Abstract
Enzymic properties have been compared in the following five genetic variants of glucose-6-phosphate dehydrogenase from human erythrocytes: the two common variants with normal activity, A and B; the common variant associated with enzyme deficiency, A-; and two new rare variants, “Ijebu-Ode” and “Ita-Bale.”
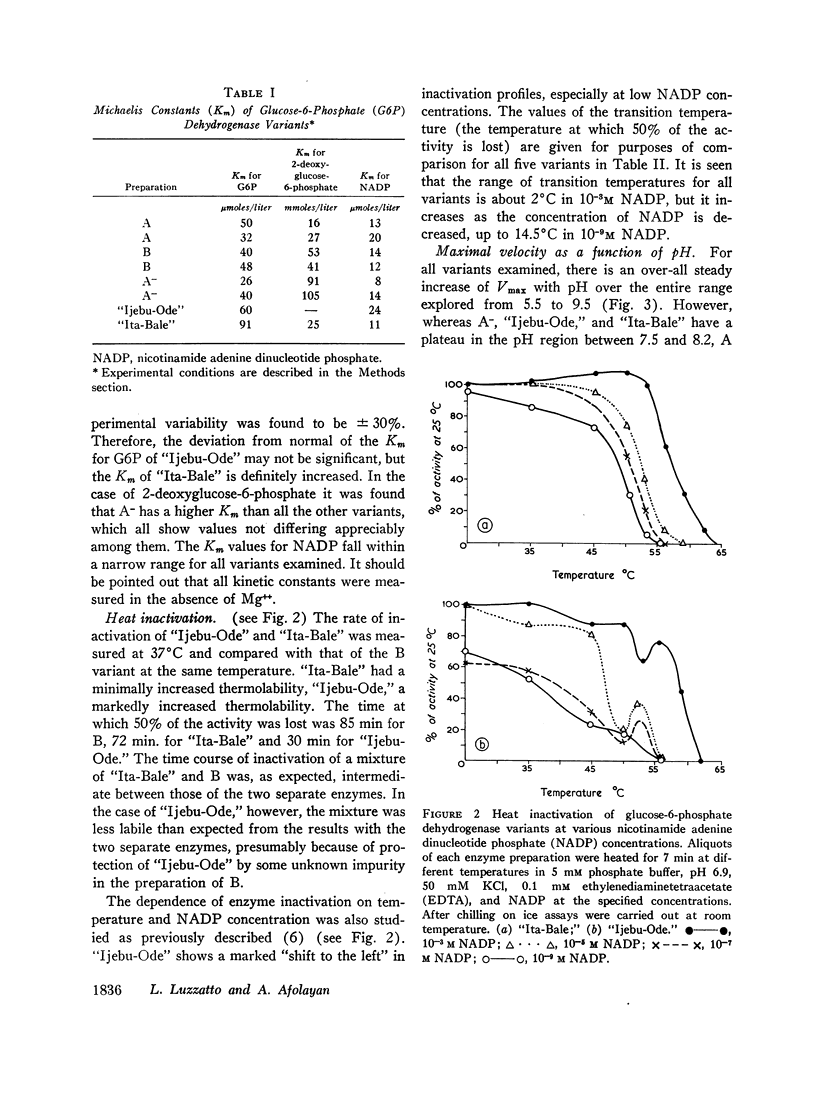
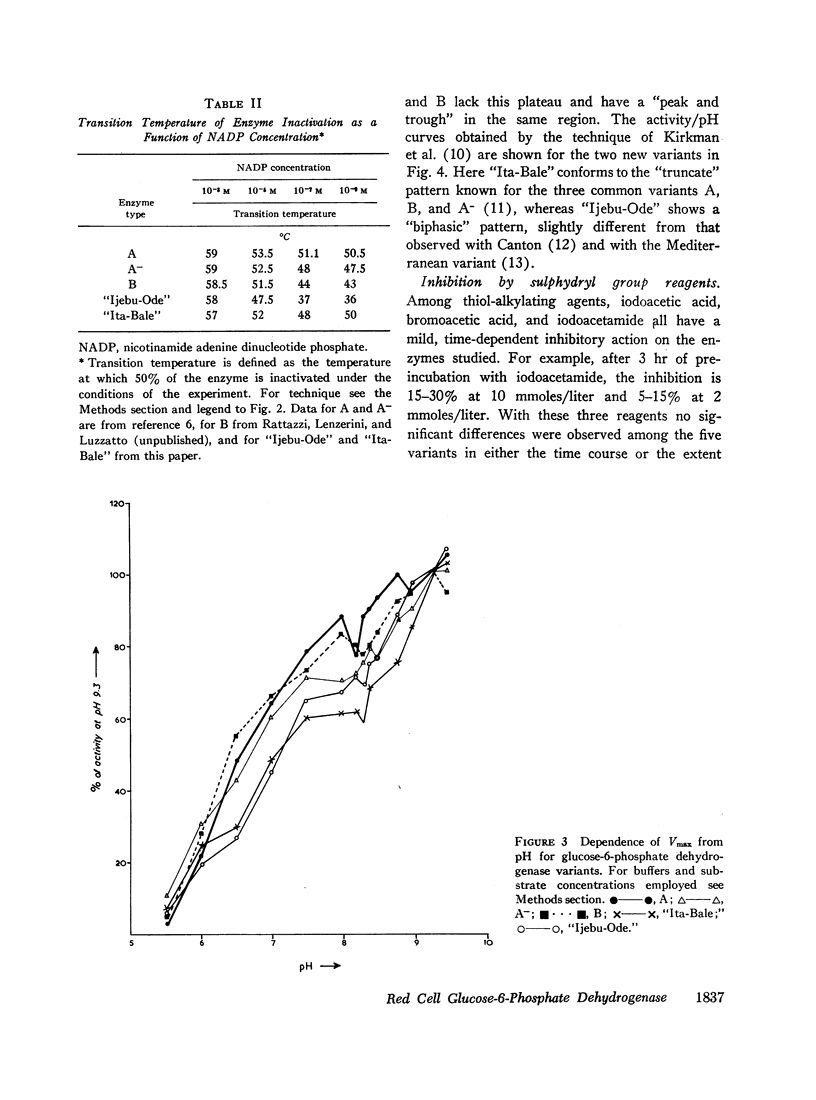
The maximal velocity of the enzyme reaction (Vmax) increases steadily with pH over the entire range explored (from pH 5.5 to 9.5) for all enzyme variants when buffers are used that show no specific ion effects on enzyme activity. Small differences are found among the variants in the pH range 7.5-8.2, where A and B show a “peak and trough,” while A-, “Ijebu-Ode,” and “Ita-Bale” exhibit a plateau.
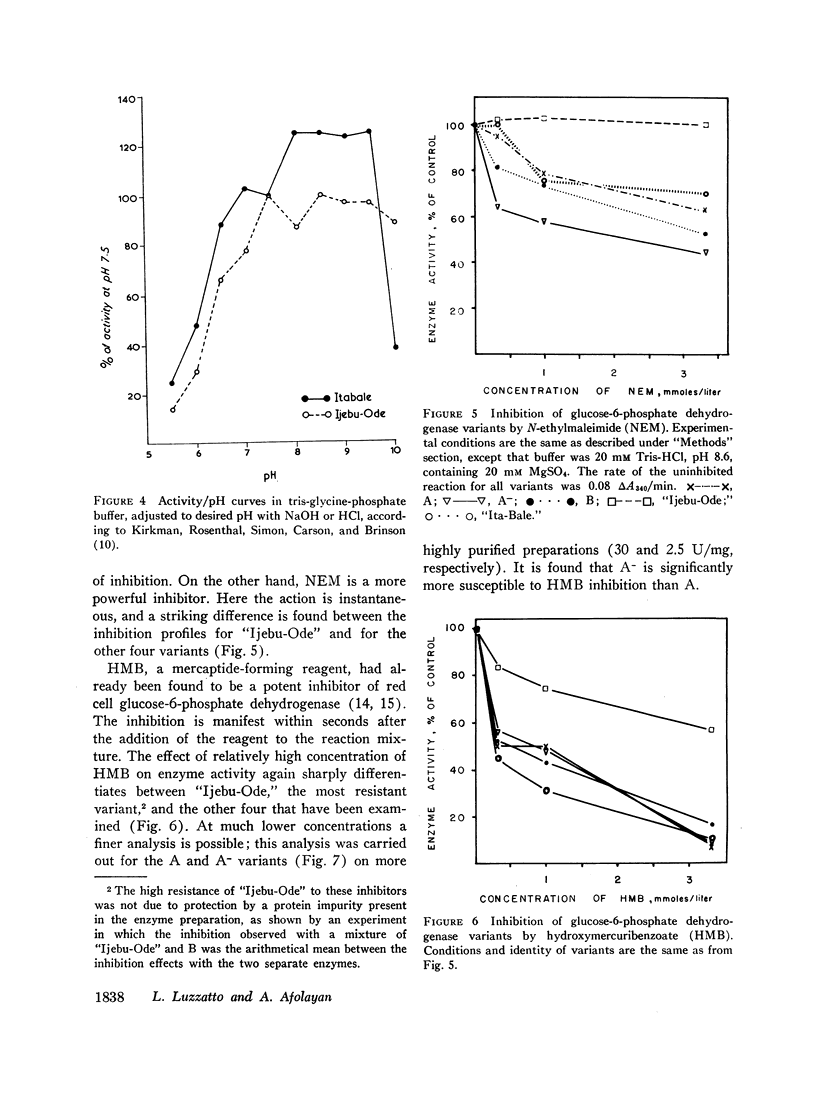
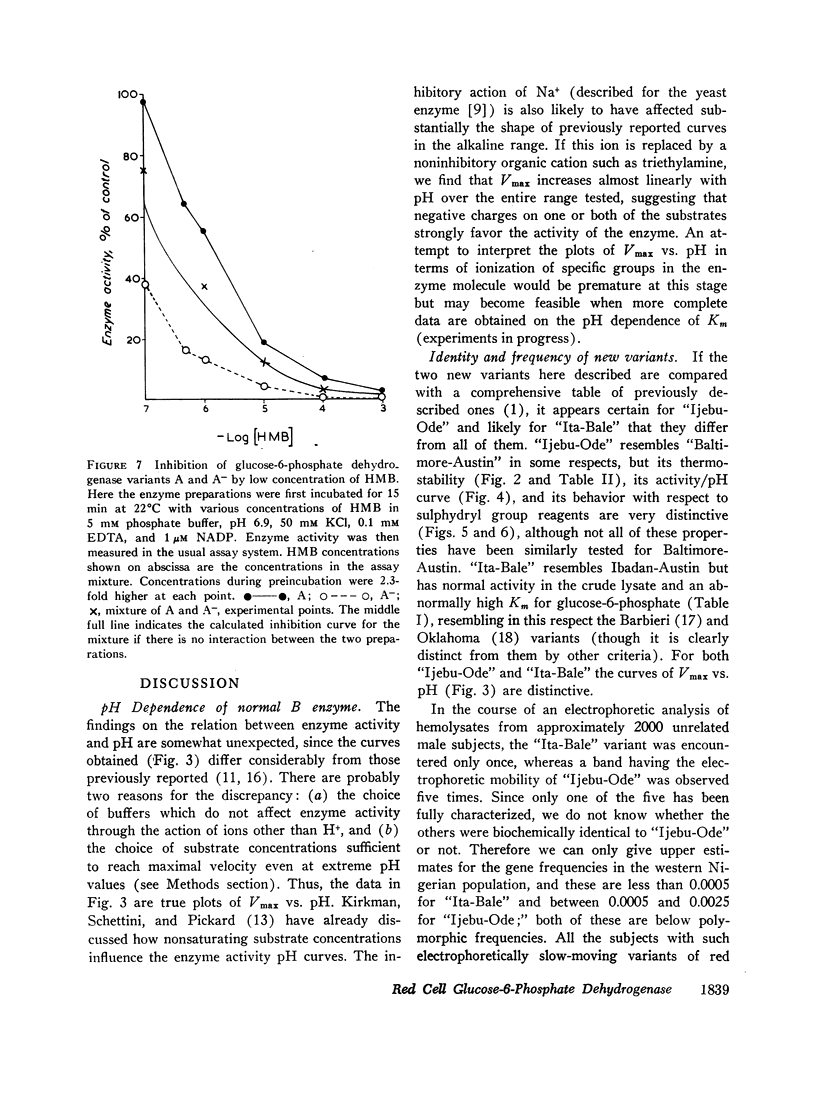
When the effects of reagents that bind to sulphydryl groups are compared, iodoacetate, bromoacetate, and iodoacetamide are weak inhibitors, while N-ethylmaleimide (NEM) and hydroxymercuribenzoate (HMB) are potent inhibitors. The last two reagents have differential inhibitory action on different variants; one of these, “Ijebu-Ode,” is strikingly resistant to HMB and totally resistant to NEM (up to 3 mmoles/liter).
The enzyme inactivation as a function of temperature exhibits distinctive profiles for all variants examined.
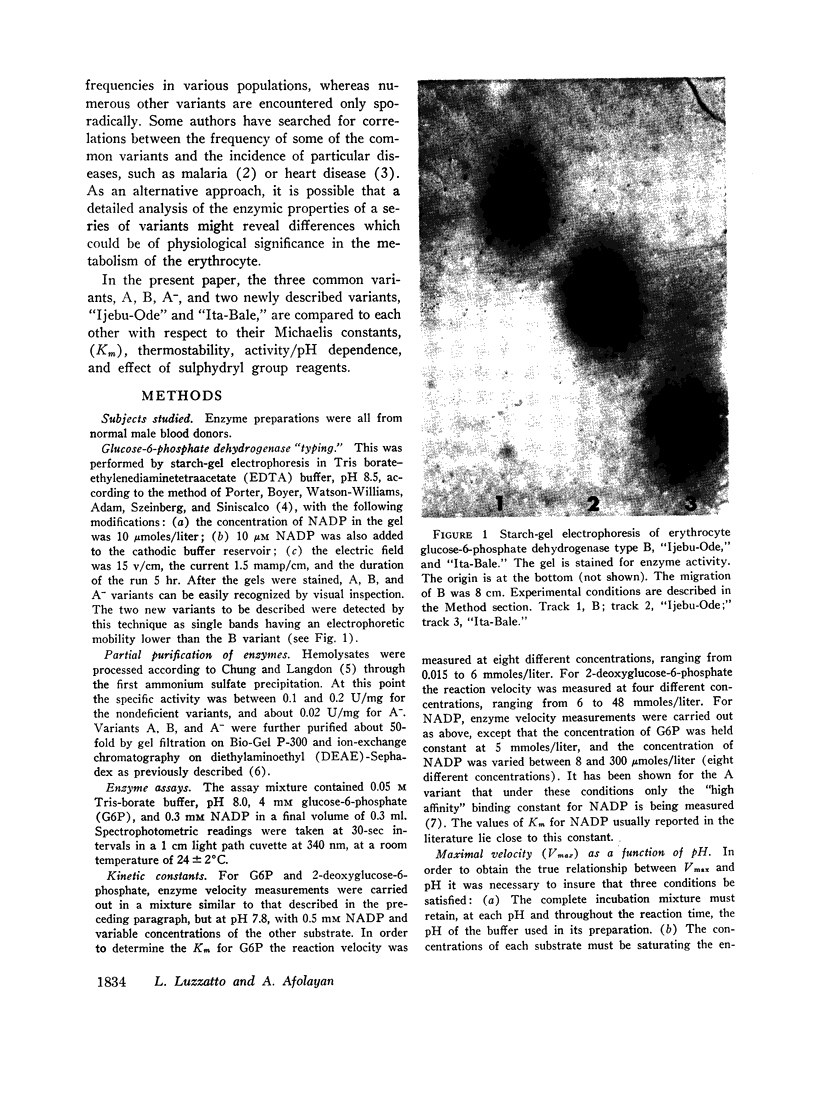
Both of the new variants described differ significantly from the normal B type in several respects: “Ijebu-Ode” in electrophoretic mobility, thermostability, dependence of Vmax on pH, and resistance to sulphydryl group reagents; “Ita-Bale” in electrophoretic mobility, Michaelis constant (Km) for glucose-6-phosphate, and dependence of Vmax on pH. When these data are compared with those available in the literature, both variants are different from all those previously described. The estimated frequencies of the corresponding genes in western Nigeria are between 0.0005 and 0.0025 for “Ijebu-Ode” and less than 0.0005 for “Ita-Bale”.
The A- variant, compared to A, has a distinctly higher Km for 2-deoxyglucose-6-phosphate and is more inhibited by very low concentrations of HMB. These are the first observed differences in kinetic properties between A and A-.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHUNG A. E., LANGDON R. G. Human erythrocyte glucose 6-phosphate dehydrogenase. I. Isolation and properties of the enzyme. J Biol Chem. 1963 Jul;238:2309–2316. [PubMed] [Google Scholar]
- CHUNG A. E., LANGDON R. G. Human erythrocyte glucose 6-phosphate dehydrogenase. II. Enzyme-coenzyme interrelationship. J Biol Chem. 1963 Jul;238:2317–2324. [PubMed] [Google Scholar]
- ESCOBAR M. A., HELLER P., TROBAUGH F. E., Jr "COMPLETE" ERYTHROCYTE GLUCOSE-6-PHOSPHATE DEHYDROGENASE DEFICIENCY. Arch Intern Med. 1964 Mar;113:428–434. doi: 10.1001/archinte.1964.00280090114019. [DOI] [PubMed] [Google Scholar]
- FORNAINI G., SEGNI P., LEONCINI G., BONSIGNORE D. PROPRIET'A DELLA GLUCOSO-6-FOSFATO DEIDROGENASI DEGLI ERITROCITI DI SOGGETTI UMANI NORMALI E SENSIBILI ALLE FAVE. Enzymol Biol Clin (Basel) 1963;45:137–157. [PubMed] [Google Scholar]
- KIRKMAN H. N. Charasteristics of glucose-6-phosphate dehydrogenase from normal and primaquine-sensitive erythrocytes. Nature. 1959 Oct 24;184:1291–1292. doi: 10.1038/1841291a0. [DOI] [PubMed] [Google Scholar]
- KIRKMAN H. N. Glucose 6-phosphate dehydrogenase from human erythrocytes. I. Further purification and characterization. J Biol Chem. 1962 Jul;237:2364–2370. [PubMed] [Google Scholar]
- KIRKMAN H. N., MCCURDY P. R., NAIMAN J. L. FUNCTIONALLY ABNORMAL GLUCOSE-6-PHOSPHATE DEHYDROGENASES. Cold Spring Harb Symp Quant Biol. 1964;29:391–398. doi: 10.1101/sqb.1964.029.01.041. [DOI] [PubMed] [Google Scholar]
- KIRKMAN H. N., RILEY H. D., Jr Congenital nonspherocytic hemolytic anemia. Am J Dis Child. 1961 Sep;102:313–320. doi: 10.1001/archpedi.1961.02080010315006. [DOI] [PubMed] [Google Scholar]
- KIRKMAN H. N., ROSENTHAL I. M., SIMON E. R., CARSON P. E., BRINSON A. G. "CHICAGO I" VARIANT OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE IN CONGENITAL HEMOLYTIC DISEASE. J Lab Clin Med. 1964 May;63:715–725. [PubMed] [Google Scholar]
- Kirkman H. N., Simon E. R., Pickard B. M. Seattle variant of glucose-6-phosphate dehydrogenase. J Lab Clin Med. 1965 Nov;66(5):834–840. [PubMed] [Google Scholar]
- LETTIN A. W. ADDICTION TO PLASTER? Lancet. 1964 Apr 11;1(7337):795–795. doi: 10.1016/s0140-6736(64)92983-6. [DOI] [PubMed] [Google Scholar]
- Long W. K., Wilson S. W., Frenkel E. P. Associations between red cell glucose-6-phosphate dehydrogenase variants and vascular diseases. Am J Hum Genet. 1967 Jan;19(1):35–53. [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L., Allan N. C. Different properties of glucose 6-phosphate dehydrogenase from human erythrocytes with normal and abnormal enzyme levels. Biochem Biophys Res Commun. 1965 Dec 21;21(6):547–554. doi: 10.1016/0006-291x(65)90520-6. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Okoye V. C. Resolution of genetic variants of human erythrocyte glucose 6-phosphate dehydrogenase by thin-layer chromatography. Biochem Biophys Res Commun. 1967 Dec 15;29(5):705–709. doi: 10.1016/0006-291x(67)90274-4. [DOI] [PubMed] [Google Scholar]
- Luzzatto L. Regulation of the activity of glucose-6-phosphate dehydrogenase by NADP+ and NADPH. Biochim Biophys Acta. 1967 Sep 12;146(1):18–25. doi: 10.1016/0005-2744(67)90069-1. [DOI] [PubMed] [Google Scholar]
- MARKS P. A., SZEINBERG A., BANKS J. Erythrocyte glucose 6-phosphate dehydrogenase of normal and mutant human subjects: properties of the purified enzymes. J Biol Chem. 1961 Jan;236:10–17. [PubMed] [Google Scholar]
- McCurdy P. R., Kirkman H. N., Naiman J. L., Jim R. T., Pickard B. M. A Chinese variant of glucose-6-phosphate dehydrogenase. J Lab Clin Med. 1966 Mar;67(3):374–385. [PubMed] [Google Scholar]
- NANCE W. E. GENETIC TESTS WITH A SEX-LINKED MARKER: GLUCOSE-6-PHOSPHATE DEHYDROGENASE. Cold Spring Harb Symp Quant Biol. 1964;29:415–425. doi: 10.1101/sqb.1964.029.01.043. [DOI] [PubMed] [Google Scholar]
- Pinto P. V., Newton W. A., Jr, Richardson K. E. Evidence for four types of erythrocyte glucose-6-phosphate dehydrogenase from G-6-PD-deficient human subjects. J Clin Invest. 1966 Jun;45(6):823–831. doi: 10.1172/JCI105398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell R. D., Brewer G. J., DeGowin R. L., Carson P. E. Effects of glucose-6-phosphate dehydrogenase deficiency upon the host and upon host-drug-malaria parasite interactions. Mil Med. 1966 Sep;131(9 Suppl):1039–1056. [PubMed] [Google Scholar]
- Yoshida A. A single amino Acid substitution (asparagine to aspartic Acid) between normal (b+) and the common negro variant (a+) of human glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1967 Mar;57(3):835–840. doi: 10.1073/pnas.57.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A. Glucose 6-phosphate dehydrogenase of human erythrocytes. I. Purification and characterization of normal (B+) enzyme. J Biol Chem. 1966 Nov 10;241(21):4966–4976. [PubMed] [Google Scholar]
- Yoshida A., Stamatoyannopoulos G., Motulsky A. G. Negro variant of glucose-6-phosphate dehydrogenase deficiency (A-) in man. Science. 1967 Jan 6;155(3758):97–99. doi: 10.1126/science.155.3758.97. [DOI] [PubMed] [Google Scholar]