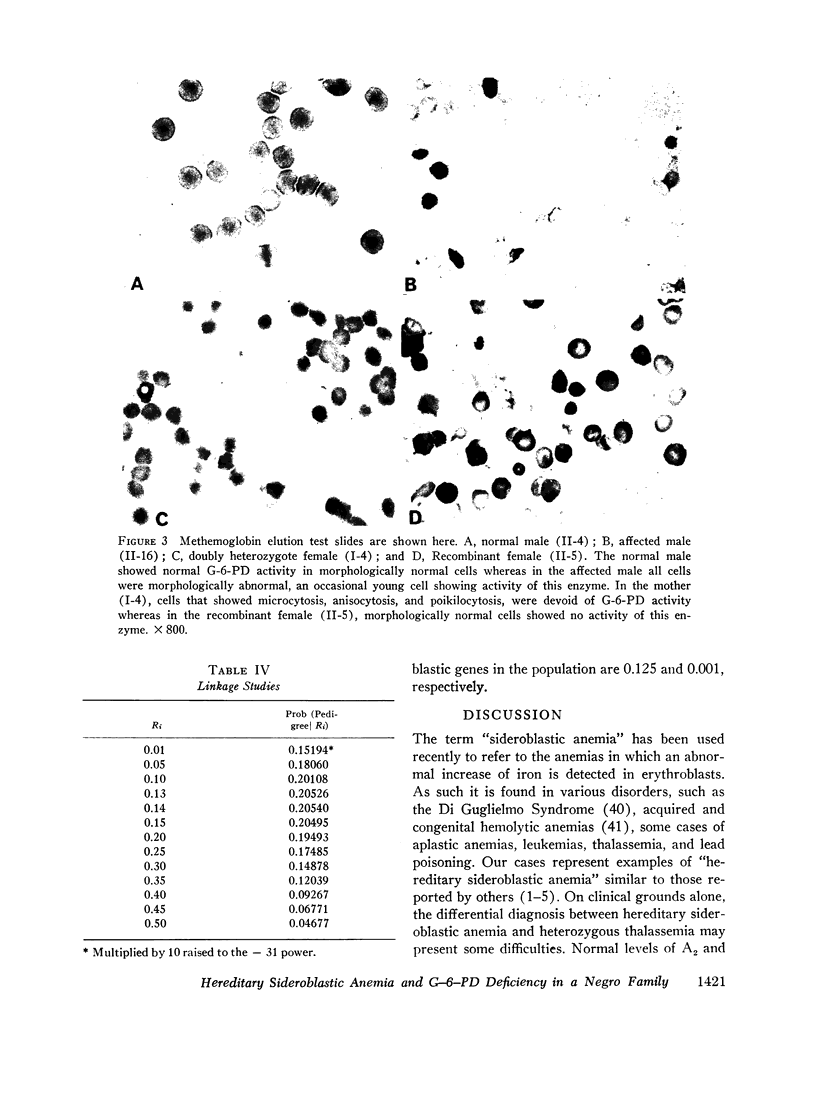
Studies of G-6-PD involved the methemoglobin elution test for G-6-PD activity of individual erythrocytes, quantitative G-6-PD assay, and electrophoresis. In the pedigree, linkage information was obtained from a doubly heterozygous woman, four of her sons, and five of her daughters. Three sons were doubly affected, and one was normal. One daughter appeared to be a recombinant. The genes appeared to be linked in the coupling phase in the mother. The maximum likelihood estimate of the recombination value was 0.14.
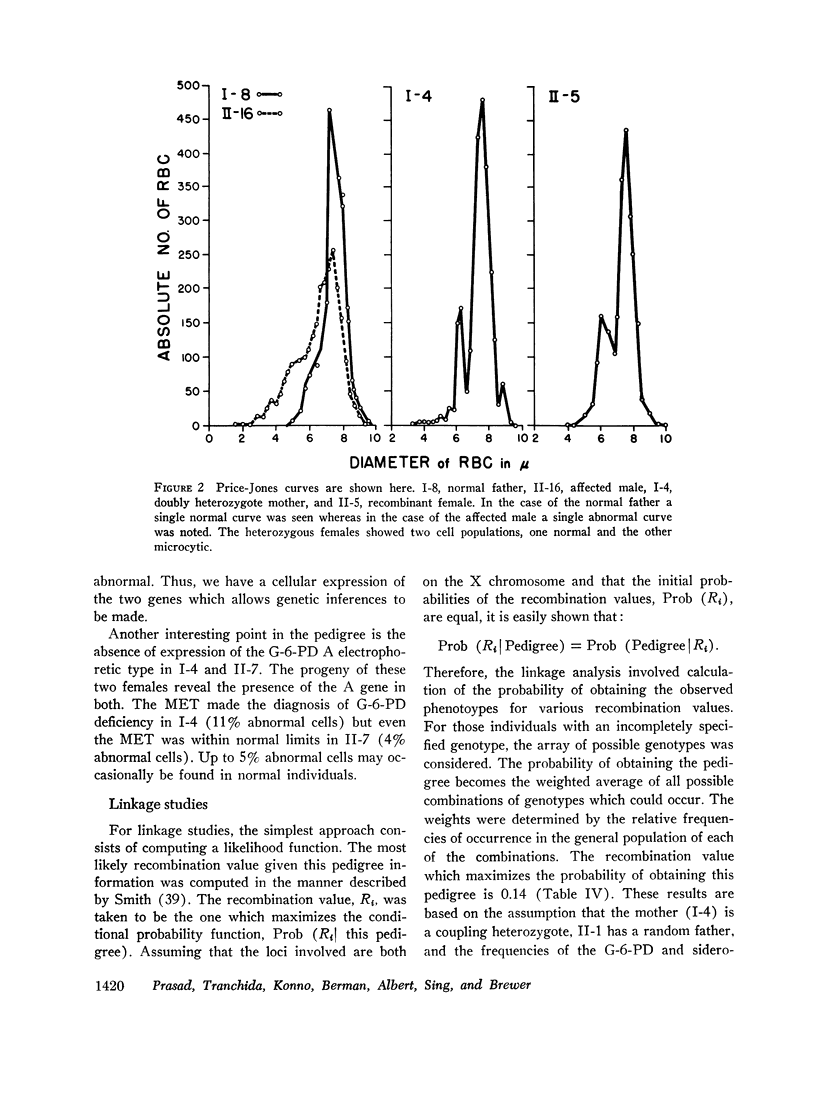
By means of Price-Jones curves, the microcytic red cells in peripheral blood were quantitated in female carriers. The sideroblast count in the bone marrow in the mother corresponded closely to the percentage of microcytic cells in peripheral blood. This is the second example in which the cellular expression of a sex-linked trait has been documented in the human red cells, the first one being G-6-PD deficiency. The coexistence of the two genes in doubly heterozygous females has made it possible to study correlations in cell counts; our studies showed a strong positive correlation except in the probable recombinant in which a reciprocal relation held which indicated that X-inactivation was at least regional, rather than locus by locus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E., YEH M., FAIRBANKS V. F. The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker. Proc Natl Acad Sci U S A. 1962 Jan 15;48:9–16. doi: 10.1073/pnas.48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BICKERS J. N., BROWN C. L., SPRAGUE C. C. Pyridoxine responsive anemia. Blood. 1962 Mar;19:304–312. [PubMed] [Google Scholar]
- BOURNE M. S., ELVES M. W., ISRAUELS M. C. FAMILIAL PYRIDOXINE-RESPONSIVE ANAEMIA. Br J Haematol. 1965 Jan;11:1–10. doi: 10.1111/j.1365-2141.1965.tb00077.x. [DOI] [PubMed] [Google Scholar]
- BYRD R. B., COOPER T. Hereditary iron-loading anemia with secondary hemochromatosis. Ann Intern Med. 1961 Jul;55:103–123. doi: 10.7326/0003-4819-55-1-103. [DOI] [PubMed] [Google Scholar]
- Briere R. O., Golias T., Batsakis J. G. Rapid qualitative and quantitative hemoglobin fractionation. Cellulose acetate electrophoresis. Am J Clin Pathol. 1965 Dec;44(6):695–701. doi: 10.1093/ajcp/44.6.695. [DOI] [PubMed] [Google Scholar]
- CAROLI J., BERNARD J., BESSIS M., COMBRISSON A., MALASSENET R., BRETON J. Hemochromatose avec anémie hypochrome et absence d'hémoglobine anormale; etude au microscope electronique. Presse Med. 1957 Dec 4;65(88):1991–1996. [PubMed] [Google Scholar]
- DACIE J. V., SMITH M. D., WHITE J. C., MOLLIN D. L. Refractory normoblastic anaemia: a clinical and haematological study of seven cases. Br J Haematol. 1959 Jan;5(1):56–82. doi: 10.1111/j.1365-2141.1959.tb04013.x. [DOI] [PubMed] [Google Scholar]
- DAMESHEK W., BALDINI M. The Di Guglielmo syndrome. Blood. 1958 Feb;13(2):192–194. [PubMed] [Google Scholar]
- DAVIDSON R. G., NITOWSKY H. M., CHILDS B. DEMONSTRATION OF TWO POPULATIONS OF CELLS IN THE HUMAN FEMALE HETEROZYGOUS FOR GLUCOSE-6-PHOSPHATE DEHYDROGENASE VARIANTS. Proc Natl Acad Sci U S A. 1963 Sep;50:481–485. doi: 10.1073/pnas.50.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBAUGH F. G., Jr, EMERSON C. P., ROSS J. F. The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination or red cell survival in vivo. J Clin Invest. 1953 Dec;32(12):1260–1276. doi: 10.1172/JCI102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARBY L., SJOLIN S., VAHLQUIST B. Chronic refractory hypochromic anaemia with disturbed haem-metabolism. Br J Haematol. 1957 Jan;3(1):55–67. doi: 10.1111/j.1365-2141.1957.tb05771.x. [DOI] [PubMed] [Google Scholar]
- GARDNER F. H., NATHAN D. G. Hypochromic anemia and hemochromatosis. Response to combined testosterone, pyridoxine, and liver extract therapy. Am J Med Sci. 1962 Apr;243:447–457. doi: 10.1097/00000441-196204000-00005. [DOI] [PubMed] [Google Scholar]
- GELPI A. P., ENDE N. An hereditary anemia with hemochromatosis; studies of an unusual hemopathic syndrome resembling thalassemia. Am J Med. 1958 Aug;25(2):303–314. doi: 10.1016/0002-9343(58)90036-6. [DOI] [PubMed] [Google Scholar]
- Gall J. C., Brewer G. J., Dern R. J. Studies of Glucose-6-Phosphate Dehydrogenase Activity of Individual Erythrocytes: The Methemoglobin-Elution Test for Identification of Females Heterozygous for G6PD Deficiency. Am J Hum Genet. 1965 Jul;17(4):359–368. [PMC free article] [PubMed] [Google Scholar]
- HANSEN H. A., WEINFELD A. Hemosiderin estimations and sideroblast counts in the differential diagnosis of iron deficiency and other anemias. Acta Med Scand. 1959 Dec 5;165:333–356. doi: 10.1111/j.0954-6820.1959.tb14509.x. [DOI] [PubMed] [Google Scholar]
- HUFF R. L., HENNESSY T. G., AUSTIN R. E., GARCIA J. F., ROBERTS B. M., LAWRENCE J. H. Plasma and red cell iron turnover in normal subjects and in patients having various hematopoietic disorders. J Clin Invest. 1950 Aug;29(8):1041–1052. doi: 10.1172/JCI102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOSOWSKY M. S., HALL R. HEREDITARY SIDEROBLASTIC ANAEMIA. Br J Haematol. 1965 Jan;11:70–85. doi: 10.1111/j.1365-2141.1965.tb00086.x. [DOI] [PubMed] [Google Scholar]
- LUEDIN H. [Sideroachrestic anemias]. Praxis. 1962 May 24;51:534–541. [PubMed] [Google Scholar]
- LUKL P., WIEDERMANN B., BARBORIK M. Hereditäre Leptocyten-Anämie bei Männern mit Hämochromatose. Folia Haematol (Frankf) 1958 Sep;3(1):17–45. [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Sex chromatin and gene action in the mammalian X-chromosome. Am J Hum Genet. 1962 Jun;14:135–148. [PMC free article] [PubMed] [Google Scholar]
- MAMMEN E. F., AOKI N., OLIVEIRA A. C., BARNHART M. I., SEEGERS W. H. PROVEST AND BLOOD COAGULATION TESTS. Int J Fertil. 1963 Jul-Sep;8:653–663. [PubMed] [Google Scholar]
- MILLS H., HUFF R. L., KRUPP M. A., GARCIA J. F. Hemolytic anemia secondary to a familial (hereditary) defect in hemoglobin synthesis; report of a case with radioiron studies. AMA Arch Intern Med. 1950 Nov;86(5):711–726. doi: 10.1001/archinte.1950.00230170064006. [DOI] [PubMed] [Google Scholar]
- MOLLIN D. L. SIDEROBLASTS AND SIDEROBLASTIC ANAEMIA. Br J Haematol. 1965 Jan;11:41–48. doi: 10.1111/j.1365-2141.1965.tb00082.x. [DOI] [PubMed] [Google Scholar]
- REDMOND A. O., ROBERTSON J. H., NELSON M. G. Familial hypochromic anaemia with hyperferricaemia. A study of two families. Br Med J. 1963 Jul 13;2(5349):89–93. doi: 10.1136/bmj.2.5349.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ MEDAL L., ELIZONDO J., TORRES GALLARDO J., GITTLER C. Pyridoxine-responsive anemia: report of 2 cases in brothers and a review of the literature. Blood. 1961 May;17:547–561. [PubMed] [Google Scholar]
- SHOWS T. B., Jr, TASHIAN R. E., BREWER G. J., DERN R. J. ERYTHROCYTE GLUCOSE-6-PHOSPHATE DEHYDROGENASE IN CAUCASIANS: NEW INHERITED VARIANT. Science. 1964 Sep 4;145(3636):1056–1057. doi: 10.1126/science.145.3636.1056. [DOI] [PubMed] [Google Scholar]
- SMITH C. A. Some comments on the statistical methods used in linkage investigations. Am J Hum Genet. 1959 Dec;11:289–304. [PMC free article] [PubMed] [Google Scholar]
- TANAKA K. R., VALENTINE W. N., MIWA S. Pyruvate kinase (PK) deficiency hereditary nonspherocytic hemolytic anemia. Blood. 1962 Mar;19:267–295. [PubMed] [Google Scholar]
- VERLOOP M. C., BIERENGA M., DIEZERAAD-NJOO A. Primary or essential sideroachrestic anaemias. Pathogenesis and therapy. Acta Haematol. 1962;27:129–145. doi: 10.1159/000206775. [DOI] [PubMed] [Google Scholar]
- VERLOOP M. C., RADEMAKER W. Anaemia due to pyridoxine deficiency in man. Br J Haematol. 1960 Jan;6:66–80. doi: 10.1111/j.1365-2141.1960.tb06218.x. [DOI] [PubMed] [Google Scholar]
- ZINKHAM W. H., LENHARD R. E., Jr Metabolic abnormalities of erythrocytes from patients with congenital nonspherocytic hemolytic anemia. J Pediatr. 1959 Sep;55:319–336. doi: 10.1016/s0022-3476(59)80228-6. [DOI] [PubMed] [Google Scholar]