In their mechanistic examination of the role of the aryl hydrocarbon receptor (AHR, also known as the dioxin receptor) in genome shuffling, Okudaira et al. (1) chose to use 6-formylindolo[3,2-b]carbazole (FICZ), a candidate endogenous agonist of this receptor, instead of the more commonly used anthropogenic agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). FICZ was discovered serendipitously when the amino acid l-tryptophan was used to absorb UV B radiation and thereby act as a photosensitizer in experiments designed to produce unique agonists of AHR for quantitative structure–activity relationship analyses (2). On the basis of its formation by photolysis of tryptophan with both visible and UV light, its formation in cells exposed to UV light, and its ability to activate the AHR transiently at concentrations of a few picomolar, FICZ has been proposed to be an endogenous signaling molecule that mediates biological responses to light (ref. 3 and references therein). In marked contrast to TCDD, FICZ is an ideal substrate for the previously orphan cytochrome P450 enzymes CYP1A1, CYP1A2, and CYP1B1, thereby participating in autoregulatory feedback that maintains its own steady-state concentrations at low levels (3).
In PNAS, Okudaira et al. (1) document the unexpected observation that FICZ stimulates retrotransposition efficiently without involvement of the AHR. Almost one half of each mammalian genome is now thought to consist of retrotransposable elements that are silenced epigenetically (reviewed in refs. 4, 5) and whose amplification via reverse transcriptase and insertion into coding genes causes mutations that have contributed to evolution. Currently ongoing retrotransposon activity (RTP) involving the long interspersed nuclear elements-1 (L1), the most abundant retrotransposons in the human genome, is strikingly high; moreover, the DNA of tumor cells contains a larger number of L1 insertions than does the DNA in surrounding normal tissue (6–9). As a consequence of observations such as these, more and more effort is being focused on elucidating the molecular mechanisms by which L1s are amplified in the human genome, and in their present work, Okudaira et al. (1) propose a previously undescribed mechanism for the integration of L1 cDNA in connection with L1-RTP. Their findings indicate that the aryl hydrocarbon nuclear translocator (ARNT) [a basic helix-loop-helix (bHLH-PAS) heterodimeric transcription factor], MAPK, and FICZ are all important and previously unidentified participants in this process. Their data suggest that binding of FICZ to an as yet unidentified bHLH-PAS partner protein activates ARNT, which, in turn, modulates L1-RTP.
L1s are ≈6 kb in length, harbor an internal polymerase II promoter, and encode two ORFs: ORF1 coding for a 40-kDa basic RNA-binding protein with RNA chaperone activity and ORF2 coding for a 150-kDa protein with endonuclease and reverse transcriptase activities. Together with L1 RNA, the ORF1 and ORF2 proteins form a complex that is translocated to the nucleus, where ORF2 cleaves DNA and then uses the resulting nicked ends to prime RT of the L1 RNA, leading ultimately to the integration of L1 cDNA into the genome. Recruitment of additional transcription factors required may be regulated by, for example, epigenetic modifications of and damage to the DNA.
Although earlier investigations have shown that DNA-damaging agents and other environmental stressors, including cisplatin, etoposide, benzo(a)pyrene, and UV and γ-irradiation, can stimulate L1-RTP, the mechanism(s) involved are far from being understood. The fact that a number of these environmental agents generate reactive oxygen species (ROS), together with redox-sensitive regulation of L1 RNA transcription involving antioxidant-responsive elements suggests that L1 expression may be one adaptive response to ROS (ref. 10 and references therein). Rather than enhancing levels of L1 mRNA as oxidants do, FICZ is described here as promoting interaction of ORF1p with ARNT and subsequent recruitment of ORF1p to chromatin in a manner dependent on the activation of MAPK. Thus, a key finding of this study is that FICZ induces L1-RTP via a mechanism that differs from that induced by DNA damage (1).
FICZ appears to exert a variety of effects on mammalians cells, most of which are mediated through its high-affinity binding to the AHR, the highest of all small molecules tested to date (reviewed in ref. 11), and subsequent activation of the transcription of numerous genes (Fig. 1). For example, it is via this receptor that FICZ coordinates several different pathways for the adaptation of human keratinocytes and melanocytes to stress, including internalization of epidermal growth factor receptor and expression of COX2 (12) and melanin production (13), respectively. The current report by Okudaira et al. (1) identifies a role for this photoproduct of tryptophan in the organization of the genome. The AHR has been implicated previously in L1-RTP (references in ref. 14), such that the lack of dependence of this function of FICZ on this receptor was totally unexpected. An additional AHR-independent effect recently shown to be caused by FICZ is activation of the liver X-receptor (15). FICZ also appears to influence both pro- and antiinflammatory processes (16–18) and, in general, may act as a physiologically important hormone/vitamin-like molecule.
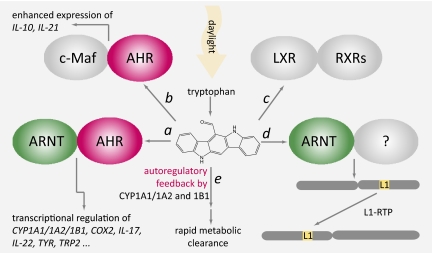
Fig. 1.
Biological processes known to be stimulated in an AHR-dependent and/or AHR-independent manner by the tryptophan photoproduct FICZ. (A) FICZ acts as an agonist of AHR, thereby stimulating the canonical AHR-ARNT signaling pathway that leads to rapid and transient enhancement of the transcription of various sets of genes in different types of cells (2, 3, 12, 13, 17, 18). (B) In murine T cells stimulated by IL-27, FICZ promotes the binding of AHR to c-Maf and, consequently, expression of IL-10 and IL-21 (16). (C) FICZ activates human LXRα and LXRβ with EC50 values similar to those of endogenous activators of these same receptors, such as oxysterols (15). (D) FICZ activates L1-RTP, as described by Okudaira et al. (1). (E) Human cytochrome P450 enzymes CYP1A1, CYP1A2, and CYP1B1 convert FICZ to ring-hydroxylated metabolites that are further metabolized via sulfoconjugation (3).
The important mechanistic insight concerning how FICZ activates ARNT- and MAPK-mediated retrotransposition provided here by Okudaira et al. (1) raises several key questions. Most importantly, the protein that interacts with ARNT needs to be identified and the proposal that this protein is related to the circadian bHLH-PAS protein PER, as indicated by the toxicogenomic studies of Ramos (14), needs to be examined.
Acknowledgments
The research on FICZ-mediating signaling in my own laboratory is supported by grants from the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning.
Footnotes
The author declares no conflict of interest.
See companion article on page 18487.
References
- 1.Okudaira N, et al. Induction of long interspersed nucleotide element-1 (L1) retrotransposition by 6-formylindolo[3,2-b] carbazole (FICZ), a tryptophan photoproduct. Proc Natl Acad Sci USA. 2010;107:18487–18492. doi: 10.1073/pnas.1001252107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rannug A, et al. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262:15422–15427. [PubMed] [Google Scholar]
- 3.Wincent E, et al. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009;284:2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- 4.Kazazian HH., Jr. Mobile elements: Drivers of genome evolution. Science. 2004;303:1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- 5.Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- 6.Beck CR, et al. LINE-1 retrotransposition activity in human genomes. Cell. 2010;141:1159–1170. doi: 10.1016/j.cell.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewing AD, Kazazian HH., Jr. High-throughput sequencing reveals extensive variation in human-specific L1 content in individual human genomes. Genome Res. 2010;20:1262–1270. doi: 10.1101/gr.106419.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CR, et al. Mobile interspersed repeats are major structural variants in the human genome. Cell. 2010;141:1171–1182. doi: 10.1016/j.cell.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iskow RC, et al. Natural mutagenesis of human genomes by endogenous retrotransposons. Cell. 2010;141:1253–1261. doi: 10.1016/j.cell.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos KS, et al. Computational and biological inference of gene regulatory networks of the LINE-1 retrotransposon. Genomics. 2007;90:176–185. doi: 10.1016/j.ygeno.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritsche E, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luecke S, et al. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755-148X.2010.00762.x. doi: 10.1111/j.1755-148X.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramos KS. Unraveling genetic regulatory networks of mammalian retroelements. BMC Proc. 2009;3(Suppl 2):S3. doi: 10.1186/1753-6561-3-s2-s3. Available online at http://www.biomedcentral.com/1753-6561/3/S2/S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reschly EJ, et al. Ligand specificity and evolution of liver X receptors. J Steroid Biochem Mol Biol. 2008;110:83–94. doi: 10.1016/j.jsbmb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]