Abstract
The biogeochemical role of phytoplanktonic organisms strongly varies from one plankton type to another, and their relative abundance and distribution have fundamental consequences at the global and climatological scales. In situ observations find dominant types often associated to specific physical and chemical water properties. However, the mechanisms and spatiotemporal scales by which marine ecosystems are organized are largely not known. Here we investigate the spatiotemporal organization of phytoplankton communities by combining multisatellite data, notably high-resolution ocean-color maps of dominant types and altimetry-derived Lagrangian diagnostics of the surface transport. We find that the phytoplanktonic landscape is organized in (sub-)mesoscale patches (10–100 km) of dominant types separated by physical fronts induced by horizontal stirring. These physical fronts delimit niches supported by water masses of similar history and whose lifetimes are comparable with the timescale of the bloom onset (few weeks). The resonance between biological activity and physical processes suggest that the spatiotemporal (sub-)mesoscales associated to stirring are determinant in the observation and modeling of marine ecosystems.
Keywords: biogeochemical cycles, chaotic stirring, marine biogeography, marine ecology, phytoplankton functional types
Phytoplankton play several key roles in the ocean and climate systems, supporting the marine food chain and taking part in chemical cycles, including CO2 recycling (1, 2). The specific biogeochemical role of planktonic organisms and their response to environmental changes strongly depend on the functional type they belong to: For instance, sinking diatoms efficiently export carbon to the deep ocean; coccolithophorids’ biocalcification reduces sea water alkalinity and is critically affected by ocean acidification; Phaeocystis produces dimethyl sulphide, influencing the Earth’s climate through sulphate aerosol formation. A widespread characteristic of planktonic populations is that a huge number of species are present at any location, but only a few dominate the biomass (3). At the basin-scale, the dominance of a specific type is generally understood in terms of adaptation to the local water properties (4–7) and a climatological emergence of plankton biogeography has been documented in both models and field studies (3, 6, 8). At a smaller scale, remote-sensing and in situ observations of tracers such as sea surface temperature and chlorophyll concentration evidence strong contrasts in the upper ocean layer. Here ∼100 km-large and ∼month-lasting mesoscale turbulence structures organize biogeochemical tracers in filaments of mesoscale length but of thinner width (∼10 km) and shorter lifetimes (days/weeks) (9–14). In the following, we will refer to this spatiotemporal domain as the (sub-)mesoscale. How this variability is reflected by the biogeography of planktonic communities is not known, but is of key concern for fishery management, conservation ecology, tuning next-generation high-resolution climate models, and in general, for the observation and modeling of biogeophysical interactions on the ecological timescale of a bloom.
We explored the structuring role of surface mesoscale turbulence on the spatiotemporal organizations of planktonic communities by combining two remote-sensing datasets: Nine-kilometer ocean-color images from Sea-Viewing Wide Field-of-View Sensor (SeaWiFS) reprocessed with the PHYSAT algorithm (15, 16), and altimetry-derived currents (at nominal resolution of 1/3°) reanalyzed with Lagrangian diagnostics (14, 17) (see Materials and Methods). These two datasets provided high-resolution, daily information of dominant-type distribution, and information on the stirring induced by mesoscale turbulence, respectively. We focused on the bloom occurring at the confluence of the Brazil and Malvinas currents. This region is rich in spatial structures (Fig. 1A) and is of particular importance for its biogeochemical and ecological features—namely, high photosynthetic carbon uptake, productivity, and presence of fisheries (18–20). This region has been also identified as a hot spot of enhanced phytoplanktonic diversity by a global ocean circulation and ecosystem model (8). Here, satellite-derived chlorophyll-a images indicate a bloom starting typically in August (see Fig. S1) with clear signatures of mesoscale patterns.
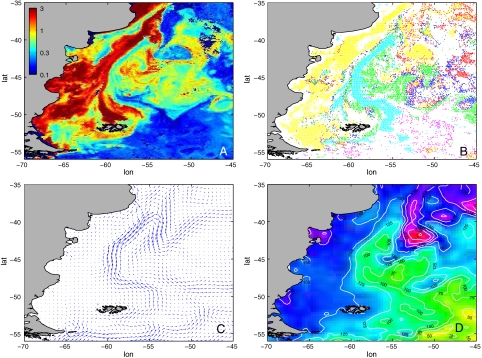
Fig. 1.
Ecological and physical (sub-)mesoscale structure from satellite data. (A) Total chlorophyll distribution (μg/L). (B) Dominant types identified from 8-d SeaWiFS composite by the PHYSAT algorithm during the spring bloom (November 24–December 1, 2001). Colors in B indicate diatoms (green), Prochlorococcus (red), Synechococcus (dark blue), nanoeukaryotes (yellow), Phaeocystis (magenta), and coccolithophorids (cyan). Boundaries among dominant types do not separate necessarily chlorophyll patches of different concentration, but may appear in regions of relatively homogeneous total Chl values (see Fig. S2). (C) Mean circulation. (D) Sea surface height (contours indicate millimeters of height above the reference geoid).
Results
A PHYSAT image at 9-km resolution during the spring bloom of November 2001 and the mesoscale surface circulation are shown in Fig. 1 B and C. This image displays a complex and highly contrasted landscape of well-defined, compact (sub-)mesoscale patches of dominant types (Prochlorococcus, Synechococcus, nanoeukariotes, diatoms, Phaeocystis, and coccolithophorids) that cannot be distinguished on the basis of chlorophyll concentration alone (Fig. S2). The coexistence of these dominant types is a recurrent feature of the spring and autumn blooms in this region and occurred during all years of our study (2000–2003), as shown in Figs. S3–S6. The dominant types identified by the PHYSAT algorithm, as well as their spatial location, are consistent with in situ campaign studies (19, 21) (see SI Text and Fig. S7 for details). The comparison with mean circulation and sea surface height (Fig. 1 C and D) shows that many patches were reminiscent of large- and mesoscale circulation structures, suggesting that the horizontal transport plays a prominent role in the spatial organization of the planktonic community on the timescale of the bloom.
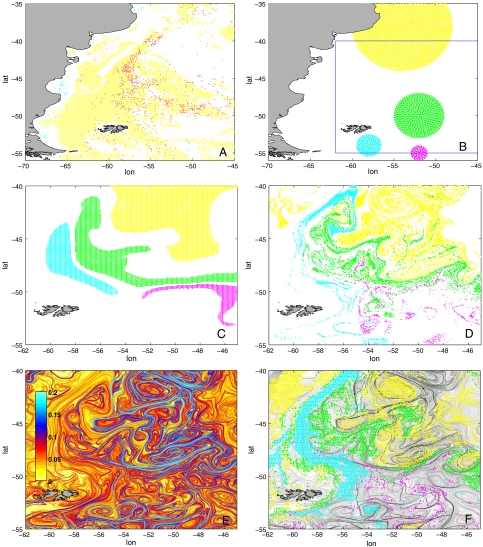
We tested this hypothesis with a numerical model, comparing the observed biological patterns with the physical patterns generated when the regional water masses are stirred by altimetry-derived surface velocities. Simulations were initialized at the very beginning of the bloom (end of August), 3 mo ahead of the days previously analyzed. At this initial date, PHYSAT-reprocessed satellite images show no pattern of prevalence, the whole region being dominated by picophytoplankton and nanoeukariotes (Fig. 2A). We labeled with different colors three regions of subpolar and one of subtropical origin, as displayed in Fig. 2B, and we advected forward in time for 3 mo the points within these circles (Fig. 2D). We estimated the initial position of these patches by a back-trajectory analysis (Fig. S8). The comparison of Fig. 2 D and F shows a striking similarity between the ecological landscape from PHYSAT analysis (Fig. 2F) and the physical landscape of passive tracers numerically advected with altimetry-derived velocities (Fig. 2D). Water patches sustaining the same dominant type, even if spread far apart in the basin late in November, appear to have the same origin and advection history. Therefore, patches associated to the same type share their climatological prebloom characteristics. They also come in contact with the same environmental conditions (like nutrient enrichment at the shelf break; ref. 22) along their common pathway. These observations indicate horizontal stirring as a viable mechanism for structuring the ocean surface in fluid dynamical niches, i.e., in water patches of contrasted physicochemical characteristics, capable of sustaining the emergence of different dominant types.
Fig. 2.
Identification and history of the fluid dynamical niches. (A) PHYSAT-detected dominant types at the beginning of the bloom season (August, 20, 2001). (B) Initial distribution of numerical tracers (August 20, 2001). (C) Distribution of the numerical tracers advected by the altimetry-derived large-scale circulation (altimetry filtered in space and time by 200 km and 3 mo) after 3 mo (November 28, 2001). (D) Distribution of the numerical tracers advected by altimetry-derived geostrophic currents after 3 mo (November 28, 2001). (E) Stirring rates (altimetry-derived finite-size Lyapunov exponents) in the region of interest. (F) Observed dominant phytoplankton types; yellow here is an aggregation of picoplankton (Prochlorococcus and Synecococcus) and nanoeukariotes, other colors as in Fig. 1. Superimposed in gray, physical fronts of panel E.
The role of the mesoscale surface turbulence can be pinpointed by repeating the advection experiment with only the large-scale component of the geostrophic currents, obtained by averaging the geostrophic velocities in space and time (respectively, 200 km and 3 mo) (Fig. 2C). In this case, the lack of mesoscale turbulence removed systematically interpatch intrusion filaments, reducing for each patch the extent and number of contacts between planktonic types.
The position and boundaries of the patches of dominant types evolve in the course of time. A direct observation of the temporal evolution of individual patches from PHYSAT data is not possible due to cloud episodes. Models have shown that planktonic fronts can be controlled by both physical forcing (23) and by the ecological dynamics of invasion/substitution processes (24). In our analysis, the borders of the observed patches compared well to the fronts generated by the horizontal stirring and separating water masses of different origin.
In order to analyze the temporal evolution of the plankton patches, we turned to the dynamics associated to the stirring process. In general, stirring creates filaments that are stretched in progressively thinner structures and are eventually dispersed by small-scale turbulence. Stirring intensity and the location of filaments it induces can be estimated by computing the largest finite-size Lyapunov exponent (FSLE) from the geostrophic velocities. An FSLE map (Fig. 2E) is obtained by measuring the backward-in-time divergence of particle trajectories initialized nearby. Highest FSLE values are found along the boundaries that separate water masses coming from regions far apart and match remarkably well the ecological boundaries of dominant phytoplanktonic types in this region as well as in other confluence regions of the world ocean (Figs. S3–S6, S9, and S10). Besides locating fronts induced by horizontal transport, the value of the FSLE yields the exponential rate at which a water mass is stretched along a front (17), or equivalently the timescale of filament formation. By considering the stretching process as a precondition of mixing, we estimate the lifetime of a fluid dynamical niche as the time necessary for shrinking mesoscale patches (∼100 km) down to a width of ∼1 km, where small-scale turbulent processes dominate and mixing occurs. This calculation* provides typical niche lifetimes of days–weeks, indicating that the structuring effects of horizontal stirring occurs on the same timescale of planktonic blooms and has the potential of affecting key ecological processes like competition and exclusion.
Discussion
Our analysis is based on the comparison of two independent datasets providing, respectively, information on stirring by surface mesoscale currents and on the phytoplankton community structure in terms of dominant types. Our first result is the observation of a spatial structure in the distribution of dominant types, which appear to be organized in mesoscale and smaller filamentary patches. Our second result is the primary role of fluid dynamics—and, more precisely, horizontal stirring—in shaping and maintaining such ecological landscape. This conclusion is based on the observed coincidence between the boundaries of the ecological patches and the physical boundaries induced by horizontal stirring on the timescale of a planktonic bloom. Our observations are consistent with recent circulation model experiments which indeed seem to indicate that transport is one of the main factors which create biodiversity hot spots, bringing together species that prosper in environments far apart (25).
Remote-sensing data do not allow one to follow ecological interactions inside filaments, such as the dynamics of competition and succession. Nevertheless, we can speculate on the effect of the temporal resonance between fluid dynamics and planktonic blooms with the following temporal scale argument.
By creating a landscape of water patches with contrasted characteristics, stirring by mesoscale turbulence can maintain niches with contrasted water properties long enough for types locally adapted to be selected and become largely predominant. However, in the course of a bloom, physical and biological timescales are entangled: On the same timescale over which dominant types can emerge (days/weeks), fluid dynamical niches are stretched into progressively thinner filaments and intrude into regions far apart from their origin; on a longer timescale, filaments are mixed and so is the planktonic community they sustain. This mechanism prevents complete exclusion from taking place, because the initially most adapted type would find itself in a different environment, where neutral coexistence of multiple types may occur. With this two-step mechanism—segregation and intrusion during the bloom, followed by mixing—populations that have possibly emerged within different filaments are eventually dispersed and homogenized, building up a highly diverse and widely distributed background of plankton types at the basin scale. We suggest that such mechanism of landscape structuring in (sub-)mesoscale ephemeral niches is the basis of the maintenance of both local dominance and large-scale dispersal of planktonic types (26), as summarized by Baas Becking’s dictum, “Everything is everywhere, but environment selects”.
The large latitudinal displacement values for the 3-mo advection period and the dramatic deformation of the patches (Fig. 2 D and E, and Fig. S8) indicate the dynamical nature of the dominant types’ landscape, where fluid dynamical niches are advected and interleaved in the course of the bloom. A patch of a dominant type can deform and travel a distance equivalent to its width in just a few days. This characteristic brings in a synopticity problem and renders these patches particularly elusive for in situ observations: Without transport information, fixed stations or transect samplings can misinterpret the spatial variability of the planktonic community as temporal variability. An exception to this problem are the patches associated to the cores of eddies with a slow temporal variability compared to the days/weeks timescale associated to the stirring process, like the one centered in 45°S, 55°E. The exception to the synopticity problem of water patches associated to eddies with slow temporal variability is consistent with the fact that mesoscale patchiness in phytoplankton communities has been typically associated in the past to persistent, slow-moving eddies in cruise observations (27, 28) or to vorticity filaments in model studies (29). Our observational and modeling results, however, indicate that community structuring by mesoscale turbulence is a much more general—and more dynamical—phenomenon, of which the segregation by persistent eddies is just a special case suitable for long-term, fixed observations.
The patches of dominant types are not necessarily associated to chlorophyll anomalies (Fig. S2), so that the combination of physical and biological information from remote sensing is needed for providing synoptic images of dominant type variability. When provided in near real time, this information may support an adaptive in situ sampling strategy. In turn, such in situ observations are needed to address some questions that our approach is not able to resolve. In simulating the horizontal dynamics of the water masses, indeed, we are not able to assess the relative impact on the biological activity of the initial prebloom conditions with respect to physical events—for instance, vertical fluxes or atmospheric exchanges such as dust deposition—occurring in the course of the advection and contributing to the definition of the abiotic environment. Moreover, our results are based on surface measurements. In the case of SeaWiFS data in particular, data are sparse in time due to cloud events, and their analysis through PHYSAT provides hints only on the most abundant phytoplanktonic type. The possibility of tracking and monitoring in situ a fluid dynamical niche would thus complement the remote-sensing-derived information with observations of the water column, the community structure, and its temporal dynamics beyond a most dominant type criterion.
Conclusions
The interaction between marine ecosystems and the environment takes place on multiple spatial and temporal scales. At the basin scale, environmental features have been identified as exerting a strong control over planktonic ecotypes (6–8). In confluence regions, water masses with different properties are stirred, creating contrasted physicochemical conditions which favor the emergence of complex community distributions. We have shown that, in such regions, the landscape of dominant planktonic groups is structured by mesoscale turbulence in fluid dynamical niches that can be retrieved with remarkable precision by a combined multisatellite analysis. These niches have a lifetime comparable with that of the bloom onset, sustain different communities, and thus should be considered a key element for the dynamics and maintenance of planktonic biodiversity in both observations and models.
Materials and Methods
Ocean-color data have been obtained by 8-d composites of SeaWiFS level 3 product at 9-km resolution (from Ocean Color Web). Geostrophic currents have been obtained by the Ssalto/Duacs altimetry product that for 2001 combines the measurements of Topex/Poseidon, European Remote Sensing, and Geosat Follow-On satellites. These data have a nominal spatial and temporal resolution of 1/3° and 1 wk, respectively.
Dominant phytoplankton types have been estimated by applying the PHYSAT algorithm to each individual SeaWiFS pixel. The PHYSAT approach is based on the labelization of some specific signatures in the spectral measurements of ocean-color sensors (five channels from 412 to 555 nm, for SeaWiFS). This empirical method has been established by comparing ocean-color water leaving radiance to a large in situ database of pigments (see ref. 15 for details). Phytoplankton types are generally characterized by some specific pigments which have been used to retrieve dominance information from in situ measurements and then associated them to a specific remote-sensing signal. In the construction of the PHYSAT algorithm, a type has been defined “dominant” for situations in which a given phytoplantkon type contributes to more than 60% to the total pigment. In others cases, pixels are associated to a “nonclassified” type. The large-scale component of the surface circulation has been obtained by averaging geostrophic velocities with a running window 200-km wide in space and 3 mo in space. Advection of numerical tracers by altimetry-derived currents has been achieved with a fourth-order Runge-Kutta integrator, interpolating linearly the velocities in space and time. The fronts and transport barriers induced by the mesoscale eddies have been calculated by finding the Lagrangian coherent structures embedded in the velocity field with the finite-size Lyapunov exponent technique (see refs. 14 and 17 for details).
Supplementary Material
Acknowledgments
The authors acknowledge NASA SeaWiFs project for access to SeaWiFs L3 data. The altimeter products were produced by Ssalto/Duacs and distributed by Aviso with support from the Centre National d’Études Spatiales.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 18235.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004620107/-/DCSupplemental.
*Given a Lyapunov exponent λ, the thinning of a patch of initial size δ0 due to horizontal stirring is δ = δ0 exp(-λt). Setting δ0 ∼ 100 km, δ ∼ 1 km, and solving for the time t, one finds t = 1/λ log(δ/δ0) ∼ 4.6/λ.
References
- 1.Watson AJ, Robinson C, Robinson JE, Williams PJ le B, Fasham MJR. Spatial variability in the sink for atmospheric carbon dioxide in the North Atlantic. Nature. 1991;350:50–53. [Google Scholar]
- 2.Allen JT, et al. Diatom carbon export enhanced by silicate upwelling in the northeast Atlantic. Nature. 2005;437:728–732. doi: 10.1038/nature03948. [DOI] [PubMed] [Google Scholar]
- 3.Fuhrman JA. Microbial community structure and its functional implications. Nature. 2009;459:193–199. doi: 10.1038/nature08058. [DOI] [PubMed] [Google Scholar]
- 4.Litchman E, Klausmaier CA, Shofield OM, Falkowski PG. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol Lett. 2007;10:1170–1181. doi: 10.1111/j.1461-0248.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 5.Giovannoni SJ, Stingl U. Molecular diversity and ecology of microbial plankton. Nature. 2005;437:343–348. doi: 10.1038/nature04158. [DOI] [PubMed] [Google Scholar]
- 6.Johnson ZI, et al. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science. 2006;311:1737–1740. doi: 10.1126/science.1118052. [DOI] [PubMed] [Google Scholar]
- 7.Bouman HA, et al. Oceanographic basis of the global surface distribution of Prochlorococcus ecotypes. Science. 2006;312:918–921. doi: 10.1126/science.1122692. [DOI] [PubMed] [Google Scholar]
- 8.Follows M, Dutkiewicz S, Grant S, Chisholm SW. Emergent biogeography of microbial communities in a model ocean. Science. 2007;315:1843–1846. doi: 10.1126/science.1138544. [DOI] [PubMed] [Google Scholar]
- 9.Williams RG, Follows MJ. Eddies make ocean deserts bloom. Nature. 1998;394:228–229. [Google Scholar]
- 10.McGillucuddy DJ, et al. Influence of mesoscale eddies on new production in the Sargasso Sea. Nature. 1998;394:263–266. [Google Scholar]
- 11.Lévy M. The modulation of biological production by oceanic mesoscal turbulence. Lect Notes Phys. 2008;744:219–261. [Google Scholar]
- 12.Martin AP. Phytoplankton patchiness: The role of lateral stirring and mixing. Prog Oceanogr. 2003;57:125–174. [Google Scholar]
- 13.Smetaceck V, Klaas C, Menden-Deur S, Rynearson TA. Mesoscale distribution of dominant diatom species relative to the hydrographical field along the Antarctic Polar Front. Deep-Sea Res Pt II. 2002;49:3835–3848. [Google Scholar]
- 14.Lehahn Y, d’Ovidio F, Lévy M, Heifetz E. Stirring of the Northeast Atlantic spring bloom: a Lagrangian analysis based on multi-satellite data. J Geophys Res. 2007;112:C08005. [Google Scholar]
- 15.Alvain S, Moulin C, Dandonneau Y, Bréon FM. Remote sensing of phytoplankton groups in case 1 waters from global SeaWiFS imagery. Deep-Sea Res Pt I. 2005;52:1989–2004. [Google Scholar]
- 16.Alvain S, Moulin C, Dandonneau Y, Loisel H. Seasonal distribution and succession of dominant phytoplankton groups in the global ocean: A satellite view. Global Biogeochem Cy. 2008;22:GB3001. [Google Scholar]
- 17.d’Ovidio F, López C, Hernández-García E, Fernández V. Mixing structures in the Mediterranean sea from finite-size Lyapunov exponents. Geophys Res Lett. 2004;31:L17203. [Google Scholar]
- 18.Garcia CAE, Sarma YVB, Mata MM, Garcia VMT. Chlorophyll variability and eddies in the Brazil-Malvinas confluence region. Deep-Sea Res Pt II. 2004;51:159–172. [Google Scholar]
- 19.Signorini SR, et al. Seasonal and interannual variability of calcite in the vicinity of the Patagonian shelf break (38°S–52°S) Geophys Res Lett. 2006;33:L16610. [Google Scholar]
- 20.Saraceno M, Provost C, Piola AR. On the relationship between satellite-retrieved surface temperature fronts and chlorophyll a in the western South Atlantic. J Geophys Res. 2005;110:C11016. [Google Scholar]
- 21.Garcia VC, et al. Environmental factors controlling the phytoplantkon blooms at the Patagonia shelf-break in spring. Deep-Sea Res Pt I. 2008;55:1150–1166. [Google Scholar]
- 22.Marcoval MA, Villafane VE, Helbling EW, Walter E. Combined effects of solar ultraviolet radiation and nutrients addition on growth, biomass and taxonomic composition of coastal marine phytoplankton communities of Patagonia. J Photoch Photobio. 2008;91:157–166. doi: 10.1016/j.jphotobiol.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Abraham ER. The generation of plankton patchiness by turbulent stirring. Nature. 1998;391:577–580. [Google Scholar]
- 24.Levin SA, Segel LA. Hypothesis for origin of planktonic patchiness. Nature. 1976;256:659. [Google Scholar]
- 25.Barton A, Dutkiewicz S, Flierl G, Bragg J, Follows MJ. Patterns of diversity in marine phytoplankton. Science. 2010;327:1843–1846. doi: 10.1126/science.1184961. [DOI] [PubMed] [Google Scholar]
- 26.Cermeño P, Falkowski PG. Controls on diatom biogeography in the ocean. Science. 2009;325:1539–1541. doi: 10.1126/science.1174159. [DOI] [PubMed] [Google Scholar]
- 27.Benitez-Nelson C, et al. Mesoscale eddies drive increased silica export in the subtropical Pacific Ocean. Science. 2007;316:1017–1021. doi: 10.1126/science.1136221. [DOI] [PubMed] [Google Scholar]
- 28.McGillicuddy DJ, Jr, et al. Eddy/wind interactions stimulate extraordinary mid-ocean plankton blooms. Science. 2007;316:1021–1026. doi: 10.1126/science.1136256. [DOI] [PubMed] [Google Scholar]
- 29.Bracco A, Provenzale A, Sheuring I. Mesoscale vortices and the paradox of the plankton. Proc Roy Soc Lond B. 2000;267:1795–1800. doi: 10.1098/rspb.2000.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.