Abstract
Networks of marine reserves are increasingly a major component of many ecosystem-based management plans designed to conserve biodiversity, protect the structure and function of ecosystems, and rebuild and sustain fisheries. There is a growing need for scientific guidance in the design of network-wide monitoring programs to evaluate the efficacy of reserves at meeting their conservation and management goals. Here, we present an evaluation of the Channel Islands reserve network, which was established in 2003 off the coast of southern California. This reserve network spans a major environmental and biogeographic gradient, making it a challenge to assess network-wide responses of many species. Using fish community structure data from a long-term, large-scale monitoring program, we first identified persistent geographic patterns of community structure and the scale at which sites should be grouped for analysis. Fish communities differed most among islands with densities of individual species varying from 3- to 250-fold. Habitat structure differed among islands but not based on reserve status. Across the network, we found that, after 5 years, species targeted by fishing had higher densities (1.5×) and biomass (1.8×) inside reserves, whereas nontargeted species showed no significant differences. Examining trophic groups, piscivore and carnivore biomass was significantly greater inside reserves (1.8× and 1.3× more, respectively), whereas the biomass of planktivores and herbivores was similar inside and out. A framework for incorporating biogeographic variation into reserve network assessments is critical as we move from the evaluation of single reserves to networks of reserves.
Keywords: ecosystem-based management, kelp forest ecosystem, marine protected areas, monitoring, response ratios
Efforts to prevent and reverse declining trends in coastal marine ecosystems and the fisheries and other services these ecosystems support increasingly advocate the use of ecosystem-based management (EBM) (1–3). One common element of the EBM framework includes the use of marine protected areas (MPAs) (4–6). Results of synthetic analyses routinely show that single MPAs are generally effective at biodiversity conservation by increasing density, size, biomass, and diversity of species within their borders (7–9). Emerging evidence indicates that adult spillover (10, 11) and larval subsidies (12, 13) may benefit fished areas outside MPAs. Following the successes of single marine reserves, these management tools have gained popularity in coastal zones and stimulated the drive to establish regional networks of MPAs (4, 14). Networks of MPAs represent an integrated system of multiple protected areas, often designed to conserve regional biodiversity and ecosystem function across habitats, buffer against catastrophes, connect populations on ecological timescales, and provide sustained socioeconomic benefits (15, 16). In some cases, scientific guidance has been used to establish size and spacing guidelines in MPA network design, with the goal to create MPA networks that protect species and habitats while enhancing larval replenishment of populations open and closed to harvest (17–20).
Increasingly, the development of MPA networks includes monitoring programs designed to evaluate the effectiveness of MPAs at meeting their conservation and management goals. One intent of these evaluations is to inform the adaptive management of individual MPAs and whole networks. In addition, monitoring programs facilitate the role of MPAs as tools for EBM. Comparisons inside and outside of MPAs provide fishery managers with estimates of the effects of fishing on the structure and functions of populations, communities, and ecosystems. Such comparisons can also inform stock assessments and estimate the relative response and resiliency of fished and unfished systems to natural and anthropogenic perturbations. Thus, there is growing demand for guidance in the design of monitoring programs, especially those that capitalize on the opportunities (e.g., replication, variation) provided to scientists and decision makers by the multiple MPAs that constitute networks.
In 2003, the California Department of Fish and Game established a network of 10 State Marine Reserves (fully no-take) and two State Marine Conservation Areas (some limited fishing activities allowed) covering approximately 12% of state waters within the Channel Islands National Marine Sanctuary [CINMS; (Fig. 1) hereafter referred to as the “reserve network”]. In 2007, the network was expanded into federal waters. The reserve network covers 488 km2 in state waters, including reserves on five islands, and was the product of a stakeholder-initiated process in which scientific and socioeconomic guidance were used to identify the number, size, spacing, and locations of reserves according to ecological design criteria (15).
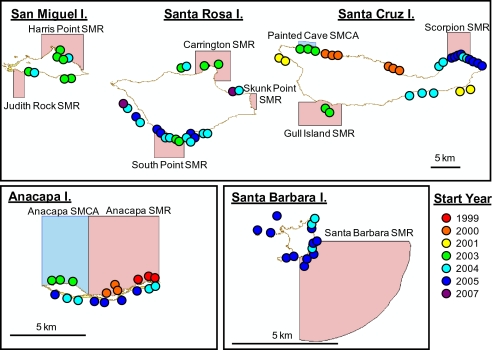
Fig. 1.
Map of the Channel Islands showing the locations of fish community surveys and the first year that fish surveys occurred. The MPA network is highlighted on the map (red, fully no-take State Marine Reserve; blue, limited-take State Marine Conservation Area). Note that some sites were not surveyed every year following the start year of sampling.
Over a relatively short geographic scale (approximately 100 km), the CINMS encompasses a number of biogeographic regions (see ref. 15 and its reference list), caused in part by strong environmental gradients in sea surface temperature (SST), productivity, wind stress, and wave exposure (21–23). The CINMS sits at the confluence of two major currents, with the cool, nutrient-rich California Current bathing the western islands from the north, whereas the warmer Southern California Countercurrent bathes the eastern islands from the south. Satellite-derived estimates of SST during the past 28 years show a strong gradient throughout the CINMS (Fig. 2A). In addition, a major biogeographic break occurs at Pt. Conception, influencing the abundance patterns of many species (24). When designing MPA networks to maximize biodiversity protection, biogeographic representation is often one of the top ecological criteria (16, 20). In the Channel Islands process, three main biogeographic regions emerged when the area was subdivided according to physical and biological differences using information available at the time (15). Ultimately, multiple reserves were placed in each of three bioregions: the Oregonian Province in the west, the Transition Zone, and the Californian Province in the east.
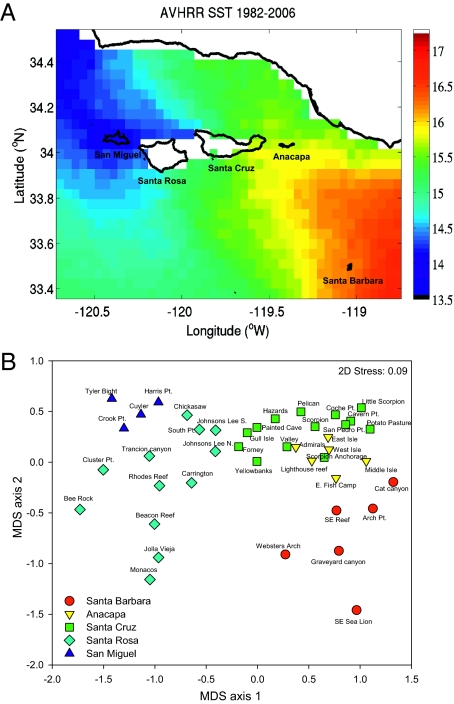
Fig. 2.
(A) Map of the Channel Islands showing long-term (1982–2006) average SST recorded by Advanced Very High Resolution Radiometer satellite. The sharp gradient in SST between the western and eastern islands is apparent. (B) Nonmetric MDS analysis depicting similarities in fish community structure among survey sites. Most sites group at the island scale, suggesting similarities among fish communities at this scale. MDS axis 1 is positively correlated with average SST at each site (r = 0.88; P < 0.0001).
Inherent variation in environmental factors, habitats, species abundances, and community composition provide a distinct challenge for evaluating the strength of biological responses across large MPA networks, such as the Channel Islands. If species vary greatly in abundance as a result of biogeography, these differences should be taken into account when assessing overall network responses to ensure that the detection of biological responses is a result of protection. Using biogeographic inferences may also help to identify appropriate bioregions and reserve and nonreserve reference sites for comparison. The framework we present here will provide guidance for evaluating MPA network performance in light of biogeographic effects in many systems. We illustrate this analytical approach for the Channel Islands marine reserve network after 5 years of protection.
Since 1999, we have conducted annual surveys of kelp forest ecosystems in the CINMS, and monitoring efforts were greatly expanded in 2003 to evaluate the effects of reserves (Fig. 1). Unfortunately, detailed fish community data were not available for the many reserve and reference locations before reserve establishment, which is likely to be the case for most networks, as reserve locations are seldom known far in advance. We first used surveys of kelp forest communities to identify geographic patterns of community structure (i.e., bioregions) and to define appropriate scales over which to group sites for reserve performance evaluations. We then examined the density and biomass response of common fish species inside and outside of reserves and asked whether species’ responses to protection differed based on target status or trophic group. We tested the hypotheses that targeted (i.e., fished) species would show positive responses across the reserve network compared with nontargeted species and that higher trophic level predators (e.g., piscivores and carnivores) would show stronger responses to protection because these species are commonly fished.
Results
A nonmetric multidimensional scaling (MDS) analysis of the 30 most common fish species indicated similarities in fish community structure by the relative distance between sites in 2-D space (Fig. 2B). In general, sites on each of the five islands grouped together and were more similar to each other than they were to sites on other islands (Tables S1 and S2). These groupings followed a pattern similar to that of SST (MDS axis 1 is strongly positively correlated with average SST; r = 0.88; P < 0.0001), with the largest differences in fish community structure occurring between the coolest (San Miguel) and the warmest (Santa Barbara) islands surveyed (Tables S1 and S2). Similar site groupings emerged in cluster and MDS analyses of invertebrate and macroalgal community structure measured from the same monitoring program. Both targeted and nontargeted fish species ranged from being three times to 250 times more abundant on particular islands (Table S3), and some species were absent on one or more islands. Both of these factors suggest the importance of controlling for biogeographic differences in MPA network evaluations.
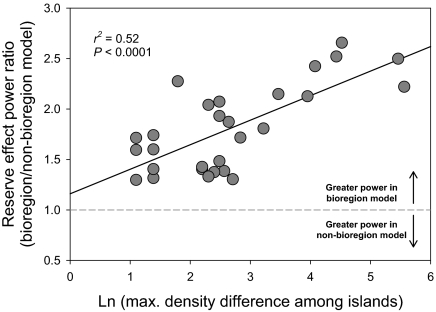
The statistical benefit of considering biogeography within the Channel Islands reserve network was evaluated by comparing statistical models including bioregions (i.e., island term) to ones where bioregions were ignored. For all of the response variables considered in this study (as detailed later), a statistical model incorporating this island-level source of variation proved to have greater statistical power, after adjusting the degrees of freedom in cross-model comparisons (Materials and Methods; Fig. 3). The improvement in power to detect reserve effects correlated positively with the magnitude to which a species varied in density across islands (r2 = 0.52; P < 0.0001; Fig. 3). We did not find evidence of systematic habitat differences between sites inside and outside reserves across the network (Fig. S1). Although we did detect differences in habitat among the islands, reserve and nonreserve sites did not differ in physical relief or substrate type, beyond nonreserves having higher cover of flat relief (Table S4).
Fig. 3.
Relationship between the reserve effect power ratio (power in bioregion model/power in nonbioregion model) and the maximum difference in a species’ density across the Channel Islands (values from Table S3). In all cases, the power to detect reserve effects is improved by controlling for biogeography (i.e., all points occur above the dashed line, indicating improved power in the bioregion model). Statistical power increases most for species that exhibit strong biogeographic differences in abundance.
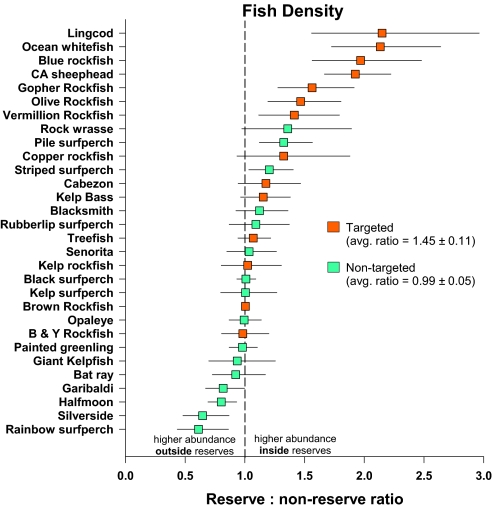
Biological responses were concordant with the predicted effects of reserves. Targeted fish species are more abundant inside reserves (average response ratio [ARR], 1.45 ± 0.11), and nontargeted fish species are equally abundant (ARR, 0.99 ± 0.05) inside and outside reserves (Fig. 4). In fact, 13 of 14 targeted species have ARRs >1 (Binomial test, P = 0.01), whereas only 8 of 16 nontargeted species have ARRs >1 (Binomial test, P > 0.05), indicating no significant pattern in the ARRs of nontargeted species. After controlling for biogeographic differences in densities, a number of targeted species were significantly more abundant inside reserves (e.g., lingcod, California sheephead, copper rockfish, cabezon, ocean whitefish, kelp bass; Table S5), whereas nontargeted species did not show differences based on reserve status. The biomass (a product of density and size) of individual targeted species showed an even stronger difference across the reserve network (ARR, 1.78 ± 0.22), whereas nontargeted species had equal biomass inside and outside (ARR, 0.98 ± 0.05; Table S6 and Fig. S2).
Fig. 4.
ARRs (density inside/density outside) for individual targeted and nontargeted species of fish in the Channel Islands reserve network. Values represent the back-transformed average of the log response ratios for each island. Error bars are ± SE.
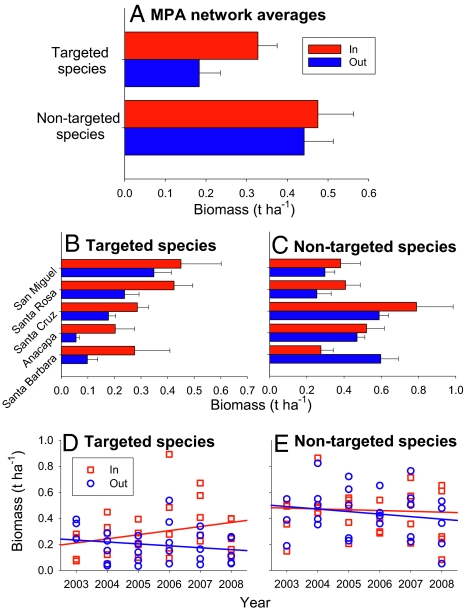
We assessed differences in the total biomass of targeted and nontargeted species inside and outside reserves (Fig. 5A). Targeted species biomass was approximately two times higher inside reserves than outside (by ANOVA, reserve F1,30 = 11.6, P = 0.0019; island F4,30 = 3.7, P = 0.015; reserve × island F4,30 = 0.16, P = 0.96). For nontargeted species, on average there was no difference in fish biomass inside versus outside reserves (by ANOVA, reserve F1,30 = 0.59, P = 0.45; island F4,30 = 3.4, P = 0.02; reserve × island F4,30 = 2.3, P = 0.08). These patterns also differed among the islands, with some islands showing stronger differences than other islands (Fig. 5 B and C). The biomass trajectories of targeted species inside and outside of reserves diverged through time, with targeted species increasing inside reserves relative to outside (by ANCOVA, reserve F1,53 = 4.95, P = 0.03; year F1,53 = 0.42, P = 0.51; reserve × year F1,53 = 3.65, P = 0.06; Fig. 5D). In contrast, nontargeted species did not show temporal trends either inside or outside of reserves (by ANCOVA, reserve F1,53 = 0.15, P = 0.69; year F1,53 = 0.54, P = 0.47; reserve × year F1,53 = 0.14, P = 0.71; Fig. 5E). Despite annual fluctuations in biomass among islands, ARRs of targeted species increased during the 5 years following reserve establishment, but showed no trend for nontargeted species (Fig. S3).
Fig. 5.
Spatial and temporal patterns of biomass for targeted and nontargeted species inside (red bars or squares) and outside (blue bars or circles) of reserves in the Channel Islands. (A) Averages across all islands in the reserve network, (B) targeted species biomass for each island, (C) nontargeted species biomass for each island, (D) time series of targeted species biomass, and (E) time series of nontargeted species biomass. Biomass is reported in metric tons per hectare. Error bars are ± SE.
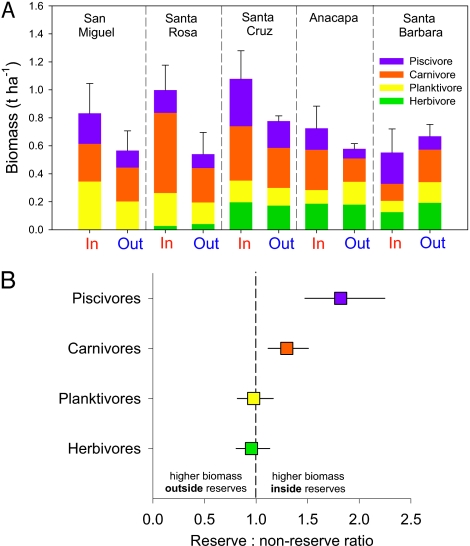
We also found that differences inside and outside reserves varied by trophic role across the islands (Fig. 6). On average, piscivores showed the largest difference, with 1.8 times higher biomass inside reserves (by ANOVA, reserve F1,30 = 6.3, P = 0.018; island F4,30 = 1.6, P = 0.20; reserve × island F4,30 = 0.13, P = 0.97; Fig. 6B). We recorded 1.3 times higher biomass of carnivores inside reserves, but also detected significant differences in carnivore biomass among islands and a significant reserve/island interaction (by ANOVA, reserve F1,30 = 5.0, P = 0.033; island F4,30 = 4.0, P = 0.01; reserve × island F4,30 = 3.2, P = 0.027). Planktivores showed no significant difference in biomass inside and outside reserves, but there were differences between islands (by ANOVA, reserve F1,30 = 0.53, P = 0.47; island F4,30 = 3.2, P = 0.026; reserve × island F4,30 = 1.6, P = 0.19). Herbivores showed equal biomass inside and outside of reserves (by ANOVA, reserve F1,30 = 0.8, P = 0.38; island F4,30 = 23.2, P < 0.001; reserve × island F4,30 = 1.4, P = 0.25). We also detected a strong difference among islands, likely driven by biogeographic factors that limit herbivory in the cool western islands.
Fig. 6.
(A) Biomass of different trophic groups of fishes at sites inside and outside of reserves on each island in the Channel Islands reserve network. (B) ARRs (biomass inside/biomass outside) for each trophic group across the Channel Islands reserve network. Response ratios are the back-transformed average of the log response ratios. Error bars are ± SE.
Discussion
Networks of relatively large MPAs are being implemented in many locations across the globe, but few networks have been evaluated as such. One important question is how to appropriately assess biological responses across the network, given potential biogeographic and habitat differences among MPAs and between MPAs and reference areas. We used biogeographic information to identify the scale at which sites should be compared, and this was roughly the island-scale in our system. The bioregions identified in our analyses differed slightly from the three bioregions identified during the design phase of the reserve network, which synthesized the available literature (15). In particular, sites on Santa Barbara Island did not group with sites on the south side of Santa Rosa and Santa Cruz Islands (i.e., the Transition Zone). Instead, sites on each island grouped most closely with other sites on that same island, although Anacapa Island overlapped with sites on the eastern end of Santa Cruz Island. Biogeographic variation in community structure occurred over a relatively small spatial scale (approximately 100 km) in the Channel Islands, was strongly correlated with SST (similar to ref. 25 for intertidal algae), and likely influenced by other environmental gradients (21–23). However, the critical spatial scales of community similarity will likely vary from network to network. The utility and importance of considering biogeographic structure in analyses of reserve effects was demonstrated in this study by an increase in statistical power to detect reserve effects when sites were grouped by bioregion (i.e., island). Importantly, the increase in power was most pronounced for precisely those species that showed strong biogeographic differences in abundance (Fig. 3). We recommend that researchers consider these biogeographic effects in future MPA network evaluations, especially as large-scale MPA networks become established more commonly (e.g., ref. 26).
After 5 years of protection, we recorded significantly greater densities and biomass of targeted fish species inside reserves compared with outside, but did not find differences among nontargeted species. Network-wide performance was assessed with meta-analysis techniques (i.e., ARRs) that translated density and biomass differences into the standard currency of effect sizes while controlling for biogeographic variation. Despite the lack of detailed “before” data across the network, these differences were not likely pre-existing or an artifact of reserve placement, as habitat did not systematically differ between reserve and nonreserve sites, and because only the biomass of targeted fish species increased over time. If sites with better habitat quality were originally sited inside reserves, we would expect both targeted and nontargeted species to show higher biomass inside reserves, which was not the case here. In addition, synthetic analyses of reserve responses across the globe have commonly reported that before/after effects are comparable to inside/outside differences (7, 9). The lack of before data are likely to be the norm for many MPA networks because reserve boundaries are often not known far in advance of reserve establishment.
The overall biological responses after 5 years are likely explained by a reduction in fishing mortality inside reserves and potentially by a redistribution of effort outside reserves (both effects may explain the rapid increase in biomass of targeted species after the first year of protection; Fig. S3). Qualitatively similar patterns in density, size, biomass, and predicted reproductive output were reported for many of these same species in a number of small southern California reserves that have been protected for 17 to 35 years (27). These responses by targeted and nontargeted species fit expectations and suggest that the reserve network is effective in meeting biodiversity protection goals (i.e., rebuilding the abundances of targeted species). It remains to be seen whether the Channel Islands reserve network enhances fisheries through spillover and larval subsidy effects. However, the increased biomass and associated increase in reproductive output of targeted species inside reserves should supplement fishery production outside, although this may be difficult to detect in practice (13). In addition, modeling and empirical results from other southern California reserves suggest spillover is likely to occur (28).
Piscivorous and carnivorous functional groups accounted for the largest differences in fish biomass between reserve and nonreserve areas across the network (1.8 and 1.3 times more biomass inside, respectively). Many of the species in these groups are targeted by fishing and would be expected to respond most rapidly to reserve protection, as has been reported across a collection of Mediterranean (8) and Hawaiian (29) MPAs. Piscivores and carnivores both can play critical roles in structuring temperate kelp forest ecosystems. For example, fished species such as California sheephead and spiny lobsters are important consumers of sea urchins (30, 31) and help to prevent the transition of productive kelp beds into unproductive urchin barrens (32). Indeed, higher urchin predator abundance in the Anacapa Island Ecological Reserve (established in 1978) has maintained a persistent kelp forest state, in contrast to nonreserve sites (33). Kelp forest ecosystems support more diverse communities, complex food webs, and abundant populations of fished species than urchin barrens (34). Top-down ecosystem-level changes in temperate systems in response to reserve protection may take decades to cascade through the food web, as predators disproportionately benefit from a reduction in fishing pressure (35). As the biomass of key predators increases further inside reserves in the Channel Islands, we expect continued changes to the ecosystems they support, similar to other temperate reserves (8, 27, 35–37). Assessing the aggregate responses of different functional groups to MPAs may provide managers with tools to forecast ecosystem-level impacts.
Here we present one of the first evaluations of the performance of a large-scale network of marine reserves. After 5 years, response ratio and biomass trajectories indicate targeted species are increasing in biomass inside reserves relative to outside, with no differences in nontargeted species. Despite matching our a priori predictions, the probability associated with this interaction between target status and survey year was not quite significant, likely because of high site-level variance in biomass, which may be attributed in part to annual monitoring and associated sampling error (e.g., variation in visibility, current, and swell). Given sampling constraints, this result suggests a tradeoff between spatial coverage and sampling frequency that may affect the time required to conclusively attribute observed differences in biological responses to a reserve effect. On the Great Barrier Reef, Russ et al. (38) reported a similarly rapid increase in the abundance of fished species across the world's largest MPA network, which spans more than 1,000 km of coastline and was implemented in 2004. Many other regional studies have analyzed the biological responses of collections of reserves and reported similar increases in abundance and biomass of fished taxa (e.g., refs. 8, 27, 29, 37). However, not all species showed positive responses to reserve protection in the Channel Islands, and these tended to be nontargeted species. The causes of these patterns require further study, but may reflect species interactions such as decreases in prey following increases in their predators inside reserves (39). Interestingly, we detected spatial variation in the strength of the biological responses of reserves across the different island groups and responses of targeted species were greatest in the eastern (i.e., warmer water) islands (Fig. 5B). As other recent studies have shown, marine reserve responses may be context dependent and spatially variable as a result of differences in physical and environmental factors, reserve size, the degree and time of reserve protection, and the intensity of fishing pressure outside (37, 39–41).
Is a network simply a collection of MPA replicates or does it function as more than the sum of its parts? The concept of a network directly implies connectivity among MPAs and areas open to harvest (20). However, given natural recruitment variability, it may be extremely difficult to demonstrate larval export and connectivity empirically (13; but see ref. 42), especially within the confines of standard MPA monitoring plans. However, research is progressing to use monitoring data (e.g., population density, size structure, and estimates of larval production) to parameterize spatially explicit multispecies MPA models, which are in turn coupled with data on fishing intensity and oceanographic models of larval connectivity patterns. These models may then be used to generate null expectations of changes in abundance, biomass, and community structure that can be compared with monitoring time series. Linking monitoring data with models will be a critical step in establishing expectations of biological responses in individuals MPAs and across whole networks (e.g., ref. 43). With monitoring data, we can assess the overall performance of MPA networks as a set of replicate reserves. However, “typical” ecological monitoring programs generally do not allow us to directly measure network connectivity and larval subsidy effects over these large scales. The development of new technologies and tools in ocean circulation modeling and larval tracking will help to close that gap (e.g., refs. 42, 44). EBM and adaptive management require an understanding of which MPAs in a network are outperforming others and how differences in design criteria (e.g., MPA size, spacing, and allowed human activities) and potential drivers (e.g., biogeography, productivity, fishing pressure outside) correspond to observed differences in ecological and socioeconomic responses. Knowledge of these factors will help improve the design of MPA networks in the future.
Materials and Methods
Community Survey Methodology.
The Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO) has conducted community monitoring in nearshore kelp beds and rocky reefs in the CINMS since 1999 and sites have been steadily added through time (www.piscoweb.org; Fig. 1). At each site, PISCO conducts eight to 12 fish transects that are 30 × 2 × 2 m at multiple levels in the water column: benthic, midwater, and canopy (when present). Transects are laid out in a stratified random design, with multiple nonpermanent transects located in fixed strata (i.e., outer, middle, and inner edges of the reef). At each level in the water column, one diver per transect counts and sizes all fish to the nearest centimeter (total length), excluding small cryptic fishes (e.g., gobies). In addition, PISCO conducts four to six benthic transects at each site to characterize community structure of invertebrates and macroalgae along 30 × 2 m swaths and percent cover, substrate type, and physical relief using uniform point contact at 30 points along each transect line. Transects are conducted on SCUBA in depths shallower than 20 m once per year at each site.
We used published length/weight relationships for each species or closest congener (ref. 45; see also www.fishbase.org) to estimate the biomass of every individual. We used published diet studies (45, 46) and personal observations to assign each species to various functional groups (herbivores, planktivores, carnivores, and piscivores). Some species were assigned to different functional groups at different sizes to reflect ontogenetic changes in diet. Species were also categorized as being generally targeted or nontargeted by commercial and/or recreational fishing activities, by examining California Department of Fish and Game landings records from areas in the CINMS and consulting with fisheries managers and fishermen. Using the count or biomass data for each fish species or group, summed over the different levels, we calculated the average density or biomass per site per year. These estimates represented the lowest level of replication for further analyses.
Biogeographic Considerations in MPA Network Analyses.
Because of the historic placement of PISCO survey sites throughout the islands before reserve designation, the large sizes of reserves (Table S7), and variation in habitat over those scales, many sites outside reserves were not designed as references for particular sites inside reserves. More importantly, as a result of biogeographic factors, some species are abundant at particular islands and are rare or absent at other islands (Table S3). Therefore, we used biogeographic inferences to identify bioregions and the scales at which sites should be compared. Using the years in which PISCO surveyed the full complement of sites (2005–2008; see Fig. 1), we calculated average abundances of the 30 most common species. After creating a Bray-Curtis similarity index from fourth root–transformed data, we performed a MDS analysis in PRIMER 6.0 to visualize similarities and differences in fish community structure. As islands were identified in the MDS analysis as appropriate biogeographic units to use in further analyses, we tested for significant differences in community structure among islands (i.e., analysis of similarity) and the success of jackknifed reclassification of sites to their island of origin (i.e., canonical analysis of principal coordinates) using PRIMER 6.0 (Tables S1 and S2).
To evaluate whether statistical power to detect differences inside and outside of reserves would be improved by grouping sites according to these predetermined biogeographic regions, we compared an ANOVA model that included a bioregion (i.e., island) term to a model that did not. The bioregion model tested the terms “island,” “reserve,” “year,” and all interactions, whereas the nonbioregion model tested only “reserve,” “year,” and the “reserve × year” interaction. Degrees of freedom for the error term are automatically reduced in the bioregion model by the addition of the island term and its interactions, so improved statistical power occurs in cross-model comparisons when the error term is small enough to offset this loss; in other words, when the following inequality is true:
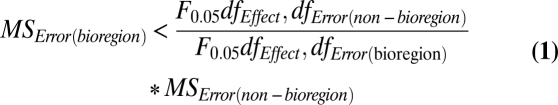 |
The relative power of the two models to detect both reserve and reserve × year effects was compared using each of the response variables examined in this study, and the bioregion model was found to have greater power in all instances (Fig. 3).
Reserve Responses.
After identifying islands as the appropriate grouping scale, we examined the response of fish species to reserve establishment by comparing the average response of all sites inside reserves to the average response of all sites outside reserves on each island in a given year. We relied primarily on inside versus outside comparisons because of a lack of sufficient “before” data. We calculated log response ratios (inside/outside) as the log of the average response (i.e., density or biomass) at all sites inside reserves to the average response of all sites outside reserves for each island in each year, for the years 2005 through 2008 (47). To examine general responses across the collective network, we took the averages of the log response ratios across each island and calculated SEs in those ARRs. The log response ratios and limits of the errors were back-transformed for presentation purposes (i.e., ARR). Species with ARRs >1 are more abundant inside reserves, whereas species with ARRs <1 are more abundant outside reserves. To assess the statistical significance of individual species responses to reserves, we used the mean island-year density or biomass and conducted two-way ANOVAs with the factors of reserve, island, and reserve × island.
We compared the total fish biomass of all targeted and nontargeted species and conducted a two-way ANOVA with the factors of reserve, island, and reserve × island. While acknowledging that sampling was more limited in 2003 and 2004 (the first years following reserve designation), we used the available data in each year to examine temporal trajectories of changes in biomass of targeted and nontargeted species across the reserve and nonreserve sites using ANCOVA with the factors of reserve, year, and reserve × year. We also conducted an ANCOVA on the ARR trajectories for targeted and nontargeted species with the factors of fish group, year, and fish group × year. For trophic group analyses, we summed the biomass for each trophic group at each site and compared ARRs and biomass responses of the different trophic groups as mentioned earlier. All analyses were conducted in JMP 7.0.
Supplementary Material
Acknowledgments
The authors thank the Channel Islands MPA working group for helpful insight and suggestions. B. Broitman created the SST map for Fig. 2 and P. Raimondi advised on statistical analysis. We thank the legions of divers who conducted the fieldwork over the years, but in particular, M. Sheehy, C. Svedlund, M. Kay, M. Readdie, J. Patterson, A. Parsons-Field, and K. Davis. Funding to S. Hamilton and D. Malone was provided by the Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO) and the Commonweal Ocean Policy Program. Funding and support for fieldwork was provided by the National Park Service, the Ocean Protection Council, the Channel Islands National Marine Sanctuary, the California Department of Fish and Game, and PISCO, which is supported by the Gordon and Betty Moore Foundation and the David and Lucile Packard Foundation. This is PISCO contribution number 351.
Footnotes
This article is a PNAS Direct Submission. S.D.G. is a guest editor invited by the Editorial Board.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908091107/DCSupplemental.
References
- 1.Rosenberg AA, McLeod KL. Implementing ecosystem-based approaches to management for the conservation of ecosystem services. Mar Ecol Prog Ser. 2005;300:270–274. [Google Scholar]
- 2.Crowder LB, et al. The impacts of fisheries on marine ecosystems and the transition to ecosystem-based management. Annu Rev Ecol Evol Syst. 2008;39:259–278. [Google Scholar]
- 3.McLeod KL, Leslie H. Managing for Resilience: New Directions in Marine Ecosystem-Based Management. Washington, DC: Island Press; 2009. [Google Scholar]
- 4.Lubchenco J, Palumbi SR, Gaines SD, Andelman S. Plugging a hole in the ocean: the emerging science of marine reserves. Ecol Appl. 2003;13:S3–S7. [Google Scholar]
- 5.Roberts CM, Hawkins JP, Gell FR. The role of marine reserves in achieving sustainable fisheries. Phil Trans R Soc B. 2005;360:123–132. doi: 10.1098/rstb.2004.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruckelshaus M, Klinger T, Knowlton N, DeMaster DP. Marine ecosystem-based management in practice: Scientific and governance challenges. Bioscience. 2008;58:53–63. [Google Scholar]
- 7.Halpern BS. The impact of marine reserves: do reserves work and does reserve size matter. Ecol Appl. 2003;13(suppl):S117–S137. [Google Scholar]
- 8.Guidetti P, Sala E. Community-wide effects of marine reserves in the Mediterranean Sea. Mar Ecol Prog Ser. 2007;335:43–56. [Google Scholar]
- 9.Lester SE, et al. Biological effects within no-take marine reserves: a global synthesis. Mar Ecol Prog Ser. 2009;384:33–46. [Google Scholar]
- 10.Roberts CM, Bohnsack JA, Gell F, Hawkins JP, Goodridge R. Effects of marine reserve on adjacent fisheries. Science. 2001;294:1920–1923. doi: 10.1126/science.294.5548.1920. [DOI] [PubMed] [Google Scholar]
- 11.Russ GR, Alcala AC, Maypa AP, Calumpong HP, White AT. Marine reserve benefits local fisheries. Ecol Appl. 2004;14:597–606. [Google Scholar]
- 12.Kaiser MJ, Blyth-Skyrme RE, Hart PJB, Edwards-Jones G, Palmer D. Evidence for greater reproductive output per unit area in areas protected from fishing. Can J Fish Aquat Sci. 2007;64:1284–1289. [Google Scholar]
- 13.Pelc RA, Warner RR, Gaines SD, Paris CB. Detecting larval export from marine reserves. Proc Natl Acad Sci USA. 2010;107:18266–18271. doi: 10.1073/pnas.0907368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood LJ, Fish L, Laughren J, Pauly D. Assessing progress towards global marine protection targets: shortfalls in information and action. Oryx. 2008;42:340–351. [Google Scholar]
- 15.Airamé S, et al. Applying ecological criteria to marine reserve design: a case study from the California Channel Islands. Ecol Appl. 2003;13:S170–S184. [Google Scholar]
- 16.Roberts CM, et al. Application of ecological criteria in selecting marine reserves and developing reserve networks. Ecol Appl. 2003;13:S215–S228. [Google Scholar]
- 17.Botsford LW, Micheli F, Hastings A. Principles for the design of marine reserves. Ecol Appl. 2003;13:S25–S31. [Google Scholar]
- 18.Gell F, Roberts CM. Benefits beyond boundaries: the fishery effects of marine reserves. Trends Ecol Evol. 2003;18:448–455. [Google Scholar]
- 19.Shanks AL, Grantham BA, Carr MH. Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl. 2003;13:S159–S169. [Google Scholar]
- 20.Gaines SD, et al. Designing marine reserve networks for both conservation and fisheries management. Proc Natl Acad Sci USA. 2010;107:18286–18293. doi: 10.1073/pnas.0906473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harms S, Winant CD. Characteristic patterns of circulation in the Santa Barbara Channel. J Geophys Res. 1998;103:3041–3065. [Google Scholar]
- 22.Dever EP. Objective maps of near-surface flow states near Point Conception, California. J Phys Oceanogr. 2004;34:441–461. [Google Scholar]
- 23.Blanchette CA, Broitman BR, Gaines SD. Intertidal community structure and oceanographic patterns around Santa Cruz Island, CA, USA. Mar Biol. 2006;149:689–701. [Google Scholar]
- 24.Horn MH, Allen LG. A distributional analysis of California coastal marine fishes. J Biogeogr. 1978;5:23–42. [Google Scholar]
- 25.Murray SN, Littler MM. Biogeographical analysis of intertidal macrophyte floras of southern California. J Biogeogr. 1981;8:339–351. [Google Scholar]
- 26.McCook L, et al. Adaptive management and monitoring of the great barrier reef reserve network: a globally significant case study in marine conservation. Proc Natl Acad Sci USA. 2010;107:18278–18285. doi: 10.1073/pnas.0909335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetreault I, Ambrose RF. Temperate marine reserves enhance targeted but not untargeted fishes in multiple no-take MPAs. Ecol Appl. 2007;17:2251–2267. doi: 10.1890/06-0161.1. [DOI] [PubMed] [Google Scholar]
- 28.Kellner JB, Tetrault I, Gaines SD, Nisbet RM. Fishing the line near marine reserves in single and multispecies fisheries. Ecol Appl. 2007;17:1039–1054. doi: 10.1890/05-1845. [DOI] [PubMed] [Google Scholar]
- 29.Friedlander AM, Brown EK, Monaco ME. Coupling ecology and GIS to evaluate efficacy of marine protected areas in Hawaii. Ecol Appl. 2007;17:715–730. doi: 10.1890/06-0536. [DOI] [PubMed] [Google Scholar]
- 30.Cowen RK. The effect of sheephead (Semicossyphus pulcher) predation on red sea urchin (Stronglycentrotus franciscanus) populations: an experimental analysis. Oecologia. 1983;58:249–255. doi: 10.1007/BF00399225. [DOI] [PubMed] [Google Scholar]
- 31.Tegner MJ, Levin LA. Spiny lobsters and sea urchins: analysis of a predator-prey interaction. J Exp Mar Biol Ecol. 1983;73:125–150. [Google Scholar]
- 32.Tegner MJ, Dayton PK. Ecosystem effects of fishing in kelp forest communities. ICES J Mar Sci. 2000;57:579–589. [Google Scholar]
- 33.Behrens MD, Laffert KD. Effects of marine reserves and urchin disease on southern Californian rocky reef communities. Mar Ecol Prog Ser. 2004;279:129–139. [Google Scholar]
- 34.Graham MH. Effects of local deforestation on the diversity and structure of southern California giant kelp forest food webs. Ecosystems (N Y. Print) 2004;7:341–357. [Google Scholar]
- 35.Shears NT, Babcock RC. Continuing trophic cascade effects after 25 years of no-take marine reserve protection. Mar Ecol Prog Ser. 2003;246:1–16. [Google Scholar]
- 36.Sala E, Zabala M. Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Mar Ecol Prog Ser. 1996;140:71–81. [Google Scholar]
- 37.Barrett NS, Edgar GJ, Buxton CD, Haddon M. Changes in fish assemblages following 10 years of protection in Tasmanian marine protected areas. J Exp Mar Biol Ecol. 2007;345:141–157. [Google Scholar]
- 38.Russ GR, et al. Rapid increase in fish numbers follows creation of world's largest marine reserve network. Curr Biol. 2008;18:515–543. doi: 10.1016/j.cub.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Micheli F, Halpern BS, Botsford LW, Warner RR. Trajectories and correlates of community change in no-take marine reserves. Ecol Appl. 2004;14:1709–1723. [Google Scholar]
- 40.Shears NT, Babcock RC, Salomon AK. Context-dependent effects of fishing: variation in trophic cascades across environmental gradients. Ecol Appl. 2008;18:1860–1873. doi: 10.1890/07-1776.1. [DOI] [PubMed] [Google Scholar]
- 41.Claudet J, et al. Marine reserves: size and age do matter. Ecol Lett. 2008;11:481–489. doi: 10.1111/j.1461-0248.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 42.Planes S, Jones GP, Thorrold SR. Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci USA. 2009;106:5693–5697. doi: 10.1073/pnas.0808007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Botsford LW, et al. Connectivity and resilience of coral reef metapopulations in marine protected areas: matching empirical efforts to predictive needs. Coral Reefs. 2009;28:327–337. doi: 10.1007/s00338-009-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowen RK, Paris CB, Srinivasan A. Scaling of connectivity in marine populations. Science. 2006;311:522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- 45.Cailliet GM, et al. Biological characteristics of nearshore fishes of California: a review of existing knowledge and proposed additional studies. Sacramento: California Dept of Fish and Game; 2000. [Google Scholar]
- 46.Love MS. Probably more than you want to know about the fishes of the Pacific Coast. Really Big Press, Santa Barbara; 1996. [Google Scholar]
- 47.Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.