Abstract
The global increase in the prevalence of obesity and metabolic disorders coincides with the increase of exposure to light at night (LAN) and shift work. Circadian regulation of energy homeostasis is controlled by an endogenous biological clock that is synchronized by light information. To promote optimal adaptive functioning, the circadian clock prepares individuals for predictable events such as food availability and sleep, and disruption of clock function causes circadian and metabolic disturbances. To determine whether a causal relationship exists between nighttime light exposure and obesity, we examined the effects of LAN on body mass in male mice. Mice housed in either bright (LL) or dim (DM) LAN have significantly increased body mass and reduced glucose tolerance compared with mice in a standard (LD) light/dark cycle, despite equivalent levels of caloric intake and total daily activity output. Furthermore, the timing of food consumption by DM and LL mice differs from that in LD mice. Nocturnal rodents typically eat substantially more food at night; however, DM mice consume 55.5% of their food during the light phase, as compared with 36.5% in LD mice. Restricting food consumption to the active phase in DM mice prevents body mass gain. These results suggest that low levels of light at night disrupt the timing of food intake and other metabolic signals, leading to excess weight gain. These data are relevant to the coincidence between increasing use of light at night and obesity in humans.
Keywords: circadian rhythms, light pollution, metabolic syndrome, mice, obesity
During the past 2 decades, obesity has shifted from an epidemic centered in the United States to a global issue. Although well-documented factors such as caloric intake, dietary choices, and lack of exercise are known to contribute to the prevalence of obesity and metabolic disorders, additional environmental factors are now considered critical in the development and maintenance of obesity (1). The increase of light at night (LAN) during the 20th century coincides with increasing rates of obesity and metabolic disorders throughout the world. Artificial lighting allows people to extend daytime activities into the night but as a consequence produces significant environmental light pollution caused by light straying into the atmosphere and brightening the nighttime sky.
Circadian regulation of energy homeostasis is controlled by an endogenous biological clock, located in the suprachiasmatic nuclei (SCN) of the hypothalamus, that is synchronized by photic information that travels directly from light-sensitive ganglion cells in the retina to the SCN, thereby entraining individuals’ physiology and behavior to the external day–night cycle (2). Importantly, light is the most potent entraining signal for the circadian clock, although other factors such as food consumption influence clock signaling (3). To promote optimal adaptive functioning, the circadian clock prepares individuals for predictable events such as food availability and sleep. Shift work disrupts clock function and is linked to circadian and metabolic consequences including sleep disturbances (4), elevated body mass index (5, 6), altered plasma lipid metabolism and adiposity (7), and increased risk for cardiovascular disease (8).
Multiple studies suggest a link between the molecular circadian clock and metabolism (reviewed in ref. 9). Mice harboring a mutation in their clock genes are susceptible to obesity and metabolic syndrome (10). Clock mutants show profound changes in circadian rhythmicity as well as disrupted diurnal food intake and increased body mass. Serum leptin, glucose, cholesterol, and triglyceride levels also are increased in Clock mutants compared with wild-type mice. Mice lacking the vasoactive intestinal peptide receptor 2 pathway, which plays an important role in SCN communication (11), have metabolic abnormalities similar to those in Clock-mutant mice (12). Furthermore, consumption of a high-fat diet alters circadian rhythmicity and the cycling of circadian clock genes in mice (13).
Because multiple studies have linked disruption of the molecular circadian clock and metabolic disorders (10, 12, 14), we hypothesized that LAN exposure alters circadian organization and affects metabolic parameters. We investigated the possibility of a direct link between altered light cycles and metabolic disorder by housing mice in a standard light/dark cycle (LD; 16 h light at ∼150 lx/8 h dark at ∼0 lx), a light/dim light cycle (DM; 16 h light at ∼150 lx/8 h dim light at ∼5 lx), or 24 h of continuous lighting (LL; constant ∼150 lx) and assessing metabolic parameters. We included a DM group in addition to LL because mice in constant lighting have no temporal cue to distinguish time of day, and their biological clocks free-run. The DM group may more directly model environmental light pollution experienced in industrialized nations; however, we predicted circadian alterations in daily activity would not be as profound in a DM environment. By including the LL group, this study focused not only on the effects of light pollution but also on the effects of a desynchronized circadian system on metabolism. We hypothesized that mice housed in DM and LL conditions would alter metabolic parameters compared with mice housed in LD conditions. More specifically, we hypothesized that housing mice in DM and LL conditions would result in reduced glucose tolerance and increased body mass in comparison with LD-housed mice. We also hypothesized that mice housed in LL and DM conditions would alter stress levels, as evaluated by circulating corticosterone, and that LL would induce locomotor arrhythmicity indicative of circadian disruption.
Results
Experiment 1.
Body mass.
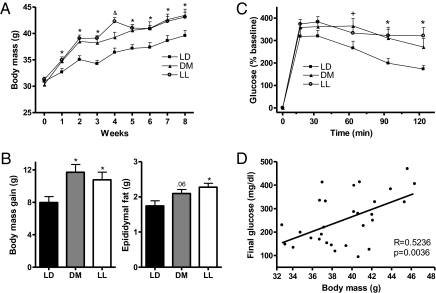
Body mass was affected differentially by light condition over the 8 experimental weeks (F24,192 = 3.457; P < 0.0001) (Fig. 1A). A significant increase in body mass among mice in the LL and DM groups, relative to the LD control group, was evident beginning 1 wk after onset of light treatment and continuing throughout the 8-wk study (F2,24 = 4.441, 10.187, 12.660, 12.232, 6.561, 4.568, and 4.293, respectively; P ≤ 0.01). The elevated body mass among the LL and DM groups resulted from increased gain in body mass (F2,24 = 4.291; P < 0.05) (Fig. 1B) rather than from initial differences in body mass among groups. Furthermore, epididymal fat pad mass was significantly greater at the end of the study in mice exposed to LAN, suggesting that the increased body mass reflected increases in white adipose tissue (F2,24 = 4.767; P < 0.05) (Fig. 1B).
Fig. 1.
Body mass, fat pad mass, and glucose tolerance were altered in mice exposed to bright or dim light at night. (A) Weekly body mass for mice throughout the study (*P ≤ 0.05 when LD differs from both LL and DM groups; &P ≤ 0.05 between all groups). Mice exposed to light at night had elevated body mass beginning 1 wk after placement in experimental light conditions and continuing throughout the remainder of the study. (B) Body mass gain and epididymal fat pad mass differed among groups at the conclusion of the study, suggesting increases in body mass may be caused by changes in body fat composition. (C) Mice exposed to either DM or LL had reduced glucose tolerance, and DM and LL mice failed to recover blood glucose as rapidly as LD mice (*P ≤ 0.05 when LD differs from both LL and DM groups; +P ≤ 0.05 when glucose level is higher in the DM group than in the LD group). (D) Body mass at the time of the GTT correlated positively with final blood glucose levels.
Glucose tolerance test.
After 4 wk in experimental light conditions, mice in the LL and DM groups displayed impaired glucose tolerance during an i.p. glucose tolerance test (GTT). Injection of glucose increased blood glucose levels in all groups following an 18-h fast (F5,23 = 151.015; P < 0.0001) (Fig. 1C), but glucose levels did not recover as rapidly in the LL and DM groups as in the LD group (F10,115 = 2.514; P < 0.01). Mice in the DM group had significantly elevated glucose levels after 60 min, and after 90 and 120 min glucose levels remained elevated in the DM and LL groups compared with the LD group (post hoc test; P < 0.05). Furthermore, the final glucose levels in the GTT correlated positively with body mass (R = 0.5236; P < 0.05) (Fig. 1D).
Locomotor activity.
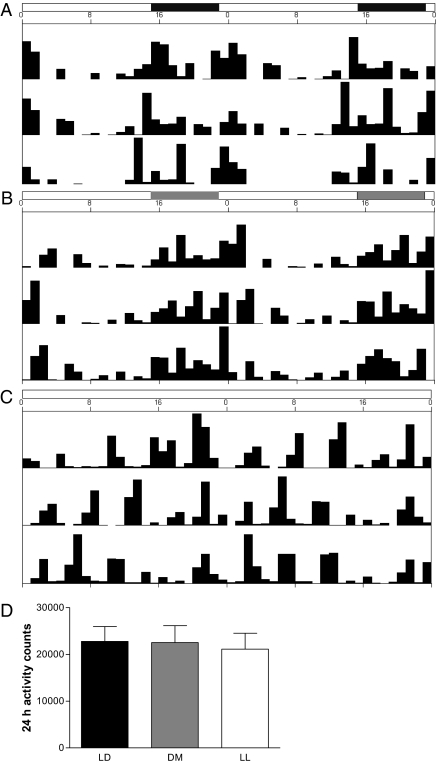
In contrast to the LD and DM groups, which displayed the typical circadian rhythm in locomotor activity, as measured in the home cage via intersecting infrared beams, seven of the eight mice in the LL group were arrhythmic as measured by Fourier analysis (Fig. 2 A–C). However, total daily locomotor activity was similar for all groups (F2,153= 0.0002; P > 0.95) (Fig. 2D).
Fig. 2.
Representative activity records from mice held in (A) LD, (B) DM, and (C) LL conditions. Home cage locomotor activity was measured by intersecting infrared beams and is shown in a double-plotted actogram. Mice in LL conditions became arrhythmic, but mice in DM conditions maintained rhythmicity. (D) All mice had equivalent total 24-h activity (P > 0.05).
Glucocorticoids.
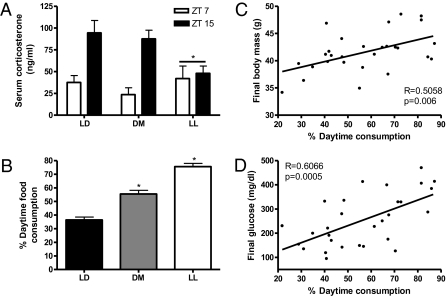
Corticosterone was decreased in the LL group (P < 0.05; Fig. 3A) but not in the mice in the DM group. These results suggest that changes in glucocorticoid concentrations were not necessary for altered metabolism.
Fig. 3.
(A) Serum corticosterone concentrations were reduced in LL mice but not in DM mice at the two time points measured, suggesting that glucocorticoids did not mediate the changes in body mass (*P ≤ 0.05 from LL and DM). (B) Mice exposed to LL and DM conditions ate more food during the light phase than during the dark phase, behavior that is atypical in nocturnal animals (*P ≤ 0.05). (C and D) Body mass (C) and blood glucose levels (D) are associated with percentage of daytime food consumption. (C) Percentage of food consumed during the day correlated with final blood glucose levels obtained in the GTT. (D) Body mass at the conclusion of the study correlated positively with percentage of daytime food intake.
Energy intake.
Total 24-h food consumption did not differ among groups (F2,24 = 0.107; P > 0.85). Although no differences in total food consumption and home cage locomotor activity were detected among groups, feeding behavior was altered in the DM group (F1,38 = 29.315; P ≤ 0.05) (Fig. 3B). Mice in the DM group consumed 55.5% of their food during the light phase, as compared with 36.5% by mice in the LD group, indicating that mice exposed to LAN ate more food during the day than at night. Mice in the LL group were not considered in this comparison because they had no temporal signal to distinguish between light and dark phases. Correlation analyses also confirmed that percentage of daytime food consumption was positively related to final body mass and final glucose levels in the GTT (R = 0.5058 and R = 0.6066, respectively; P < 0.01) (Fig. 3 C and D).
Experiment 2.
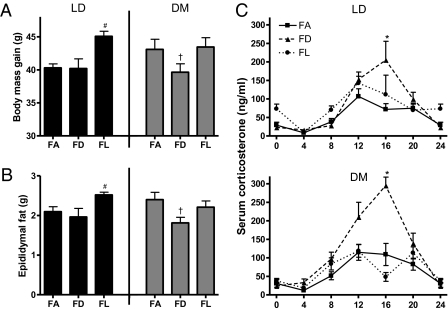
Because altered timing of food consumption may mediate changes in body mass in the DM group, we performed an additional experiment in which mice housed in either LD or DM conditions had continuous access to food (FA) or had food access limited to either the light (FL) or dark (FD) phase. Timed feeding impacted body and epididymal fat pad mass gain (F2,44 = 5.392 and 4.372, respectively; P < 0.05) (Fig. 4 A and B). In the DM group, FD feeding prevented weight and fat gain (weight: t14 = 1.940; fat: t14 = 2.526; P ≤ 0.05). Weight and fat gain in DM-FD mice was equivalent to that in LD-FD and LD-FA (weight: t14 = −0.250, t14 = −1.176; fat: t14 = −0.076, t14 = −1.004; P > 0.05). Furthermore, mice in the FL group increased body and fat pad masses (post hoc, P < 0.05); this increase was not dependent on the light/dark cycle, and LD- and DM-FL mice had comparable weight gain. Light had no effect on corticosterone concentrations at the six time points measured (P > 0.05). However, timed feeding altered corticosterone concentrations at Zeitgeber time (ZT) 16 (F2,24 = 15.316; P < 0.05) (Fig. 4C), such that FD increased corticosterone concentrations (post hoc, P < 0.05). Food consumption changed over time so that consumption decreased throughout the study (F6,258 = 664.474; P < 0.05) (Table 1). Furthermore, there was an interaction between timed feeding and food consumption over time (F12,258 = 98.051; P < 0.05), with mice in the timed-feeding groups initially consuming more than mice in the FA group.
Fig. 4.
Body mass gain and epididymal fat pad mass differed among groups at the conclusion of the study. (A) Total body mass gain and (B) epididymal fat pad mass (#P ≤ 0.05 when group differs from LD; †P ≤ 0.05 when FD differs from both FA and FL). Timed feeding during the dark in mice exposed to LAN prevented increased weight gain and elevated epididymal fat pad mass. (C) Serum corticosterone concentrations are altered by timed feeding. Feeding mice during the dark resulted in increased corticosterone concentrations at Zeitgeber time (ZT) 16, irrespective of light condition (*P ≤ 0.05 when FD differs from FA and FL).
Table 1.
Daily food intake in six experimental groups
| Daily food intake, mean ± SEM (g) |
|||||||
| Experimental group | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
| LD/FA | 4.691 ± 0.226 | 4.504 ± 0.124 | 4.233 ± 0.068 | 3.972 ± 0.118 | 3.9 ± 0.096 | 3.441 ± 0.123 | 3.657 ± 0.193 |
| LD/FD | 5.118 ± 0.220 | 4.579 ± 0.141 | 4.181 ± 0.154 | 4.296 ± 0.151 | 4.065 ± 0.103 | 3.653 ± 0.141 | 3.757 ± 0.111 |
| LD/FL | 5.739 ± 0.169 | 4.947 ± 0.119 | 4.069 ± 0.158 | 3.845 ± 0.12 | 3.844 ± 0.07 | 3.797 ± 0.075 | 3.775 ± 0.124 |
| DM/FA | 4.671 ± 0.140 | 4.615 ± 0.104 | 4.458 ± 0.160 | 4.123 ± 0.106 | 3.837 ± 0.116 | 3.585 ± 0.170 | 3.826 ± 0.193 |
| DM/FD | 5.381 ± 179 | 4.544 ± 0.152 | 4.194 ± 0.109 | 4.157 ± 0.1 | 4.048 ± 0.121 | 4.199 ± 0.12 | 4.038 ± 0.112 |
| DM/FL | 5.794 ± 0.273 | 4.795 ± 0.14 | 4.304 ± 0.144 | 3.903 ± 0.116 | 3.749 ± 0.151 | 3.656 ± 0.18 | 3.463 ± 0.19 |
Discussion
Swiss–Webster mice were housed in an LD cycle, in LL conditions, or in a DM cycle. A significant increase in body mass among mice in the LL and DM groups, relative to the LD control group, was evident beginning 1 wk after onset of light treatment and continued throughout the 8-wk study. After 4 wk, mice in the LL and DM groups displayed impaired glucose tolerance during an i.p. GTT; the mice exposed to LAN failed to recover glucose levels as effectively as the LD group. Increased body mass and reduced glucose tolerance are indicative of a prediabetic-like state (15). Thus, as little as 5 lx of light exposure during the typical dark period is sufficient to increase body mass and compromise glucose regulation.
Total daily locomotor activity, as measured in the home cage via intersecting infrared beams, was similar for all groups. However, in contrast to the LD and DM groups, which displayed the typical circadian rhythm in locomotor activity, the mice in the LL group were arrhythmic. Although no differences in total daily food consumption were detected among groups, feeding behavior was altered in the DM group. The mice in the DM group consumed 55.5% of their food during the light phase, as compared with 36.5% by mice in the LD group, indicating that mice exposed to LAN ate more food during the day than at night. LL mice were not considered in this comparison, because they had no temporal signal to distinguish the light and dark phases. Again, total 24-h consumption did not differ among groups, but food intake is substantially higher at night among nocturnal rodents (16), and altered timing of food consumption has been associated with metabolic syndrome in other animal models (10, 17). Correlation analyses confirmed that percentage of daytime food consumption was positively related to final body mass and final glucose levels in the GTT.
To establish whether the altered timing of food intake contributed to the increased weight gain, we performed an additional study with a timed feeding schedule. Because mice housed in LL probably would entrain to the time of food access, they were omitted from the food access iteration (18). Mice were housed in either LD or DM with FA, FL, or FD feeding. FD feeding prevented weight and fat gain among mice in the DM group. The weight and fat gain in DM-FD mice was equivalent to that in LD-FD mice and LD-FA mice. These results further suggest that altered timing of food consumption in the DM mice leads to increased body mass gain. As previously reported for rats and mice, FL mice increased body mass; this increase was not dependent on the light/dark cycle and LD- and DM-FL mice had comparable weight gain. Mice with limited access to food displayed an initial spike in consumption that decreased over time. With FL feeding, mice increased their food consumption during week one; however, FD-fed mice consumed more food in subsequent weeks. Again, overall food intake for the study was comparable among all groups, suggesting that the timing of food intake is a critical factor mediating increased weight gain.
Alterations in light cycles typically are considered stressful, but the results of previous research on the effects of LAN exposure on glucocorticoid concentrations are equivocal (19, 20). Because high glucocorticoid concentrations can alter metabolism, resulting in obesity, we measured circulating glucocorticoids (21). Contrary to our predictions, corticosterone was decreased in the LL mice but not the DM mice; this finding suggests that changes in glucocorticoid concentrations were unnecessary for altered metabolism. The decreased corticosterone concentrations in the LL mice probably reflect masking of the glucocorticoid rhythm. Glucocorticoid concentrations were affected by food restriction. Mice with food access during the dark phase (FA- and FD-fed mice) had elevated peak glucocorticoid concentrations. There were no differences in glucocorticoid concentrations between DM and LD mice on FA or FL feeding schedules.
These results establish that nighttime illumination at a level as low as 5 lx is sufficient to uncouple the timing of food consumption and locomotor activity, resulting in metabolic abnormalities. DM mice display desynchrony between internal metabolic activity and food intake, as demonstrated by the altered timing of food consumption; this desynchrony may be the primary factor leading to increased weight gain. Similarly, in LL mice the arrhythmic home cage activity suggests that there may be desynchrony between food intake and metabolic parameters, leading to increased weight gain caused by a similar but distinct mechanism. Mice exposed to LAN may have disrupted melatonin signaling leading to a misalignment of food intake and activity and resulting in altered fuel metabolism. Melatonin concentrations have been relatively unexplored in Swiss–Webster mice, but in one previous study (22) retinal melatonin levels were undetectable, in common with melatonin values in common strains of laboratory mice such as C57BL/6. Although previous research failed to detect melatonin in many strains of mice (23), more recent studies report attenuated but rhythmic melatonin expression in strains such as C57BL/6, which previously were thought void of melatonin (24). Melatonin rhythmicity, rather than absolute quantities of nightly melatonin secretion, plays a crucial role in metabolic function (25). For example, blunted nighttime melatonin rhythms caused by LL conditions increased visceral adiposity in rats (26), and daily administration of melatonin suppressed abdominal fat and plasma leptin levels (27). Furthermore, melatonin influences clock gene expression in peripheral tissues such as the heart (28) and similarly may modulate clock gene expression in the peripheral tissue involved in metabolism.
Mice exposed to LAN also may have disrupted clock expression leading to altered metabolism. The SCN are the primary pacemakers at the top of a hierarchy of temporal regulatory systems wherein multiple peripheral tissues contain molecular machinery necessary for self-sustaining circadian oscillation (29). In addition to becoming obese, mice fed a high-fat diet have disrupted Clock gene expression in the liver (13), and mice with mutations in clock genes have altered energy homeostasis (10). Moreover, mice with mutations in either Clock or Brain and muscle Arnt-like protein-1 (Bmal1) show impaired glucose tolerance, reduced insulin secretion, and defects in the proliferation and size of pancreatic islets (30). Several models of obesity have reported attenuated amplitude of circadian clock gene expression, and changes in the phase and daily rhythm of clock genes may cause obesity (31).
Metabolism and the circadian clock are intrinsically related (32), with desynchrony of feeding and activity causing metabolic alterations (17, 33). In humans even brief circadian misalignment results in adverse metabolic and cardiovascular consequences (34). The seemingly innocuous manipulation of environmental light used in this study that changed feeding behavior and resulted in obesity may have important implications for humans. Patients with night-eating syndrome are obese and appear to display circadian rhythm disruption (35, 36). More generally, prolonged computer use and television viewing have been identified as risk factors for obesity, diabetes, and metabolic disorders (37). For the most part, researchers considering this correlation have focused on the lack of physical activity associated with television and computer use; however, the results from the current study suggest that exposure to nighttime lighting and the resulting changes in the daily pattern of food intake and activity also may be contributing factors.
Materials and Methods
Experiment 1.
Mice.
Thirty male Swiss–Webster mice (∼8 wk of age) were obtained from Charles River Laboratories. The mice were housed individually in propylene cages (30 × 15 × 14 cm) at an ambient temperature of 22 ± 2 °C. Mice were provided with regular chow (D12450B: 10% kcal fat, 70% kcal carbohydrate, 20% kcal protein; Research Diets, Inc.) and filtered tap water ad libitum. Upon arrival, all mice were maintained under a 16:8 light/dark cycle [lights on at 23:00 Eastern Standard Time (EST) ∼130 lx] for 1 wk to allow them to entrain to local conditions and recover from the effects of shipping. A 16:8 light/dark cycle rather than a 12:12 cycle was used to avoid providing a seasonally ambiguous signal. Because Swiss–Webster mice are an outbred species and may retain some responsiveness to photoperiod, mice might interpret a 12:12 light/dark cycle as either a long or a short photoperiod, with a resulting higher variability in phenotype (38). After the habituation period, mice were assigned a number pseudorandomly and were assigned to one of three groups housed in LD, LL, or DM conditions. Mice were weighed during group assignment to establish that all groups had a similar baseline body mass; mice weighing >35 g were excluded from the experiments. After group assignment, the LL mice were placed in a constant-light room where they were exposed to ∼150 lx of continuous light. The DM mice were placed in a room with a 16:8 light/dim light cycle; during the light period they were exposed to ∼150 lx of light, and during the dim period they were exposed to ∼5 lx of light at cage level.
Experimental design.
Food intake was measured daily immediately before the onset of the dark period (15:00 EST) throughout the study, and body mass was measured weekly. After 6 wk in experimental light conditions, food intake was measure twice daily, at the onset of the dark period (15:00 EST) and at the onset of the light period (23:00 EST), to quantify timing of food consumption [expressed as a percentage: 100 × consumption light/(consumption light + consumption dark)]. Home cage activity was monitored during the fifth and sixth weeks in experimental light conditions; each room was monitored for 4 consecutive d including a weekend. At week 4 mice underwent an i.p. GTT. After 8 wk in experimental light conditions, mice were killed by cervical dislocation either directly after the onset of darkness (between 15:00 and 17:00 EST) or during the middle of the light phase (5:00–7:00 EST) to collect blood samples at the peak and nadir of locomotor activity. Epididymal fat pads also were collected as an index of white adipose tissue. All experimental procedures were approved by The Ohio State University Institutional Animal Care and Use Committee, and animals were maintained in accordance with the recommendations of the National Institutes of Health and The Guide for the Care and Use of Laboratory Animals.
GTT.
The GTT was given after 4 wk in experimental light conditions. Mice were administered an i.p. glucose bolus (1.5 g/kg body mass) at 10:00 EST after an 18-h fast. Blood samples of ∼5 μL were collected via submandibular bleed before injection and at 15, 30, 60, 90, and 120 min following injection. Blood glucose was measured immediately with the Contour blood glucose monitoring system and corresponding test strips (Bayer HealthCare).
Activity analyses.
Locomotor activity was tracked in eight mice per group using OPTO M3 animal activity monitors (Columbus Instruments) that continuously compile data using MDI software. Results from locomotor activity monitoring were used to determine whether lack of physical activity or altered rhythmicity might have affected metabolism (39).
Blood collection and hormone analyses.
A terminal blood sample was collected from the retroorbital sinus. Blood samples then were allowed to clot. The clots were removed, and samples were centrifuged at 4 °C for 30 min at 3,300 × g. Serum aliquots were aspirated and stored in sealable polypropylene microcentrifuge tubes at −80 °C for subsequent analysis. Total serum corticosterone concentrations for mice were determined in duplicate in an assay using a 125I double -antibody kit (ICN Diagnostics). The high and low limits of detectability of the assay were 1,200 and 3 ng/mL, respectively. The intraassay coefficient of variation was 7.00% for experiment 1 and 16.83% for experiment 2.
Experiment 2.
Mice.
Fifty male Swiss–Webster mice (∼8 wk of age) were obtained from Charles River Laboratories. The mice were housed individually in propylene cages (30 × 15 × 14 cm) at an ambient temperature of 22 ± 2 °C. Mice were provided with Harlan Teklad 8640 food and filtered tap water ad libitum until the start of the study, at which time they were switched to the regular chow used in experiment 1 (D12450B: 10% kcal fat, 70% kcal carbohydrate, 20% kcal protein; Research Diets, Inc.). Upon arrival, all mice were maintained under a 16:8 light/dark cycle (lights on at 18:00 EST, ∼130 lx) for 1 wk to allow them to habituate to local conditions and recover from the effects of shipping. After the habituation period, mice were assigned a number pseudorandomly and were assigned to one of six groups: Mice were housed in either LD or DM conditions with FA (food was weighed twice daily at 9:00 and 19:00 EST), FD (food in: 9:00 EST; food out: 19:00 EST), or FL (food in: 19:00 EST; food out: 9:00 EST). After mice spent 7 wk in experimental light conditions, three retroorbital blood collections to be used for corticosterone analyses (described above) were taken with at least 48 h between collection times. All entrances into the animal rooms and collections after lights off were made under dim red illumination. After 8 wk in experimental light conditions, mice were killed by cervical dislocation either directly after the onset of darkness (10:00–12:00 EST) or during the middle of the light phase (23:00–1:00 EST). Epididymal fat pads were collected.
Statistical analyses.
Hormone concentrations, total daily food consumption, and total daily activity were analyzed using a one-way ANOVA. Following a significant F score, multiple comparisons were conducted with Tukey's Highly Significant Difference tests. GTT results were analyzed with repeated-measures ANOVA with lighting condition as the within-subject factor and time as the between-subject variable. A repeated-measures ANOVA also was used to analyze change in body mass over time. Following a significant result on repeated-measures ANOVA, single time point comparisons were made using Student's t tests. The above statistical analyses were conducted with StatView software, v. 5.0.1. Fourier analysis was used to determine whether locomotor activity was rhythmic and followed 24-h periodicity using Clocklab software from Actimetrics. Mice were considered rhythmic when the highest peak occurred at approximately one cycle per day with an absolute power of at least 0.005 mV/Hz, as previously described (40). Nonlinear regression analysis was performed using GraphPad Prism software, v. 4. In all cases, differences between group means and correlation coefficients were considered statistically significant if P ≤ 0.05.
Acknowledgments
We thank D. McCarthy and S. Chen for technical assistance and A. C. DeVries, D. Crews, and I. Zucker for comments. This research was supported by the National Science Foundation and by the United States–Israel Binational Science Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 2.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 3.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohyama J. A newly proposed disease condition produced by light exposure during night: Asynchronization. Brain Dev. 2009;31:255–273. doi: 10.1016/j.braindev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 5.van Amelsvoort LG, Schouten EG, Kok FJ. Duration of shiftwork related to body mass index and waist to hip ratio. Int J Obes Relat Metab Disord. 1999;23:973–978. doi: 10.1038/sj.ijo.0801028. [DOI] [PubMed] [Google Scholar]
- 6.Parkes KR. Shift work and age as interactive predictors of body mass index among offshore workers. Scand J Work Environ Health. 2002;28:64–71. doi: 10.5271/sjweh.648. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76:424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 8.Ha M, Park J. Shiftwork and metabolic risk factors of cardiovascular disease. J Occup Health. 2005;47:89–95. doi: 10.1539/joh.47.89. [DOI] [PubMed] [Google Scholar]
- 9.Bray MS, Young ME. Circadian rhythms in the development of obesity: Potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8:169–181. doi: 10.1111/j.1467-789X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 10.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 12.Bechtold DA, Brown TM, Luckman SM, Piggins HD. Metabolic rhythm abnormalities in mice lacking VIP-VPAC2 signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R344–R351. doi: 10.1152/ajpregu.00667.2007. [DOI] [PubMed] [Google Scholar]
- 13.Kohsaka A, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 16.Zucker I. Light-dark rhythms in rat eating and drinking behavior. Physiol Behav. 1971;6:115–126. doi: 10.1016/0031-9384(71)90078-3. [DOI] [PubMed] [Google Scholar]
- 17.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mistlberger RE. Food-anticipatory circadian rhythms: Concepts and methods. Eur J Neurosci. 2009;30:1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- 19.Fonken LK, et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205:349–354. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Abílio VC, Freitas FM, Dolnikoff MS, Castrucci AM, Frussa-Filho R. Effects of continuous exposure to light on behavioral dopaminergic supersensitivity. Biol Psychiatry. 1999;45:1622–1629. doi: 10.1016/s0006-3223(98)00305-9. [DOI] [PubMed] [Google Scholar]
- 21.Dallman MF, et al. Minireview: Glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145:2633–2638. doi: 10.1210/en.2004-0037. [DOI] [PubMed] [Google Scholar]
- 22.Tosini G, Menaker M. The clock in the mouse retina: Melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 23.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 24.Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW. Melatonin in mice: Rhythms, response to light, adrenergic stimulation, and metabolism. Am J Physiol Regul Integr Comp Physiol. 2002;282:R358–R365. doi: 10.1152/ajpregu.00360.2001. [DOI] [PubMed] [Google Scholar]
- 25.Korkmaz A, Topal T, Tan DX, Reiter RJ. Role of melatonin in metabolic regulation. Rev Endocr Metab Disord. 2009;10:261–270. doi: 10.1007/s11154-009-9117-5. [DOI] [PubMed] [Google Scholar]
- 26.Wideman CH, Murphy HM. Constant light induces alterations in melatonin levels, food intake, feed efficiency, visceral adiposity, and circadian rhythms in rats. Nutr Neurosci. 2009;12:233–240. doi: 10.1179/147683009X423436. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen DD, Boldt BM, Wilkinson CW, Yellon SM, Matsumoto AM. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999;140:1009–1012. doi: 10.1210/endo.140.2.6674. [DOI] [PubMed] [Google Scholar]
- 28.Zeman M, Szántóová K, Stebelová K, Mravec B, Herichová I. Effect of rhythmic melatonin administration on clock gene expression in the suprachiasmatic nucleus and the heart of hypertensive TGR(mRen2)27 rats. J Hypertens Suppl. 2009;27(6):S21–S26. doi: 10.1097/01.hjh.0000358833.41181.f6. [DOI] [PubMed] [Google Scholar]
- 29.Kohsaka A, Bass J. A sense of time: How molecular clocks organize metabolism. Trends Endocrinol Metab. 2007;18:4–11. doi: 10.1016/j.tem.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnea M, Madar Z, Froy O. High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology. 2009;150:161–168. doi: 10.1210/en.2008-0944. [DOI] [PubMed] [Google Scholar]
- 32.Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol. 2009;16:462–467. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 34.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome; a pattern of food intake among certain obese patients. Am J Med. 1955;19:78–86. doi: 10.1016/0002-9343(55)90276-x. [DOI] [PubMed] [Google Scholar]
- 36.Benca R, et al. Biological rhythms, higher brain function, and behavior: Gaps, opportunities, and challenges. Brain Res Brain Res Rev. 2009;62:57–70. doi: 10.1016/j.brainresrev.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fung TT, et al. Leisure-time physical activity, television watching, and plasma biomarkers of obesity and cardiovascular disease risk. Am J Epidemiol. 2000;152:1171–1178. doi: 10.1093/aje/152.12.1171. [DOI] [PubMed] [Google Scholar]
- 38.Nelson RJ. Photoperiodic responsiveness in house mice. Physiol Behav. 1990;48:403–408. doi: 10.1016/0031-9384(90)90335-2. [DOI] [PubMed] [Google Scholar]
- 39.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2008;582:142–151. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 40.Kriegsfeld LJ, et al. Targeted mutation of the calbindin D28K gene disrupts circadian rhythmicity and entrainment. Eur J Neurosci. 2008;27:2907–2921. doi: 10.1111/j.1460-9568.2008.06239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]