Abstract
Canine marrow cells were incubated with transferrin-bound 59Fe, and the partition of cellular iron was studied by chromatographic and gel filtration methods. Splitting-off of iron from the stromal fraction was avoided by lysing the cells in Tris HCl buffer at pH 8.6. Cellular iron was divided into four major compartments: stroma, microsomes, main hemoglobin, and fraction I. The iron in fraction I was found in ferritin, heme proteins, and low molecular weight iron.
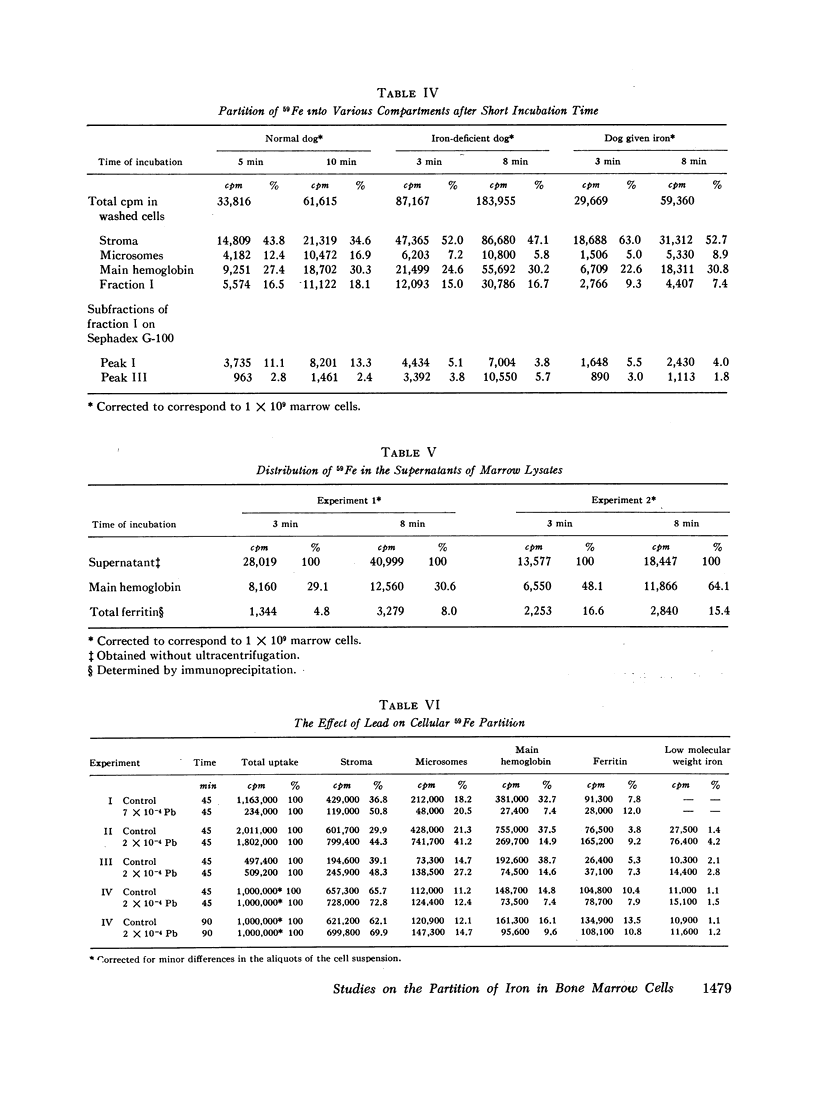
With incubation times of 3-10 min, 59Fe appeared promptly in the main hemoglobin. The entry of 59Fe into ferritin paralleled that of hemoglobin but was smaller in amount. When the marrow cells were incubated with 59Fe for 15-20 min and reincubated without radioactive iron, movement of 59Fe into main hemoglobin was observed, and essentially all this iron came from the particulate fraction (stroma, mitochondria, and microsomes). In these chase experiments there was no change in the total quantity of 59Fe in ferritin. There was no evidence of a significant hemoglobin precursor other than low molecular weight iron.
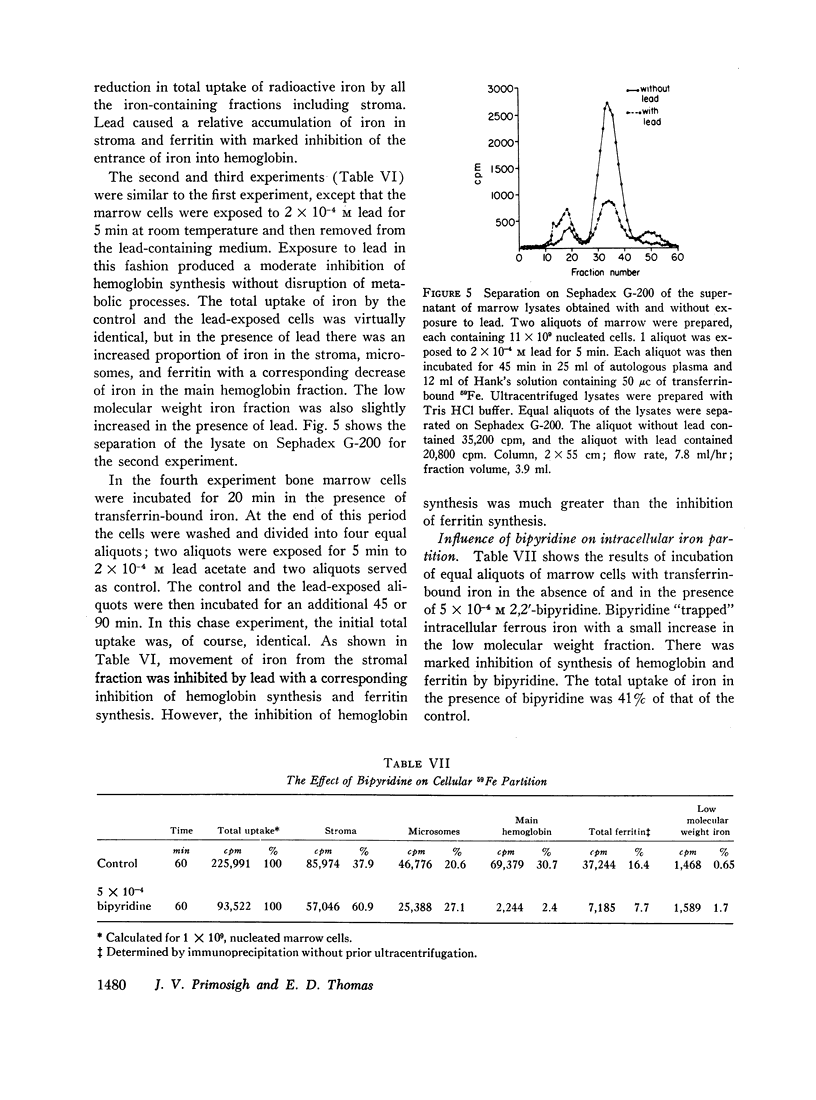
Depending upon concentration, lead was observed to inhibit cellular iron metabolism at several points: uptake of iron by the cell, movement of iron from stroma to the soluble intracellular compartment, and synthesis of hemoglobin. The most pronounced inhibitory effect of lead was always on hemoglobin synthesis with an increase in ferritin: hemoglobin ratio. Bipyridine appeared to trap intracellular ferrous iron and to inhibit synthesis of both hemoglobin and ferritin.
It was concluded that iron moves from the stroma into the soluble intracellular compartment as low molecular weight iron, probably as a complex of ferrous iron with low molecular weight components of the cytoplasm, that serves as the source of iron for both hemoglobin and ferritin synthesis.
Full text
PDF

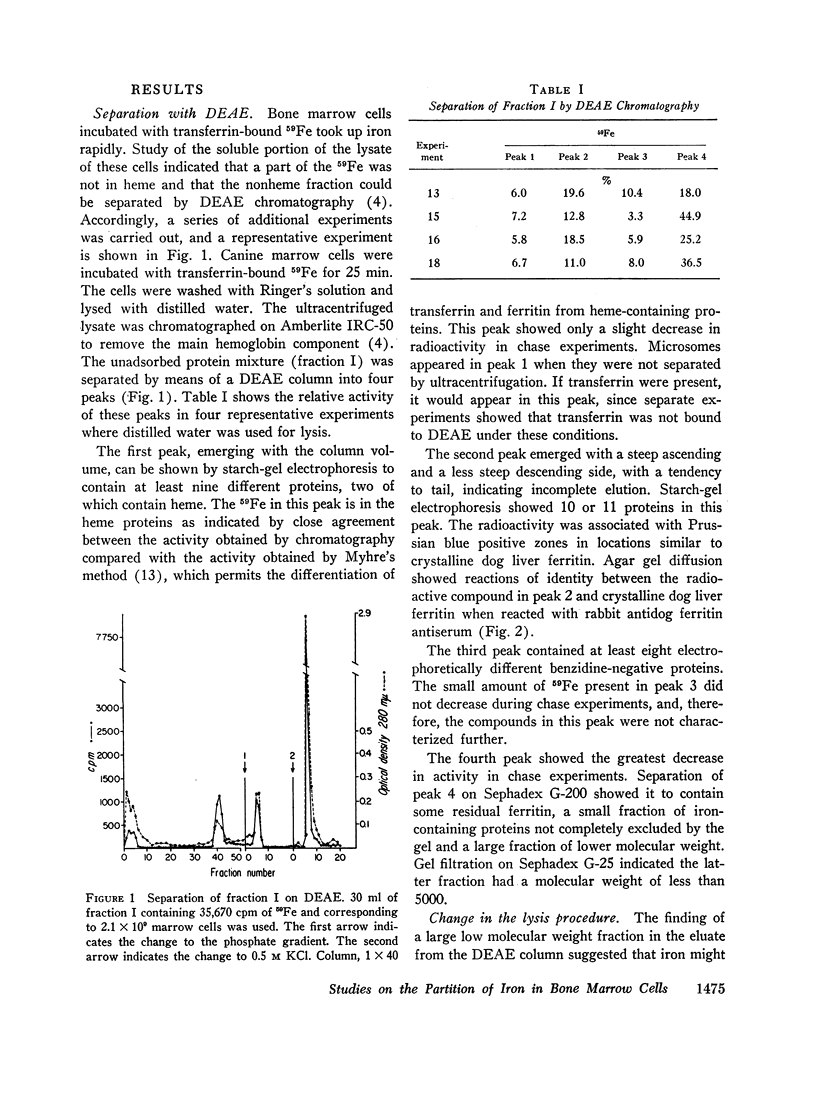


Images in this article
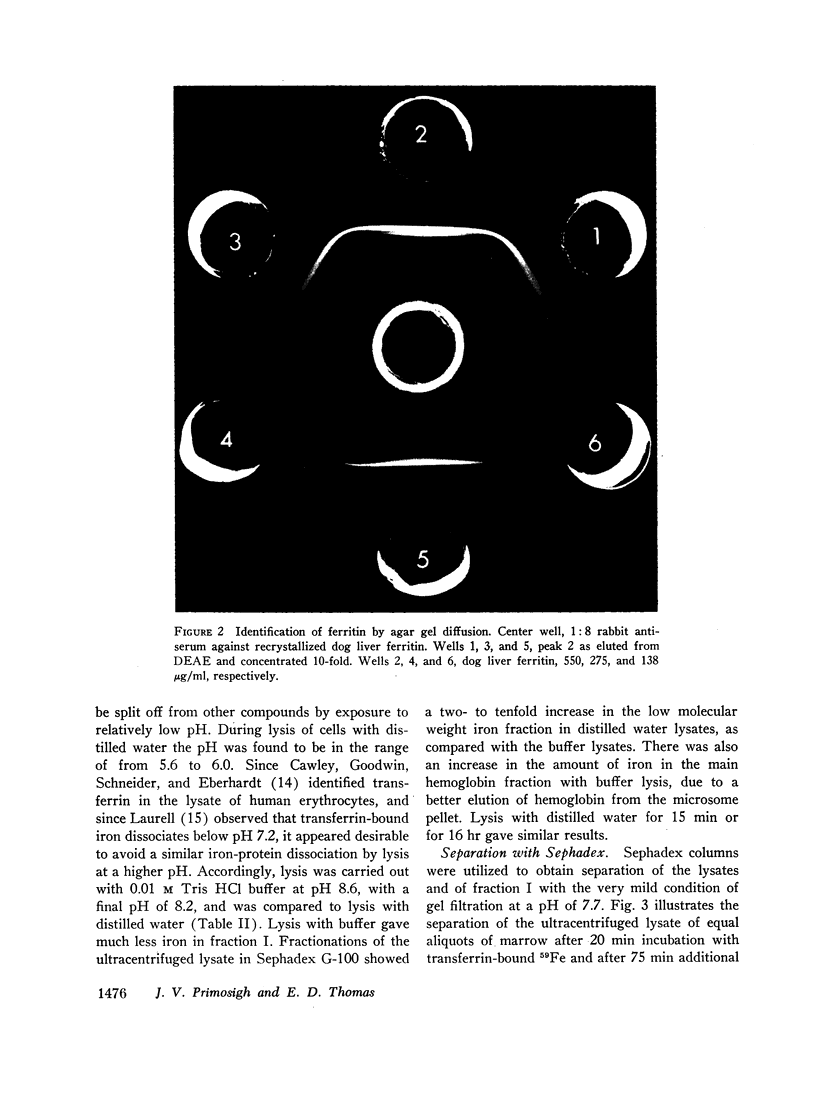
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., JANDL J. H. Kinetics of intracellular iron in rabbit reticulocytes. Blood. 1960 Jan;15:71–81. [PubMed] [Google Scholar]
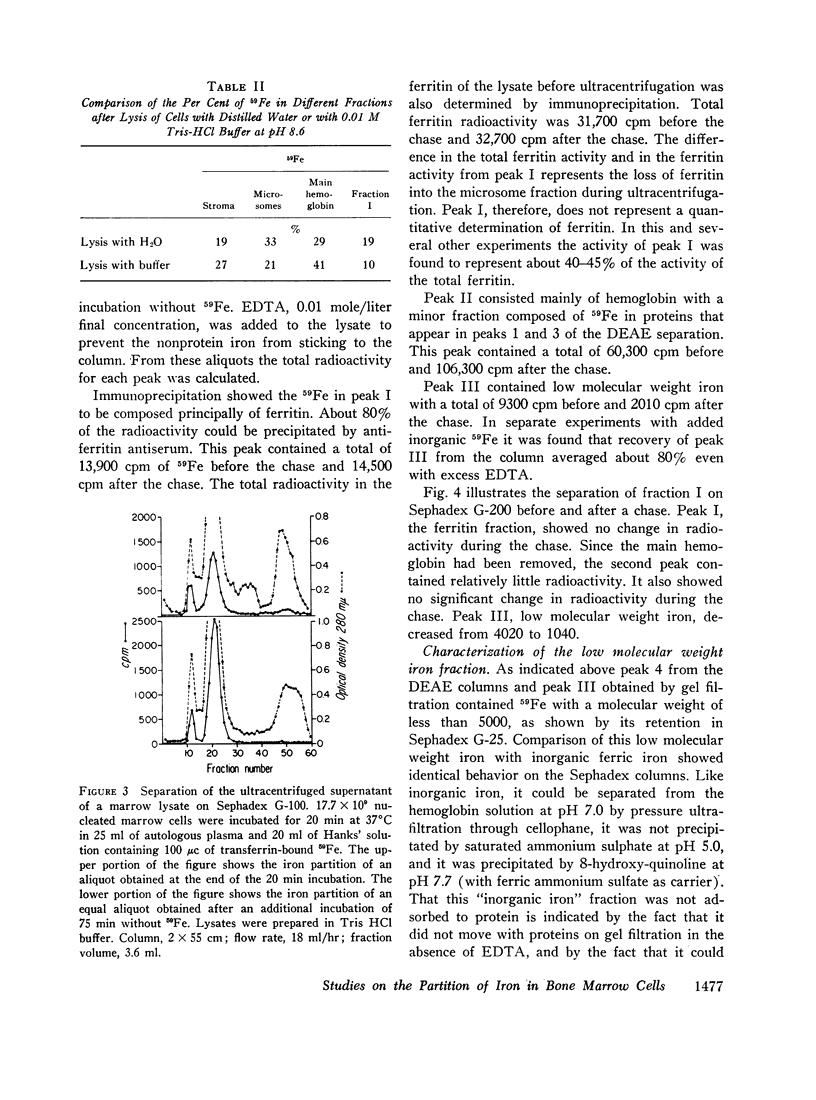
- BESSIS M. C., BRETON-GORIUS J. Iron particles in normal erythroblasts and normal and pathological erythrocytes. J Biophys Biochem Cytol. 1957 May 25;3(3):503–504. doi: 10.1083/jcb.3.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABER M., FALBE-HANSEN I. Nonhaem iron in erythrocytes as a precursor for haemoglobin. Nature. 1959 Oct 3;184:1043–1044. doi: 10.1038/1841043a0. [DOI] [PubMed] [Google Scholar]
- FALBE-HANSEN I., LOTHE K. In vivo incorporation of 59 Fe into nonhem iron and hemoglobin of red blood cells. Acta Physiol Scand. 1962 Feb;54:97–104. doi: 10.1111/j.1748-1716.1962.tb02333.x. [DOI] [PubMed] [Google Scholar]
- GRANICK S. Iron metabolism. Bull N Y Acad Med. 1954 Feb;30(2):81–105. [PMC free article] [PubMed] [Google Scholar]
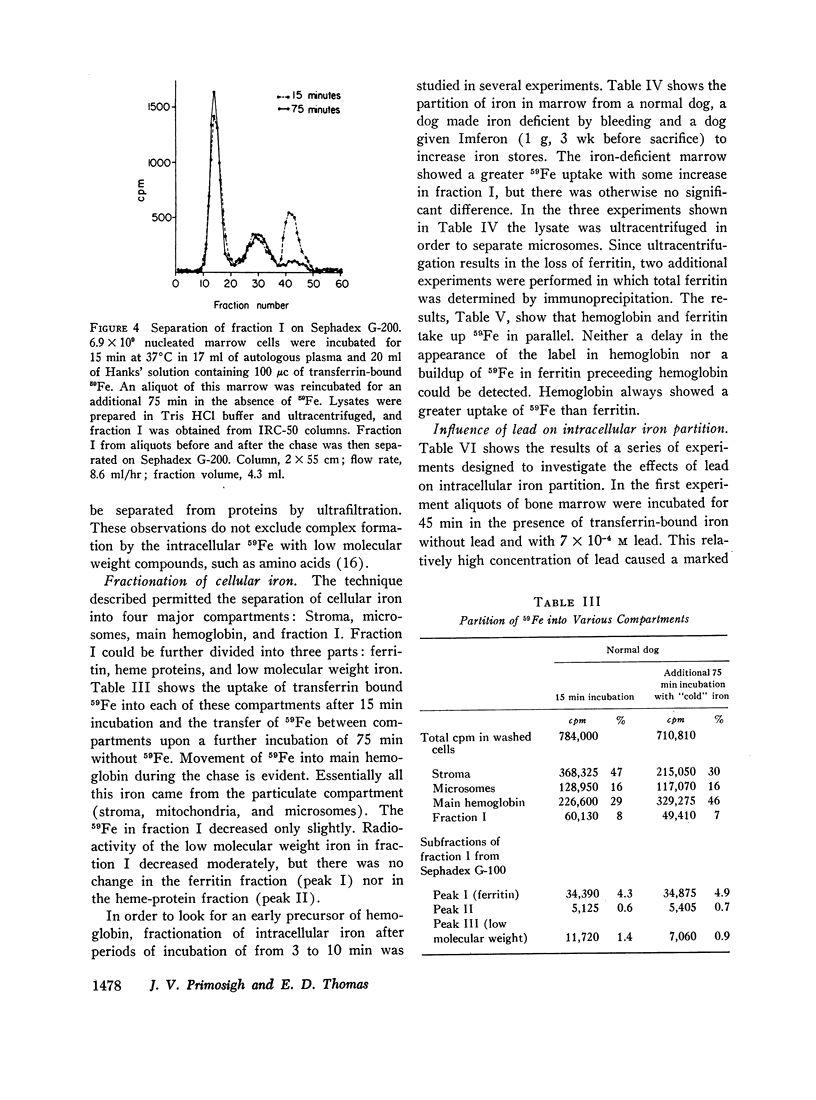
- GREENOUGH W. B., 3rd, PETERS T., Jr, THOMAS E. D. An intracellular protein intermediate for hemoglobin formation. J Clin Invest. 1962 May;41:1116–1124. doi: 10.1172/JCI104563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabuzda T. G., Gardner F. H. Observations on Fe59 labeled bone marrow ferritin. Blood. 1967 May;29(5):770–779. [PubMed] [Google Scholar]
- JANDL J. H., INMAN J. K., SIMMONS R. L., ALLEN D. W. Transfer of iron from serum iron-binding protein to human reticulocytes. J Clin Invest. 1959 Jan 1;38(1 Pt 1):161–185. doi: 10.1172/JCI103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZUR A., CARLETON A. Relation of ferritin iron to heme synthesis in marrow and reticulocytes. J Biol Chem. 1963 May;238:1817–1824. [PubMed] [Google Scholar]
- MYHRE E. IRON UPTAKE AND HEMOGLOBIN SYNTHESIS BY HUMAN ERYTHROID CELLS IN VITRO. Scand J Clin Lab Invest. 1964;16:212–221. [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- Rabinovitz M., Waxman H. S. Dependence of polyribosome structure in reticulocytes on iron; implication on the tape theory of haemoglobin synthesis. Nature. 1965 May 29;206(987):897–900. doi: 10.1038/206897a0. [DOI] [PubMed] [Google Scholar]
- Walsh R. J., Thomas E. D., Chow S. K., Fluharty R. G., Finch C. A. Iron Metabolism. Heme Synthesis in Vitro by Immature Erythrocytes. Science. 1949 Oct 14;110(2859):396–398. doi: 10.1126/science.110.2859.396. [DOI] [PubMed] [Google Scholar]
- YAKULIS V. J., HELLER P. Rapid slide technic for double diffusion agar precipitin test. Am J Clin Pathol. 1959 Apr;31(4):323–325. doi: 10.1093/ajcp/31.4.323. [DOI] [PubMed] [Google Scholar]
- ZAIL S. S., CHARLTON R. W., TORRANCE J. D., BOTHWELL T. H. STUDIES ON THE FORMATION OF FERRITIN IN RED CELL PRECURSORS. J Clin Invest. 1964 Apr;43:670–680. doi: 10.1172/JCI104952. [DOI] [PMC free article] [PubMed] [Google Scholar]



