Abstract
Pheochromocytomas are rare neoplasias of neural crest origin arising from chromaffin cells of the adrenal medulla and sympathetic ganglia (extra-adrenal pheochromocytoma). Pheochromocytoma that develop in rats homozygous for a loss-of-function mutation in p27Kip1 (MENX syndrome) show a clear progression from hyperplasia to tumor, offering the possibility to gain insight into tumor pathobiology. We compared the gene-expression signatures of both adrenomedullary hyperplasia and pheochromocytoma with normal rat adrenal medulla. Hyperplasia and tumor show very similar transcriptome profiles, indicating early determination of the tumorigenic signature. Overrepresentation of developmentally regulated neural genes was a feature of the rat lesions. Quantitative RT-PCR validated the up-regulation of 11 genes, including some involved in neural development: Cdkn2a, Cdkn2c, Neurod1, Gal, Bmp7, and Phox2a. Overexpression of these genes precedes histological changes in affected adrenal glands. Their presence at early stages of tumorigenesis indicates they are not acquired during progression and may be a result of the lack of functional p27Kip1. Adrenal and extra-adrenal pheochromocytoma development clearly follows diverged molecular pathways in MENX rats. To correlate these findings to human pheochromocytoma, we studied nine genes overexpressed in the rat lesions in 46 sporadic and familial human pheochromocytomas. The expression of GAL, DGKH, BMP7, PHOX2A, L1CAM, TCTE1, EBF3, SOX4, and HASH1 was up-regulated, although with different frequencies. Immunohistochemical staining detected high L1CAM expression selectively in 27 human pheochromocytomas but not in 140 nonchromaffin neuroendocrine tumors. These studies reveal clues to the molecular pathways involved in rat and human pheochromocytoma and identify previously unexplored biomarkers for clinical use.
Keywords: Cdkn1b, rat model, transcriptome analysis, progenitor signature
Pheochromocytomas are highly vascularized neoplasias of neural crest-derived chromaffin cells in the adrenal medulla and the sympathetic ganglia (referred to as extra-adrenal pheochromocytomas or paragangliomas). Although usually benign, ≈10 to 15% of human pheochromocytomas progress to malignancy and are refractory to curative therapy. Pheochromocytomas occur either sporadically or as part of a familial cancer syndrome (25–30% of cases) (1–3). Several autosomal dominant cancer syndromes may present with adrenal or extra-adrenal pheochromocytoma: multiple endocrine neoplasia type 2 (MEN2), von Hippel-Lindau (VHL) syndrome, neurofibromatosis type 1 (NF1), and the hereditary paraganglioma syndromes. Familial forms of pheochromocytoma and paraganglioma have been associated with germ-line mutations in RET, VHL, NF1, SDHB, and SDHD (1, 3). The molecular pathophysiology of sporadic pheochromocytoma is not fully understood. Unusually, the somatic mutation of the genes involved in familial disease is uncommon in sporadic cases (4–6).
We have identified a recessive MEN-like syndrome in the rat (termed MENX) demonstrating a phenotypic overlap with both human MEN1 and MEN2 (7). Affected rats (homozygous for the underlying mutation, and hereafter referred to as “mutant”) develop bilateral adrenal pheochromocytoma with a 100% frequency and extra-adrenal pheochromocytoma (paraganglioma) with a 68% frequency. Other tumors include multifocal anterior pituitary adenoma, parathyroid adenoma, and bilateral thyroid C-cell hyperplasia (7). Adrenal medullary hyperplasia is evident as early as 3 mo of age and progresses to pheochromocytoma by 6 to 8 mo. The genetic defect underlying the MENX syndrome is a loss-of-function mutation of Cdkn1b, encoding the cyclin-dependent kinase inhibitor p27Kip1 (hereafter referred to as p27) (8). Capitalizing on this genetic finding, we and others have identified further germ-line mutations in the CDKN1B gene in patients with MEN1-related disease (8–10), demonstrating that rats and humans share genetic pathways that predispose to endocrine malignancy.
The progression of the rat lesions offers a unique opportunity to study the molecular etiology of pheochromocytoma. We report here the alterations in gene expression that accompany the early events of pheochromocytoma development in rats and provide evidence for a common molecular pathway in the development of rat and human pheochromocytoma. Several of the deregulated genes have not been previously identified in human pheochromocytoma and may represent unique tumor markers.
Results
Differential Gene Expression of Rat Adrenal Lesions Reveals Consistent Expression Signatures.
We generated gene expression profiles of eight hyperplastic adrenomedullary lesions, four pheochromocytomas from MENX-affected rats. Adrenal medulla samples of age-matched wild-type littermates were used to generate two datasets: hyperplasia/normal and tumor/normal (Datasets S1 and S2).
Differentially expressed genes were identified by the permutation based unpaired t test with significance analysis of microarrays for equality of expression versus normal RNA. Unsupervised hierarchical clustering separated both hyperplasia and pheochromocytoma from wild-type tissues.
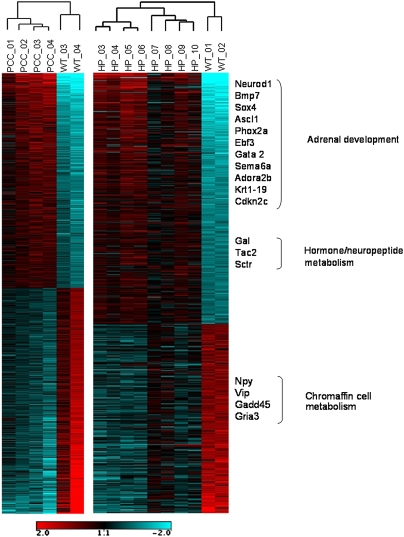
Heat-maps were generated for genes with at least twofold up- or down-regulation in both hyperplasia and tumor datasets (Fig. 1). Genes involved in pathways that are overrepresented and discussed in greater detail are highlighted in Fig. 1. A greater than twofold overexpression was seen for 196 genes in hyperplastic tissue, whereas 165 genes were underexpressed. For pheochromocytoma, 183 genes were overexpressed more than twofold and 306 were underexpressed. The gene lists are reported in Datasets S1 (hyperplasia) and S2 (tumor). The degree of overall overlap between the sets of genes dysregulated in hyperplasia and tumors was considerable (Fig. S1A). Of the 200 most dysregulated nonredundant genes, 87 were common to both datasets (P = 3.73517E-128), indicating that the majority of the gene-expression changes already take place at the hyperplastic stage of pheochromocytoma development in the MENX rats.
Fig. 1.
Heat map of the probe set identifies significantly dysregulated in hyperplasia (HP) and in pheochromocytoma (PCC). Control samples (WT_01, WT_02; and WT_03, WT_04), adrenal gland hyperplasia samples (HP 03–10), and tumors (PCC 01–04) were ordered by hierarchical clustering. Red and blue indicates higher and lower, respectively, expression level with respect to the median across all samples in each dataset. The log2 scale is provided at the bottom. Selected enriched gene ontology (GO) terms and their associated genes are shown on the right.
Neural Precursor Cell-Like Expression Signature Is a Feature of MENX-Associated Pheochromocytoma.
An overrepresentation of development-associated pathways was identified through the analysis of the 200 most up-regulated genes in hyperplastic tissue by the functional annotation tool of the Database for Annotation, Visualization, and Integrated Discovery (DAVID) software (Table 1). The Gene Ontology (GO) category “developmental process” was enriched within the set of dysregulated genes [39 of the 179 (21.8%) classified genes, P = 0.0002]. Of these genes, 18 fell into the subcategory “nervous system development” (P = 0.00017) (Fig. S1B and Table S1). These included the Mash1(Ascl1), Bmp7, Phox2a, Neurod1, Gal, Cxcr4, Cdkn2a, Cdkn2c, Gata2, Sema6a, and Sox4 genes that are all implicated in the differentiation of neural crest cells into the precursor cells of the sympathoadrenal cell lineage from which adrenal medullary cells are derived (11, 12).
Table 1.
Enriched GO categories by DAVID
| Term | Hyperplasia | Pheochromocytoma | ||
| Count | P value | Count | P value | |
| GO:0030182∼neuron differentiation | 12 | 4.91E-05 | 7 | 5.40E-02 |
| GO:0007399∼nervous system development | 18 | 1.72E-04 | 13 | 3.55E-02 |
| GO:0032502∼developmental process | 39 | 2.10E-04 | 32 | 5.21E-02 |
| GO:0048468∼cell development | 22 | 6.64E-04 | 20 | 6.94E-03 |
| GO:0048666∼neuron development | 9 | 6.78E-04 | 5 | 1.45E-01 |
| GO:0030154∼cell differentiation | 26 | 7.60E-04 | 24 | 7.48E-03 |
In pheochromocytoma, a trend toward a similar enrichment of developmental process genes is evident (Table 1), with 32 of the 200 recognized probe set identifications (16%) belonging to this category P = 0.052 (Table S2). However, the additional enrichment of “cell communication,” “cell adhesion,” and “cell proliferation” genes (Table S2) that accompanies progression from hyperplasia to tumor clearly dilutes the development-associated gene-expression signature. The enrichment of developmentally related genes in both tumor and hyperplasia was also seen using an alternative analytical tool (Gene Ontology Tree Machine) (13).
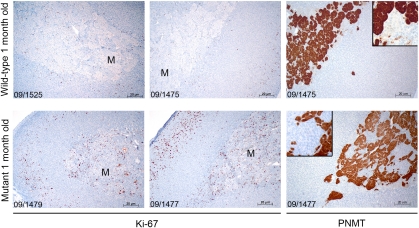
A number of genes involved in specialized adrenal cell functions were underexpressed in the rat lesions, including the glutamate receptor (Gria3), Gadd45, CREB-like protein, and the neuropeptide genes Vip and Npy (Fig. 1). A similar dedifferentiation has been reported in human familial pheochromocytoma (14). The adrenal medulla of rodents contains two cell populations of adrenergic and noradrenergic chromaffin cells that can be distinguished based on the expression of the enzyme phenylethanolamine N-methyltransferase (PNMT), which is expressed only in the former cell type. This expression also occurs in MENX-affected rats, as demonstrated by immunohistochemical staining using a specific anti-PNMT antibody (Fig. S2). During tumor progression, the cell population that expands is composed of PNMT-negative cells, in agreement with what has been previously observed in some (15), but not all (16) spontaneous or drug-induced rat pheochromocytomas. The paragangliomas that develop in the affected rats also show no immunoreactivity for PNMT.
Validation of the Microarray Analysis.
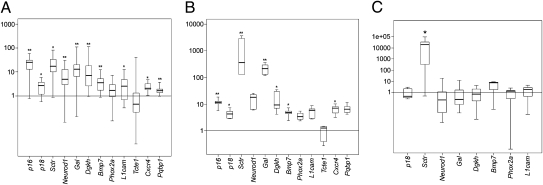
Because we were mainly interested in early gene expression changes, we focused on the hyperplasia dataset. Quantitative PCR (qRT-PCR) analysis of 12 genes overexpressed in the rat lesions was performed to validate the expression signature data. Transcripts selected were the cell-cycle inhibitors Cdkn2a (p16) and Cdkn2c (p18), Sctr (secretin receptor), Neurod1 (neurogenic differentiation factor 1), Gal (galanin), Dgkh (diacylglycerol kinase eta), Bmp7 (bone morphogenetic protein 7), Phox2a (paired-like homeobox 2a), L1cam (L1 cell adhesion molecule), Tcte1 (T-complex-associated testis-expressed protein 1), Cxcr4 (C-X-C chemokine receptor type 4), and Pqbp1 (polyglutamine-binding protein 1). These genes were selected because they belong to the most overrepresented biological process in rat hyperplasia: the nervous system development (Fig. S1). Moreover, they cover a 3- to 20-fold range of expression changes in the rat lesions. The gene encoding galanin was included because this protein was reported to be highly expressed in about 50% of human pheochromocytomas (17).
Quantitative RT-PCR analysis was performed on both the original tissue samples used for microarray analysis and additional ones (total n ≥ 25). Macrodissected adrenal medulla tissues that were mostly devoid of cortical cells were macrodissected from normal age-matched rats (n = 5) to serve as controls (18).The qRT-PCR results were largely concordant with the expression array data, although the magnitude of some of the changes detected varied between the two methods (Fig. 2A). The only exception was Tcte1, which was among the most highly overexpressed genes in the array analysis (Dataset S1) but was not up-regulated in the qRT-PCR. The discrepancy between the array and qRT-PCR results may be a result of alternative splicing of the Tcte1 transcript in adrenomedullary cells of MENX rats (Fig. S3).
Fig. 2.
Validation of selected genes by TaqMan qRT-PCR in adult and young adrenal tissues and in paraganglioma. (A) Tumor RNA was extracted from macrodissected adrenal medulla of adult mutant rats (8–10 mo of age) and from adrenal medulla of normal control rats. Quantitative RT-PCR was performed using primer and probe sets specific to the indicated rat genes. The level of mRNA in mutant tumor tissue was normalized against the value obtained for normal tissues (at least five different samples), whose average was arbitrarily set at 1. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Error bars above and below the box indicate the 90th and 10th percentiles. (B) RNA was extracted from macrodissected adrenal medulla of 1-mo-old mutant rats (n = 5) and control rats (n = 4). Quantitative RT-PCR was performed using primer and probe sets specific to the genes indicated in A. (C) RNA was extracted from paragangliomas (n = 7) of adult mutant rats and macrodissected adrenal medulla of adult wild-type rats. Quantitative RT-PCR was performed using primer and probe sets specific to the indicated genes. *P < 0.05; **P < 0.01.
A qRT-PCR assay for Mash1, which encodes a trascription factor that promotes the neuronal fate determination of neural crest stem cells (19), was not available. Consequently we applied an in situ mRNA hybridization assay to detect Mash1 transcripts (20). This process confirmed that formalin-fixed, paraffin-embedded tumor tissues expressed higher levels of Mash1 than corresponding control tissues (Fig. S4A).
Gene Expression Changes Precede Histological Changes in Mutant Adrenal Medulla.
To establish if the observed gene-expression changes precede even the histologically detected hyperplasia, we performed qRT-PCR for the 12 overexpressed genes shown in Fig. 2A on macrodissected tissues obtained from 1-mo-old mutant (n = 5) and age-matched wild-type littermates (n = 4) (Fig. 2B). We observed that all of the genes overexpressed in hyperplasia, with the exception of Tcte1 (see above), are already expressed at high—albeit variable—levels in the histologically “normal” adrenal glands of 1-mo-old affected rats (Fig. 2B). Interestingly, Sctr and Gal are expressed in these samples at levels that are even higher that those seen in hyperplasia or tumor.
The presence of altered gene expression before the histological changes may be a direct consequence of the loss of the p27 expression, rather than a tumor-progression event. To verify whether the overexpression of those genes is indeed dependent on the presence of functional p27, we assessed their expression level in the PC12 cell line (derived from a rat pheochromocytoma). PC12 cells have an endogenous wild-type Cdkn1b gene sequence and express detectable p27 protein. Interestingly, two of the genes overexpressed in MENX adrenal lesions, namely Cdkn2c and Sctr, are also more highly expressed in PC12 cells when compared with normal rat adrenal medulla (used as control) (Fig. S4B). In addition, Tcte1 is highly expressed in PC12 cells and this correlates with the presence of a correctly-spliced (and hence detectable by qRT-PCR) Tcte1 transcript in these cells (Fig. S3). These results suggest that the overexpression of Cdkn2c, Sctr, and Tcte1 is a feature of rat pheochromocytoma and is therefore not related to p27 loss-of-function, but the overexpression of the other genes may be specific for MENX-associated tumors.
Pheochromocytomas and Paragangliomas in MENX Rats Show Different Expression Signatures.
To determine whether the genes overexpressed in the MENX-associated adrenal lesions are also involved in the development of the extra-adrenal tumors (noradrenergic) in these animals, we analyzed a subset of the genes reported in Fig. 2A (i.e., Cdkn2c, Sctr, Neurod1, Gal, Dgkh, Phox2a, L1cam, and Bmp7). Of the genes analyzed by qRT-PCR in seven rat paragangliomas (Fig. 2C), only Sctr showed overexpression in these tumors. None of the other genes overexpressed in adrenal tumors was found to be overexpressed in paraganglioma compared with normal adrenal medulla. This finding suggests that, although both tumor types derive from chromaffin cells and are morphologically similar, they are the result of distinct molecular alterations within the MENX animal model.
Analysis of Human Pheochromocytomas.
A subset of the genes up-regulated in rat adrenomedullary lesions was analyzed in a series of 33 sporadic and 13 familial human pheochromocytomas (Table S2). We chose the homologs of genes already validated in the rat lesions, which belong to the highly enriched “nervous system development” GO category (i.e., GAL, DGKH, PHOX2A, L1CAM, BMP7, TCTE1, and HASH1/ASCL1, the homolog of the rat Mash1). In addition, we studied the SOX4 (SRY-sex determining region Y-box 4) and EBF3 (Early B-cell factor 3) genes because of their global role in development (21, 22). The results of the qRT-PCR showed that all of these genes are indeed also overexpressed in both sporadic and familial human pheochromocytomas, albeit with variable frequencies (Table S3). We did not observe differences in the incidence of gene overexpression between hereditary and familial cases, with the exception of DGKH, which was up-regulated in 24 of 33 sporadic tumors (72.7%) but in only 2 of 13 familial cases (15.3%). Interestingly, both the PHOX2A and L1CAM genes were frequently overexpressed, being elevated in 42 of 46 (91%) and 41 of 46 (89%) tumors, respectively. BMP7 was overexpressed in 38 of 46 tumor cases, EBF3 in 29 of 45 cases. GAL was found up-regulated, albeit at highly variable levels, in 21 of 45 samples, in agreement with previously reported data (17). In contrast to the differences in gene-expression changes between the rat MENX-associated adrenal and extra-adrenal pheochromocytoma, no significant difference in the frequency of gene up-regulation (P > 0.09) was seen between human adrenal and extra-adrenal lesions. Similarly, no difference in gene overexpression exists between adrenergic and noradrenergic tumors (P > 0.19).
Candidate Markers for Pheochromocytoma.
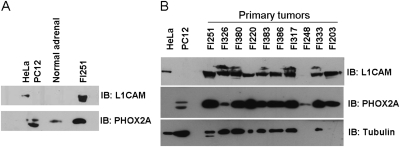
As a direct correlation between mRNA expression and protein levels does not automatically follow, we studied the protein products of two of the highly overexpressed genes, namely L1CAM and PHOX2A. The expression level of both proteins in human tumors was compared with normal human adrenomedullary tissue and cell lines: PC12 cells were used as a positive control for PHOX2A expression, and HeLa cells for L1CAM. Western blot analysis showed that L1CAM is indeed highly expressed in 9 of 10 of the human tumor samples, although it was undetectable in normal adrenal tissue. PHOX2A was expressed in 8 of 10 human pheochromocytomas at a much higher level than that seen in normal adrenal medulla (Fig. 3).
Fig. 3.
Analysis of L1CAM and PHOX2A expression in human pheochromocytomas. (A) Control membrane containing cell lines (HeLa, PC12), normal adrenal tissue (consisting of a pool of macrodissected adrenal medulla from three control individuals), and tumor sample FI251. (B) Human pheochromocytoma samples (Table S1) express at high level PHOX2A and L1CAM proteins compared with control cell lines and normal adrenal. To check for equal loading the membrane was probed with anti-α-tubulin antibody.
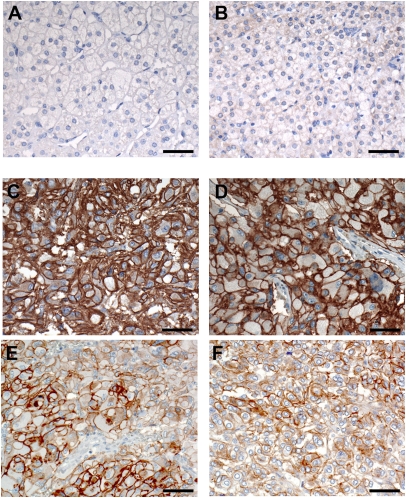
Immunohistochemical staining of human pheochromocytoma for the L1CAM protein was performed on a series of formalin-fixed, paraffin-embedded pheochromocytoma tissues: 18 from the patients already studied by qRT-PCR plus 5 additional cases (Table S4 and ref. 23). The results showed that L1CAM is expressed at high levels at the plasma membrane of the tumor cells compared with human normal adrenomedullary cells (Fig. 4). Moderate to high expression (score 2+ and 3+) was observed in all adrenal pheochromocytoma (12 sporadic and 2 familial), as well as in seven of nine extra-adrenal paragangliomas (Table S4). To examine the specificity of this putative unique marker of pheochromocytoma, we stained two tissue microarrays containing a total of 144 neuroendocrine tumors (NETs) with the anti-L1CAM antibody. Positive membranous immunoreactivity was only observed in the two samples of adrenal pheochromocytomas (scored 2+) and in one of two abdominal paragangliomas (scored 2+). Interestingly, neither two duodenal gangliocytic nonchromaffin paragangliomas, nor any of the remaining 138 nonpheochromocytoma NETs showed L1CAM immunostaining. These results attest to a highly specific and restricted expression of L1CAM in NETs that is limited to neoplasms derived from chromaffin cells.
Fig. 4.
Expression of L1CAM in pheochromocytoma. (A–F) Immunohistochemical staining with an anti-L1CAM specific antibody. (A and B) Staining of two normal adrenal medullary tissues. (C and D) Staining of two pheochromocytomas (PGL65 and PGL114) scored 3+. (E and F) Staining of two pheochromocytomas (PGL174 and PGL295) scored 2+. (Scale bars, 50 μm.) Original magnification: (A–F) 200×.
Discussion
Gene expression profiling of MENX-associated rat adrenomedullary lesions has revealed a neural precursor cell-like molecular signature and that the rat lesions share molecular mechanisms with their human counterpart.
Several genes involved in the differentiation of neural crest-derived progenitor cells of the sympathoadrenal cell lineage into endocrine chromaffin cells are among those highly expressed in the rat adrenal lesions, including Mash1 and Phox2a. Mash1/Ascl1 is a helix–loop–helix transcription factor required for the development of early precursor cells that are committed to a neuronal fate (19). Mash1/Ascl1 activates Phox2a, a homeodomain transcription factor expressed in parasympathetic neurons (24). Genes such as Mash1/Ascl and Phox2a are usually transiently expressed during development and then switched off as the cells undergo terminal differentiation at their final body location. High expression of Mash1 and Phox2a suggests that MENX-associated tumors arise from cells with a sympathoadrenal precursor-like phenotype. Other genes having a recognized role in the determination of sympathoadrenal cell lineage fate or showing a more general involvement in the formation of the neuroendocrine system, such as Gal, Bmp7, and Neurod1 (17, 25, 26), are also overexpressed in rat adrenal tumors.
The enrichment of development-related genes in our samples is reminiscent of the situation reported by Powers et al. (27), who performed microarray analysis of pheochromocytoma developing in mice harboring one defective copy of the Neurofibromatosis 1 (Nf1) gene. Within this study the authors present a list of 64 selected genes significantly overexpressed in the mouse tumors that participate in the regulation of neural development. Interestingly, seven of them are also highly expressed in MENX rat hyperplasia (Spock1, Sox9, Celsr1, Brca1, Klf7, Phox2A, Lgals1). Powers et al. proposed two alternative hypotheses to explain their results: (i) Nf1-associated pheochromocytoma could arise from persistent neural progenitors that continue to express markers associated with their cells of origin; and (ii) the expression of neuronal markers of immature precursor cells is required during neoplastic transformation. Our observation that a subset of the overexpressed genes involved in developmental processes is already highly expressed in the nontumorous adrenal medulla of 1-mo-old mutant rats lends support to the hypothesis for maintenance, rather than reacquisition, of the sympathoadrenal progenitor phenotype.
MENX mutant rats carry a homozygous germ-line mutation in Cdkn1b, which causes an extreme reduction or loss of p27 protein in affected rat tissues (8). The protein p27 is a cell-cycle regulator that regulates the interplay between proliferation and differentiation in many tissues (28, 29). The resultant loss of cell-cycle exit capability in MENX rats could promote an atypical expansion of progenitor cells or could prevent chromaffin cells from undergoing terminal differentiation. The bilateral juvenile cataracts seen in affected rats (7) supports a role for p27 in both of these processes. Indeed, histological analysis showed the presence of cell nuclei in the lens fiber compartment, a sign of ectopic cell proliferation and of failure of terminal differentiation during lens fiber formation (8). Furthermore, as we observed a higher proliferation rate in adrenomedullary cells in 1-mo-old mutant rats (Ki67 labeling index) (Fig. 5), we believe that the absence of p27 both increases proliferation and impairs terminal differentiation of neural crest-derived precursor cells with a consequent overrepresentation of cells with an immature molecular phenotype in the adrenal glands of mutant rats. The finding that some of the developmental markers identified in the MENX tumors are absent from rat PC12 cells bearing wild-type p27 further supports the hypothesis that some parts of the progenitor cell-like gene signature might be a direct result of nonfunctioning p27. However, further studies are required to prove a direct link between p27 and the expression of these selected genes.
Fig. 5.
Proliferation rate and PNMT expression in adrenal glands of young rats. Immunohistochemical staining of adrenal glands of young wild-type and mutant rats with the proliferation marker Ki67 and with PNMT. Tssue sections of adrenal glands from 1-mo-old wild-type rats (Upper, n = 2) and mutant rats (Lower, n = 2) were stained using antibodies against the Ki-67 antigen and the PNMT enzyme. M, medulla. (Scale bars, 20 μm.) Original magnification: 100×; Insets: 200×.
Importantly, some of the genes dysregulated in the rat model were also found to be up-regulated in both sporadic and hereditary human pheochromocytoma. Most of these genes have not previously been associated with human pheochromocytoma (DGHK, PHOX2A, BMP7, L1CAM, EBF3) and therefore represent potentially unique biomarkers of the disease. Assessing the function of the identified developmentally regulated neural genes in adrenomedullary tumors and normal states may help us understand the origin of these tumors and may provide us with previously unexplored targets for therapeutic regimens aimed at eradicating precursor tumor cells.
L1CAM is a neural cell adhesion molecule that interacts with other cell-surface receptors to regulate axonal projections and migration of embryonic cortical neurons (30). High L1CAM expression has been observed in various types of cancer and is associated with tumor invasion and unfavorable prognosis in carcinomas of the ovaries, endometrium, stomach, and breast (31). In contrast, expression of L1CAM correlates with favorable outcome in pediatric neuroblastoma, embryologically related to pheochromocytoma by its common derivation from the sympathoadrenal system (32). Immunohistochemical staining for L1CAM of 27 cases of adrenal and extra-adrenal pheochromocytoma and 140 human NETs of various origins showed high expression of the protein selectively in chromaffin cell-derived NETs. In our pheochromocytomas, the level of L1CAM expression was always elevated, and not obviously associated with invasive behavior: only one tumor in our series was malignant, and it scored 3+. The analysis of larger series of benign and malignant pheochromocytoma is required to determine whether a high level of L1CAM associates with a more aggressive behavior in this tumor type.
Recently, L1CAM was investigated as target antigen for radio-immunotherapy in a mouse xenograft model of human neuroblastoma by use of a radio-labeled specific anti-L1CAM antibody (33). MENX-affected rats, because of the overexpression of L1CAM in their adrenal tumors, represent a good experimental model to evaluate the efficacy of radio-labeled anti-L1CAM antibodies for functional imaging and therapy of pheochromocytoma.
The present study, together with recent genome-wide allelic imbalance data on MENX-associated pheochromocytoma (18), demonstrates that, although the rat model may not perfectly reflect the human tumors, it is a useful model of human pheochromocytoma. Considering the small number of genes selected for cross-validation in the human samples, the identification of common genetic features with MENX tumors is remarkable. Moreover, similarities in molecular features extend to human sporadic pheochromocytoma, the most frequent type and that whose molecular pathophysiology still needs to be fully deciphered. Further molecular characterization of MENX-associated pheochromocytoma will improve our understanding of the pathophysiology of this tumor and might provide novel molecular targets for early diagnosis, precise detection, and individualized therapy.
Materials and Methods
Rat Tissue Samples.
Rat tissues were snap-frozen in liquid nitrogen and stored at −80 °C until use. Histological examination by an experienced pathologist (A.P.) confirmed the diagnosis of hyperplasia or pheochromocytoma. Cryosections of adrenal tissues were macrodissected under a stereomicroscope M5-52765 (Wild) to remove the cortex. This approach gave us samples largely devoid of cortical cells, as previously demonstrated (18).
Patients.
The study was approved by the Ethics Committee of the Universities of Munich, Bern, Gliwice, and Florence and informed written consent was obtained from all patients. Phaeochromocytoma/paraganglioma patients studied included eight with MEN 2A, one with VHL syndrome, one with paraganglioma syndrome 1 (PGL1), one with PGL4, and a further 33 without evidence of an underlying hereditary condition for pheochromocytoma/paraganglioma (i.e., presumed sporadic). Clinical data of the patients used for the qRT-PCR are shown in Table S1. Cases used for immunohistochemical staining are also indicated in Table S1 or were previously reported (23).
See SI Materials and Methods for more details and associated references.
Supplementary Material
Acknowledgments
We thank E. Samson, E. Pulz, B. Geist, C. Lach, and J. Müller for excellent technical assistance. This work was supported by Grants 109223 from the Deutsche Krebshilfe, SFB 824 (DFG Sonderforschungsbereich 824) from the Deutsche Forschungsgemeinschaft, Bonn, Germany (to N.S.P.), and the Helmholtz Alliance on Systems Biology CoReNe (J.B.). S.M. was the recipient of a postdoctoral fellowship of the Centro per la Comunicazione e la Ricerca, Collegio Ghislieri, Pavia, Italy.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE21006).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003956107/-/DCSupplemental.
References
- 1.Dahia PL. Evolving concepts in pheochromocytoma and paraganglioma. Curr Opin Oncol. 2006;18:1–8. doi: 10.1097/01.cco.0000198017.45982.06. [DOI] [PubMed] [Google Scholar]
- 2.Gimenez-Roqueplo AP. New advances in the genetics of pheochromocytoma and paraganglioma syndromes. Ann N Y Acad Sci. 2006;1073:112–121. doi: 10.1196/annals.1353.012. [DOI] [PubMed] [Google Scholar]
- 3.Mannelli M, et al. Genetics and biology of pheochromocytoma. Exp Clin Endocrinol Diabetes. 2007;115:160–165. doi: 10.1055/s-2007-970407. [DOI] [PubMed] [Google Scholar]
- 4.Astuti D, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch CA, Vortmeyer AO, Zhuang Z, Brouwers FM, Pacak K. New insights into the genetics of familial chromaffin cell tumors. Ann N Y Acad Sci. 2002;970:11–28. doi: 10.1111/j.1749-6632.2002.tb04409.x. [DOI] [PubMed] [Google Scholar]
- 6.Bausch B, et al. Comprehensive mutation scanning of NF1 in apparently sporadic cases of pheochromocytoma. J Clin Endocrinol Metab. 2006;91:3478–3481. doi: 10.1210/jc.2006-0780. [DOI] [PubMed] [Google Scholar]
- 7.Fritz A, et al. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res. 2002;62:3048–3051. [PubMed] [Google Scholar]
- 8.Pellegata NS, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgitsi M, et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92:3321–3325. doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94:1826–1834. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber K. The sympathoadrenal cell lineage: Specification, diversification, and new perspectives. Dev Biol. 2006;298:335–343. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Langley K, Grant NJ. Molecular markers of sympathoadrenal cells. Cell Tissue Res. 1999;298:185–206. doi: 10.1007/pl00008810. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Schmoyer D, Kirov S, Snoddy J. GOTree Machine (GOTM): A web-based platform for interpreting sets of interesting genes using Gene Ontology hierarchies. BMC Bioinformatics. 2004;5:16. doi: 10.1186/1471-2105-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahia PL, et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tischler AS, Coupland RE. Changes in structure and function of the adrenal medulla. In: Mohr U, Dungworth CC, Capen CC, editors. Pathobiology of the Aging Rat. Vol. 2. Washington, DC: ILSI; 1994. pp. 244–268. [Google Scholar]
- 16.Powers JF, Picard KL, Nyska A, Tischler AS. Adrenergic differentiation and Ret expression in rat pheochromocytomas. Endocr Pathol. 2008;19:9–16. doi: 10.1007/s12022-008-9019-1. [DOI] [PubMed] [Google Scholar]
- 17.Bauer FE, et al. Localization and molecular forms of galanin in human adrenals: Elevated levels in pheochromocytomas. J Clin Endocrinol Metab. 1986;63:1372–1378. doi: 10.1210/jcem-63-6-1372. [DOI] [PubMed] [Google Scholar]
- 18.Shyla A, et al. Allelic loss of chromosomes 8 and 19 in MENX-associated rat pheochromocytoma. Int J Cancer. 2010;126:2362–2372. doi: 10.1002/ijc.24925. [DOI] [PubMed] [Google Scholar]
- 19.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- 21.Wilson ME, et al. The HMG box transcription factor Sox4 contributes to the development of the endocrine pancreas. Diabetes. 2005;54:3402–3409. doi: 10.2337/diabetes.54.12.3402. [DOI] [PubMed] [Google Scholar]
- 22.Dubois L, Vincent A. The COE—Collier/Olf1/EBF—transcription factors: Structural conservation and diversity of developmental functions. Mech Dev. 2001;108:3–12. doi: 10.1016/s0925-4773(01)00486-5. [DOI] [PubMed] [Google Scholar]
- 23.Pellegata NS, et al. Human pheochromocytomas show reduced p27Kip1 expression that is not associated with somatic gene mutations and rarely with deletions. Virchows Arch. 2007;451:37–46. doi: 10.1007/s00428-007-0431-6. [DOI] [PubMed] [Google Scholar]
- 24.Lo L, Tiveron MC, Anderson DJ. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;125:609–620. doi: 10.1242/dev.125.4.609. [DOI] [PubMed] [Google Scholar]
- 25.Song Q, Mehler MF, Kessler JA. Bone morphogenetic proteins induce apoptosis and growth factor dependence of cultured sympathoadrenal progenitor cells. Dev Biol. 1998;196:119–127. doi: 10.1006/dbio.1998.8847. [DOI] [PubMed] [Google Scholar]
- 26.Cho JH, Tsai MJ. The role of BETA2/NeuroD1 in the development of the nervous system. Mol Neurobiol. 2004;30:35–47. doi: 10.1385/MN:30:1:035. [DOI] [PubMed] [Google Scholar]
- 27.Powers JF, Evinger MJ, Zhi J, Picard KL, Tischler AS. Pheochromocytomas in Nf1 knockout mice express a neural progenitor gene expression profile. Neuroscience. 2007;147:928–937. doi: 10.1016/j.neuroscience.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Ohnuma S, Philpott A, Wang K, Holt CE, Harris WA. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell. 1999;99:499–510. doi: 10.1016/s0092-8674(00)81538-x. [DOI] [PubMed] [Google Scholar]
- 29.Besson A, et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–1746. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmid RS, Maness PF. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol. 2008;18:245–250. doi: 10.1016/j.conb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raveh S, Gavert N, Ben-Ze'ev A. L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett. 2009;282:137–145. doi: 10.1016/j.canlet.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Wachowiak R, et al. L1 is associated with favorable outcome in neuroblastomas in contrast to adult tumors. Ann Surg Oncol. 2007;14:3575–3580. doi: 10.1245/s10434-007-9608-0. [DOI] [PubMed] [Google Scholar]
- 33.Hoefnagel CA, et al. A comparison of targeting of neuroblastoma with mIBG and anti L1-CAM antibody mAb chCE7: Therapeutic efficacy in a neuroblastoma xenograft model and imaging of neuroblastoma patients. Eur J Nucl Med. 2001;28:359–368. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.