Abstract
Estrogen receptors, in addition to the androgen receptor (AR), are expressed at high levels in efferent ductules of the male reproductive tract and it is now well recognized that estrogen receptor (ER) α is required for the maintenance of normal structure and function of the ductules. However, little is known regarding the hormonal regulation of the receptors themselves in the male. In the present study, efferent ductule ligation and castration, followed by replacement with testosterone, dihydrotestosterone (DHT) or estradiol was used to investigate the relative importance of circulating and luminal sources of steroid for the modulation of ERα, ERβ and AR in rat efferent ductules. Uni- or bilateral castration and ligation did not affect the expression of ERα and ERβ, but bilateral castration caused down-regulation of AR. Replacement with DHT and testosterone alone or in combination with estradiol caused the recovery of AR expression to control levels. A slight recovery of AR was also observed after estrogen replacement. ERα expression was decreased to nearly undetectable levels after estrogen replacement. On the other hand, ERβ did not show evident effects following any of the treatments, suggesting a constitutive expression of this receptor. This differential modulation of the steroid hormone receptors highlights the importance of maintaining a physiological androgen-estrogen balance to regulate the structure and function of efferent ductules in the male.
Introduction
Androgens have a well-established role in regulating the male reproductive tract. However, studies in several different experimental animal models have revealed that estrogens also play an essential role in the normal structure and function of the male reproductive tract (Lubahn et al. 1993, Smith et al. 1994, Morishima et al. 1995, Hess et al. 1997a, Couse & Korach 1998, Krege et al. 1998, Fisher et al. 1999, Robertson et al. 1999, Lee et al. 2000, Turner et al. 2000, Atanassova et al. 2001, Oliveira et al. 2001, 2002, Zhou et al. 2001a, Cho et al. 2003). The effects of androgens are mediated through the androgen receptor (AR) and the estrogen actions are mediated through two estrogen receptors, ERα and ERβ, which are co-localized in some regions of the male reproductive tract (Nie et al. 2002, Zhou et al. 2002). Despite the known expression of one or both ERs in the male tract (Hess et al. 1997b, Fisher et al. 1997, Nielsen et al. 2001, Saunders et al. 2001, Nie et al. 2002, Zhou et al. 2002), the full extent of estrogen’s action is not completely understood and the interaction of the various steroid receptors in the same cells of the male has not been examined.
Disruption of ERα function caused major effects in the efferent ductules, which resulted in male infertility (Eddy et al. 1996, Hess et al. 1997a, 2001, Oliveira et al. 2001). It is consistent across species that the efferent ductule is the site having the highest concentration of ERα in the male tract (Fisher et al. 1997, Goyal et al. 1997, Hess et al. 1997b, Nielsen et al. 2001, Nie et al. 2002, Zhou et al. 2002). The functional significance of ERα in the efferent ductule is under current scrutiny (Hess et al. 1997a, Lee et al. 2001, Zhou et al. 2001a, Cho et al. 2003), but the regulation of ERα expression itself in the efferent ductules remains to be determined. Even less information is available regarding ERβ, the estrogen receptor subtype with the widest distribution in the male (Hess et al. 1997b, Atanassova et al. 2001, Saunders et al. 2001). Targeted disruption of ERβ (βERKO) did not promote significant abnormalities in the male reproductive organs (Krege et al. 1998, Dupont et al. 2000); therefore, the biological function of ERβ in these organs, as well as the factor(s) involved in ERβ regulation, are at present unknown. It should be emphasized that continued expression of ERβ in the efferent ductules of the αERKO was unable to compensate for loss of ERα (Rosenfeld et al. 1998) and the double knockout of ER receptors (αβERKO) resulted in a phenotype resembling that of αERKO.
The efferent ductules and epididymis have two sources of estrogens and androgens, either the rete testis luminal fluid or the circulating blood in the vasculature. The relative importance of each source of steroid for the efferent ductules is not known (Goyal et al. 1998). However, circulating androgens do not appear to be sufficient to maintain the structure of the initial segment epididymis which is dependent upon luminal dihydrotestosterone (DHT), but do maintain the structure in the corpus and cauda (Fawcett & Hoffer 1979, Robaire & Viger 1995). The efferent ductules have high concentrations of AR (Schleicher et al. 1984, Roselli et al. 1991) and bind DHT (Schleicher et al. 1984), but in contrast with the initial segment, there is little or no 5α-reductase activity (Roselli et al. 1991) and the testosterone concentration in the luminal fluid (29 ng/ml) is much higher than that of DHT (2 ng/ml) (Vreeburg 1975). Accordingly, very little androgen dependence has been described for the efferent ductules (Goyal & Hrudka 1980, Ilio & Hess 1994, Hess 2002). Considering that efferent ductules express greater amounts of ER than the initial segment of the epididymis (Hess et al. 1997b, Mansour et al. 2001), it is reasonable to suggest that estrogens entering the efferent ductules may play a major role in ductal epithelial function, similar to the role that DHT plays in the initial segment of the epididymis (Robaire & Viger 1995).
There is little known about the hormonal regulation of the AR and ER in efferent ductules (Ilio & Hess 1994, Hess 2002, Hess et al. 2002). The only study to have focused on ER regulation in efferent ductules was carried out in the goat (Goyal et al. 1998). That study was based on castration and testosterone replacement, but the authors did not investigate the action of testosterone metabolites, DHT and estradiol, or the regulation of ERβ expression. Therefore, an investigation of this issue in rodents is warranted. The regulation of AR and ER by androgens and estrogens shows tissue and organ specificity, with both increases and decreases in receptor mRNA and protein in reproductive and non-reproductive tissues, depending on several physiological factors (Barton & Shapiro 1988, Goyal & Williams 1988, Quarmby et al. 1990, Lauber et al. 1991, Prins 1992, Gonzalez-Cadavid et al. 1993, Prins & Birch 1997, Prins et al. 1998, Yeap et al. 1999, Agarwal et al. 2000, Lynch & Story 2000, Zhu et al. 2000, Turner et al. 2001, Zhou et al. 2001b). These studies reveal the complexity associated with steroid receptor regulation across species, as well as organ- and cell-specific responses (Yeap et al. 1999). Thus, the potential for diverse steroid receptor responses to estrogens and androgens in the efferent ductules would be expected.
The present experiments were designed to investigate whether the expressions of ERα, ERβ and AR in the rat efferent ductules are regulated by estrogens, androgens or other factors derived from testicular fluid and/or blood. For this purpose, ligation and castration, followed by replacement with testosterone, DHT or estradiol were used. Differential expression of ERα, ERβ and AR was found in the rat efferent ductules. Estrogen caused down-regulation of ERα, and androgens promoted up-regulation of AR, whereas ERβ was not affected by these hormones or by the ligation and castration procedures, suggesting a constitutive expression of this receptor in the efferent ductules.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Harlan Bioproducts, Indianapolis, IN, USA), 90 days old, were used for this study. Upon arrival, the rats were housed under constant conditions of light (12 h light: 12 h darkness) and temperature (22 °C) and allowed to adapt to the vivarium conditions for at least 7 days before the experiment. They were fed a commercial diet (Teklad Chow–Harlan Teklad, Madison, WI, USA) and tap water was available ad libitum. All animal experiments and surgical procedures were approved by the University of Illinois Division of Animal Resources and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (1996).
The rats were randomly divided into 13 groups of three animals each, which were subjected to one of the following treatments: unilateral or bilateral ligation, unilateral or bilateral orchidectomy, sham-operation, bilateral orchidectomy followed by daily replacement with different doses of testosterone, DHT or estradiol, or left intact to serve as controls.
Experimental procedures
The rats were anesthetized with an i.p. injection of sodium pentobarbital (0.08 ml/100 mg body weight (BW)) and surgery was carried out in aseptic conditions. The body weight was recorded before surgery for later comparison with the weight at death. The scrotal skin was shaved and an incision in the mid-line ventral scrotal skin was performed to expose the testis-epididymis, in order to proceed to the ligation and/or castration. With bilateral castration, deprival of both luminal and circulating hormones will be achieved, while ligation and unilateral castration will deprive the efferent ductules of the luminal but not the circulating source of sex hormones. Three animals were not submitted to any treatment and served as intact experimental controls. Sham-operations were performed in one group of males to expose, manipulate and then reinsert the testes intact into the scrotum.
Ligation
After testis-epididymis exposure, the fat surrounding the extratesticular rete testis and the initial part of the efferent ductules was carefully dissected. The ducts were carefully separated from the pampiniform plexus vessels, which were kept intact. Ligation was performed at the extratesticular rete testis level, using a nonabsorbable silk suture placed as close to the testis as possible. For unilateral ligation, the extratesticular rete testis was ligated on one side and the contralateral testis/epididymis was left intact and used as control. After ligation, the testis-epididymis was returned to the scrotum and the scrotal incision was closed by suture. The rats were killed 15 days after ligation.
Castration
The initial procedure for castration followed the same protocol as that for ligation. The extratesticular rete testis together with the testicular blood vessels were ligated, as close to the testis as possible and then the testes were removed. The ligated efferent ductules and epididymis were placed back into the scrotum and the scrotal incision was closed by suture. When unilateral castration was performed, the contra-lateral testis/epididymis remained intact and served as control. The rats were killed 15 days after castration.
At the end of surgery, the scrotal incision was closed by suture and the wound was treated with Betadine. Postoperative conditions of the animals, including food and water ingestion, defecation, and surgical site were monitored daily.
Hormone replacement
Starting on the same day as the bilateral orchidectomy, the animals were treated once per day, for 15 days, with s.c. injections of 5 mg 5α-DHT/day (Sigma, St Louis, MO, USA), 1 mg or 5 mg testosterone propionate/day (JT Baker Chemicals, Phillipsburg, NJ, USA), 75 μg or 400 μg 17β-estradiol-3-benzoate/day (Sigma) or both testosterone propionate (5 mg/day) and 17β-estradiol-3-benzoate (400 μg/day), dissolved in a volume of 0.1 ml corn oil as vehicle. The rats were killed after 15 days of treatment. The castration control group received the same volume of vehicle only.
A high dosage of estradiol (400 μg) was used because this was found to be effective for inducing complete estrogenization in male rats without inducing significant regression of the efferent ductules (Hansen et al. 1997, Tena-Sempere et al. 2000). The dosage of 75 μg estradiol was equivalent to that shown to induce a response in reproductive organs, with minimal histological alterations (Turner et al. 2001). The dose regimen of 1 mg testosterone was used to mimic physiological serum testosterone levels and that of 5 mg testosterone and DHT was based on previous studies showing that this concentration reproduces that which is normally found in the epididymis (Hansen et al. 1997, Fan & Robaire 1998, Goyal et al. 1998). DHT, the non-aromatizable metabolite of testosterone, was used to get around the problem of whether the potential effects of testosterone were direct or were dependent upon aromatization to estrogen or 5α-reduction to DHT.
Tissue preparation and morphometry
Fifteen days after surgery (ligation or castration) and initiation of hormone replacement, the rats were anesthetized (i.p. sodium pentobarbital 0.1 ml/100 g BW), weighed and perfused intracardially with 10% neutral buffer formalin (NBF). After fixation, the testis, epididymis with the attached efferent ductules, ventral prostate and seminal vesicles with coagulating glands were removed and weighed. The efferent ductules were dissected out from the epididymis, embedded in paraffin, sectioned (5 μm) and mounted on electrostatically charged glass slides. The sections were stained with hematoxylin and eosin for histological study or were used for immunohistochemistry staining. The lumens of the efferent ductules were measured at the widest diameter of five sections of tubules from the proximal area nearest the rete testis. The height of the epithelium of the efferent ductules was measured from the basement membrane to the microvillus bases in areas of straight sections from 25 cells with an evident nucleus. The luminal and epithelial measurements were performed using a calibrated ocular micrometer coupled to a 10 × and 40 × objective, respectively.
Hormone measurements
Plasma concentrations of testosterone and estradiol were measured by RIA. Blood was collected by cardiac puncture, immediately before death. The plasma was separated by centrifugation and stored at −20 °C for subsequent hormone assays. All samples were measured in duplicate. The reported concentrations of the hormones were corrected for extraction losses.
The antibody used for assaying testosterone was developed by Dr O D Sherwood (University of Illinois, Urbana, IL, USA) and the procedures have been described previously (Jackson et al. 1991, Oliveira et al. 2002). For the assay, plasma was extracted with toluene:petroleum ether (2:5 v/v). The efficiency of the extraction was 89.7%, the sensitivity of the assay was 0.2 ng/ml and the assay coefficient of variation was 8.3%.
Estradiol concentration was estimated using a double antibody ultra-sensitive estradiol RIA-DSL 4800 Kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA). This kit uses an antibody with high affinity for estradiol and low cross-reactivity with other estrogens and with testosterone. For the assay, plasma was extracted with toluene; the efficiency of the extraction was 95.3%. The limit of detection of the assay was 0.8 pg/ml and the assay coefficient of variation was 10.8%.
Immunohistochemistry
Changes in the expression of ERα, ERβ and AR in the efferent ductules were investigated by immunohistochemistry in all experimental rats, following the protocol previously described (Oliveira et al. 2003). Tissue sections from animals of each experimental group were run in parallel, and the staining was performed in three different sets using one animal of each group per set to confirm the results. Fixed tissues were embedded in paraffin; sections were subjected to microwave antigen retrieval before incubation with primary antibody. The antibodies used were a monoclonal mouse anti-human ERα antibody (NCL-ER-6F11-Novocastra Laboratories, Newcastle, UK), a polyclonal sheep anti-human/rat ERβ antibody (S-40, raised against a peptide in the hinge domain of hERβ; Saunders et al. 2000) and a polyclonal rabbit anti-rat/human androgen receptor antibody (PG21–29; Prins et al. 1991). Sections were incubated overnight at 4 °C with diluted primary antibody (1:500 for ERα and AR; 1:1000 for ERβ). For negative controls, the sections received PBS in place of the primary antibody. After washing in PBS, the sections were exposed for 1 h to a biotinylated secondary antibody - goat anti-mouse (for ERα) (Dako, Carpinteria, CA, USA), rabbit anti-sheep (for ERβ) (Vector Laboratories, Burlingame, CA, USA) and goat anti-rabbit (for AR) (Dako), all used at 1:100 dilution. After this step, the sections were incubated with the avidin-biotin complex (Vectastain Elite ABC kit, Vector Laboratories) for 30 min and the immunoreaction was visualized using diaminobenzidine containing 0.01% H2O2 in 0.05 M Tris–HCl buffer, pH 7.6. Sections stained for ERβ were slightly counterstained with Mayer’s hematoxylin.
Statistical analysis
A two-way ANOVA was applied to analyze the effect of ligation, castration and hormone replacement on testosterone and estradiol blood concentrations, body weight, efferent ductules/epididymis and accessory sex gland weights, and luminal diameter and epithelial height of efferent ductules. The post hoc Tukey’s test was used for multiple comparisons between the experimental groups.
Results
Hormone levels
After unilateral castration and efferent ductule ligation on one or both sides, as well as sham operation, testosterone levels did not differ significantly from those of the intact controls, but following bilateral orchidectomy the testosterone concentration fell to a level similar to or below the limit of detection of the assay (0.2 ng/ml) (Table 1). Similar results were observed when corn oil or estradiol was administered following castration. By contrast, supplementation with DHT or testosterone (1 or 5 mg), alone or combined with estradiol, resulted in plasma testosterone levels significantly higher than controls. This increase in testosterone level was dose dependent, reaching levels above the limit of detection (20 ng/ml) with the high dose of testosterone (5 mg). Plasma levels of estradiol were not significantly different from intact controls in any experimental group, except after estradiol replacement, alone or combined with testosterone, which resulted in an increase in plasma estradiol to levels above the upper limit of the assay (>80 pg/ml) (Table 1).
Table 1.
Effects of rat efferent ductile ligation, castration and hormonal replacement on plasma testosterone (T) and estradiol (E2) concentrations and on body weight. Values are means±S.E.M.
| Treatment | Testosterone (ng/ml) | Estradiol (pg/ml) | Body weight |
|
|---|---|---|---|---|
| Initial (g) | Final (g) | |||
| Intact control | 3.6 ± 0.5 | 17.8 ± 5.8 | ND | 396.1 ± 2.7 |
| Bilateral ligation | 3.4 ± 0.8 | 7.9 ± 1.3 | 360.4 ± 3.2 | 375.6 ± 2.4 |
| Unilateral ligation | 4.2 ± 0.8 | 8.1 ± 2.2 | 365.9 ± 8.0 | 406.3 ± 9.0 |
| Unilateral castration | 3.3 ± 0.2 | 4.9 ± 1.1 | 366.7 ± 4.4 | 389.9 ± 11.0 |
| Bilateral castration | 0.1 ± 0.0* | 5.7 ± 1.5 | 355.1 ± 16.0 | 389.0 ± 9.2 |
| Sham operation | 3.3 ± 0.7 | 3.9 ± 0.4 | 367.5 ± 3.2 | 400.2 ± 12.9 |
| Bicastration + corn oil (control) | 0.2 ± 0.0* | 4.9 ± 1.0 | 366.0 ± 5.7 | 381.9 ± 1.9 |
| Bicastration + T (1 mg) | 10.8 ± 1.3* | 7.2 ± 0.4 | 375.6 ± 4.0 | 384.8 ± 2.4 |
| Bicastration + T (5 mg) | >20.0 ± 0.0* | 6.7 ± 1.1 | 381.7 ± 2.1 | 393.2 ± 5.2 |
| Bicastration + DHT (5 mg) | 10.4 ± 0.7* | 3.9 ± 0.2 | 375.6 ± 10.4 | 400.6 ± 10.7 |
| Bicastration + E2 (75 μg) | 0.2 ± 0.0* | >80.0 ± 0.0* | 378.2 ± 3.2 | 331.5 ± 8.8* |
| Bicastration + E2 (400 μg) | 0.1 ± 0.0* | >80.0 ± 0.0* | 372.6 ± 5.7 | 284.5 ± 4.6* |
| Bicastration + T (5 mg) + E2 (400 μg) | 14.1 ± 3.4* | 69.0 ± 8.3* | 372.7 ± 2.1 | 319.9 ± 3.7* |
P ≤ 0.05 compared with intact control; ND, not determined.
Body and organ weights
No significant difference in body weight was observed when initial and final weights were compared in most experimental groups, but there was a significant (P < 0.05) loss of weight over the 15 days of estradiol replacement (12 to 24% decrease) (Table 1). Compared with the control group, the weights of the ventral prostate and paired seminal vesicle/coagulating glands, as well as the weight of the efferent ductules/epididymis were significantly diminished after bilateral castration (Table 2). Estradiol alone was not able to restore the weight of either the accessory sex glands or the efferent ductules/epididymis. Replacement with testosterone or DHT resulted in sex gland weights even higher than intact controls (Table 2). Although androgen treatment greatly increased the weight of the efferent ductules/epididymis, they were still below the intact control weight. On the other hand, when testosterone was combined with estradiol, the efferent ductules/epididymis weight was restored to control levels. Combined testosterone and estrogen also resulted in heavier sex glands than those treated with testosterone alone. Unilateral castration and uni- or bilateral ligation, as well as sham operation, did not affect significantly the weight of the accessory glands (Table 2). Similarly, the weight of the efferent ductules/epididymis was not greatly affected by sham operation, but a significant decrease in weight at the operated side was observed when unilateral ligation (32%) and unilateral castration (28%) were compared with the contra-lateral intact efferent ductules/epididymis, or when the bilateral ligated (18–22%) was compared with the intact control (Table 2).
Table 2.
Effects of efferent ductile ligation, castration and hormonal replacement on the weights of the efferent ductile/epididymis, ventral prostate, and combined seminal vesicles (SV)/coagulating glands (CG) in the rat. Values are means±S.E.M.
| Treatment | Efferent ductules + epididymis |
Ventral prostate (mg/100 g BW) | SV + CG (mg/100 g BW) | |
|---|---|---|---|---|
| Left (mg) | Right (mg) | |||
| Intact control | 170.6 ± 4.7 | 170.0 ± 2.8 | 257.1 ± 8.0 | 481.1 ± 24.0 |
| Bilateral ligation | 133.3 ± 2.7* | 136.7 ± 4.8* | 172.6 ± 16.3 | 428.3 ± 35.7 |
| Unilateral ligation | 161.6 ± 5.8 | 110.6 ± 5.9** | 203.1 ± 11.1 | 398.8 ± 20.3 |
| Unilateral castration | 169.5 ± 9.4 | 122.9 ± 5.5** | 252.9 ± 35.8 | 464.2 ± 18.1 |
| Bilateral castration | 55.4 ± 0.9* | 60.3 ± 3.2* | 26.3 ± 1.2* | 67.7 ± 4.3* |
| Sham operation | 187.8 ± 10.9 | 161.5 ± 17.8 | 196.9 ± 27.2 | 440.5 ± 14.4 |
| Bicastration + corn oil (control) | 56.8 ± 0.2* | 56.6 ± 5.7* | 25.0 ± 0.7* | 74.8 ± 2.9* |
| Bicastration + T (1 mg) | 148.4 ± 2.9* | 148.8 ± 4.9* | 348.7 ± 38.6* | 950.2 ± 33.0* |
| Bicastration + T (5 mg) | 147.5 ± 10.8* | 147.5 ± 13.2* | 379.6 ± 11.3* | 929.2 ± 3.2* |
| Bicastration + DHT (5 mg) | 147.1 ± 11.9* | 142.8 ± 8.2* | 362.4 ± 19.4* | 866.8 ± 10.8* |
| Bicastration + E2 (75 μg) | 63.8 ± 4.2* | 81.7 ± 13.7* | 42.5 ± 3.6* | 103.2 ± 15.5* |
| Bicastration + E2 (400 μg) | 69.1 ± 7.6* | 90.7 ± 18.5* | 51.0 ± 9.4* | 104.6 ± 9.0* |
| Bicastration + T (5 mg) + E2 (400 μg) | 157.6 ± 4.5 | 169.8 ± 5.1 | 466.8 ± 18.5* | 1206.9 ± 56.7* |
T, testosterone; E2, 17β-estradiol.
P ≤ 0.05 compared with intact control;
P ≤ 0.05 compared with castrated control.
Epithelial cell height
Regression of the efferent ductules, characterized by a reduction in luminal diameter and epithelial height, was observed as a result of either ligation or castration, but the effects on luminal diameter were more dramatic (Fig. 1). Efferent ductule luminal diameter was significantly increased by 400 μg estradiol, when compared with castrated control animals, but androgens had no effect. In contrast, testosterone and DHT replacement, as well as estradiol, caused a partial recovery in the height of the epithelium, but higher epithelium was seen after resupplementation with testosterone plus estradiol. However, in all cases the epithelial height was still below the control level.
Figure 1.
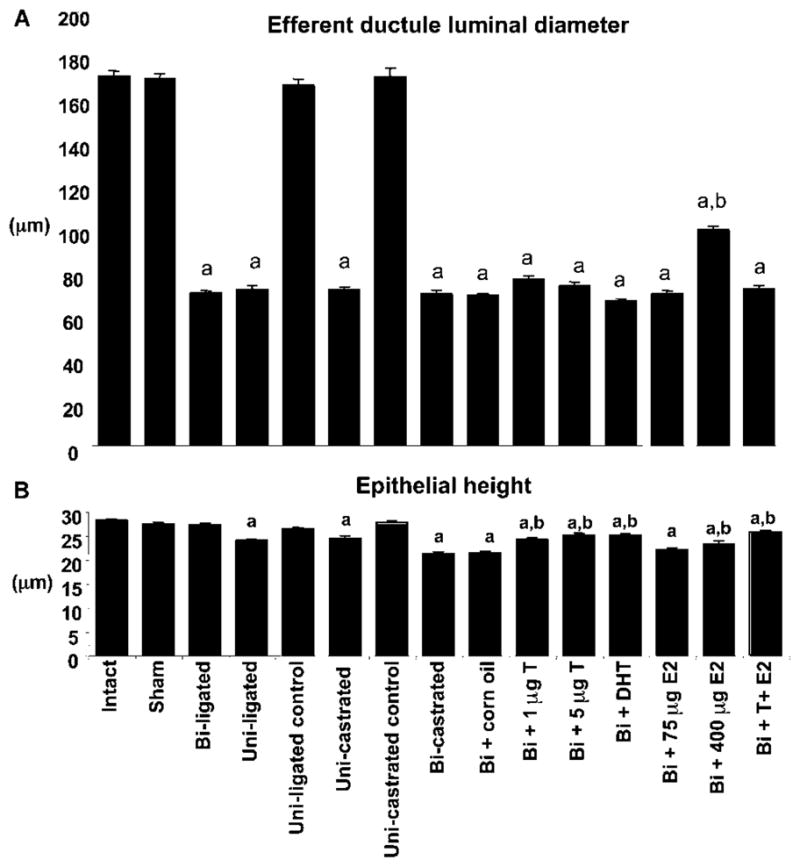
Effects of ligation, castration and hormone replacement on the efferent ductule. (A) Compared with the intact rat, the luminal diameter of the efferent ductule was significantly (a) decreased in ligated and castrated rats, following or not hormone replacement. However, compared with the castrated control animal, the luminal diameter was significantly (b) higher after treatment with the high dose of estradiol (E2; 400 μg). (B) The height of the epithelium decreased significantly (a) after unilateral ligation or castration followed or not by hormone replacement. Testosterone (T; 1 mg and 5 mg), DHT (5 mg) and estradiol (75 μg and 400 μg) caused a partial recovery of the epithelial height, compared with the castrated rat. Higher epithelium was seen after replacement with testosterone (5 mg) + estradiol (400 μg). Values represent means±S.E.M. (n = 3 in each group). a, P ≤ 0.05 compared with intact rat; b, P ≤ 0.05 compared with castrated rat. Uni, unilateral; Bi, bilateral.
Expression of androgen receptors
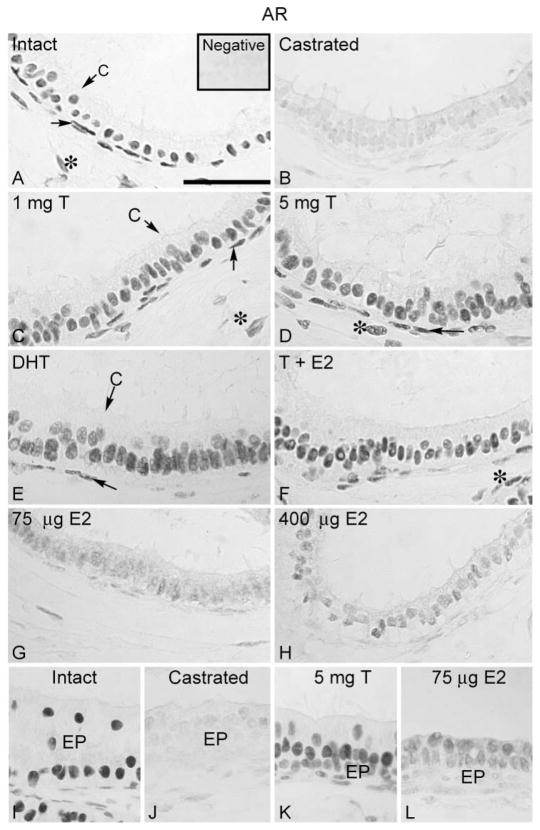
The AR expression in control efferent ductules was found in the nuclei of ciliated and nonciliated epithelial cells, in addition to the peritubular and some stromal cells (Fig. 2A). Similarly, the initial segment of the epididymis was strongly positive for AR in the epithelial, peritubular and stromal cell nuclei. AR expression was not affected by sham operation, unilateral or bilateral ligation, or unilateral castration. On the other hand, bilateral castration caused a remarkable down-regulation in AR protein expression (Fig. 2B). Testosterone, alone or associated with estradiol, as well as DHT restored the AR to the intact control levels (Fig. 2C–F). The AR immunostaining intensity in the efferent ductules was partially restored by estradiol supplementation (Fig. 2G, H). The effects on AR expression in the initial segment were similar to those of the efferent ductule, including the recovery of staining after estradiol replacement (Fig. 2I–L).
Figure 2.
Regulation of AR in the efferent ductule of rat. (A) AR was intensely expressed in the nuclei of the efferent ductule epithelial, peritubular (arrow) and some stromal cells (*) of intact control rats. C, ciliated cell. Insert represents the negative control. (B) After castration, there was a dramatic decrease in the AR expression in the efferent ductule. Replacement with (C) 1 mg testosterone (T), (D) 5 mg testosterone, (E) 5 mg DHT, as well as (F) 5 mg testosterone associated with 400 μg estradiol (E2) restored AR immunostaining to the control levels. A slight recovery of AR staining was observed after estradiol (75 μg and 400 μg) replacement in the efferent ductules (G, H). The initial segment of the epididymis (EP) was positive for AR (I). The AR staining decreased after castration (J), but testosterone replacement restored the positive reaction (K). Estradiol replacement also induced a slight recovery of AR staining in the initial segment (L). The results regarding the efferent ductule of sham-operated, unilateral or bilateral ligated and unilateral castrated rats were similar to those of intact controls (not shown). Bar = 100 μm.
Expression of ERα
Immunohistochemical localization of ERα in the efferent ductules was restricted to the ciliated and nonciliated cell nuclei (Fig. 3A). ERα immunostaining in the efferent ductules after unilateral or bilateral ligation and castration was unchanged when compared with that of intact controls (Fig. 3B). The administration of corn oil, testosterone and DHT following castration had no effect on ERα expression (Fig. 3C, D, E). However, ERα was greatly reduced in the epithelium of the efferent ductules after estradiol replacement, in a dose-dependent manner (Fig. 3G, H). The higher dose of estradiol (400 μg) promoted down-regulation of ERα to nearly undetectable levels, both when used alone and in combination with testosterone (Fig. 3F, H). The lower dose of estradiol (75 μg) caused less, but still a dramatic, reduction in immunodetectable ERα in the efferent ductules (Fig. 3G). The initial segment of the epididymis was negative for ERα (Fig. 3I); however resupplementation with estradiol induced slight ERα immunoexpression in the epithelium (Fig. 3J).
Figure 3.
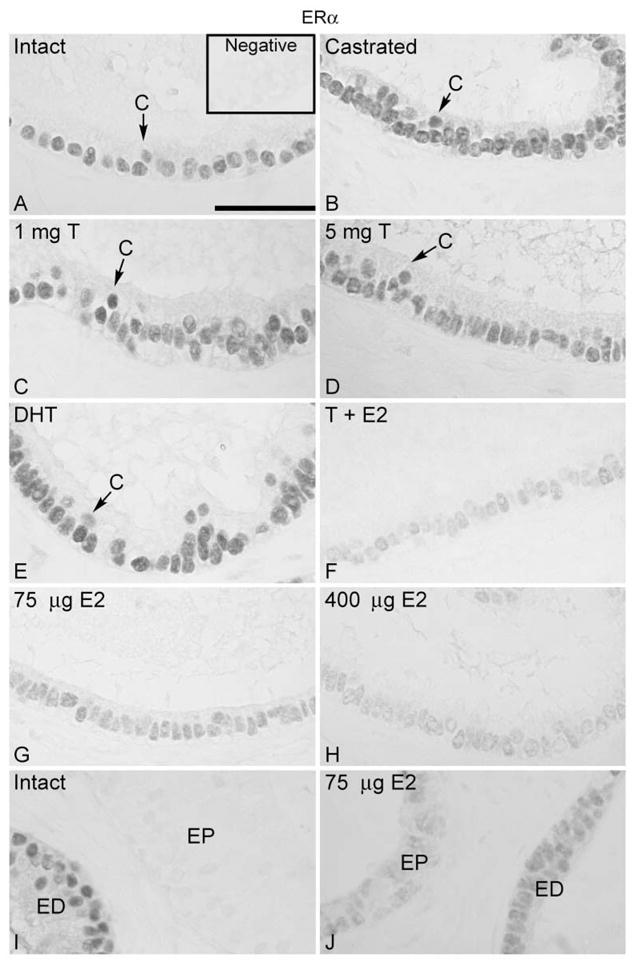
Regulation of ERα in the efferent ductule of the rat. (A) ERα was expressed in the nuclei of the ciliated (C) and nonciliated cells of efferent ductule epithelium in intact control rats. Insert represents the negative control. Castration (B) or castration followed by androgen replacement (C, D, E) did not affect ERα expression. After replacement with 400 μg estradiol (E2) associated with 5 mg testosterone (T) (F), or with estradiol (75 μg and 400 μg) alone (G, H) there was a remarkable decrease in ERα expression. The higher dose of estradiol induced a greater decrease in ERα (F, H). (I) The initial segment of the epididymis (EP) was negative for ERα in control animals. ED, distal efferent ductules. (J) After replacement with estradiol (75 μg and 400 μg) a slight staining for ERα was found in the initial segment epithelium (EP). The results from efferent ductules of sham-operated, unilateral or bilateral ligated and unilateral castrated rats were similar to those of intact controls (not shown). Bar = 100 μm.
Expression of ERβ
The pattern of ERβ localization in efferent ductules and initial segment was similar to that of the AR, with positive staining detected in the nuclei of epithelial cells, as well as in peritubular and some stromal cells (Fig. 4A). Bilateral and unilateral ligation or castration, as well as hormonal replacement did not change ERβ expression or, at the very least, caused a very slight decrease in ERβ (Fig. 4B-H). A decrease in staining was observed after testosterone treatment.
Figure 4.
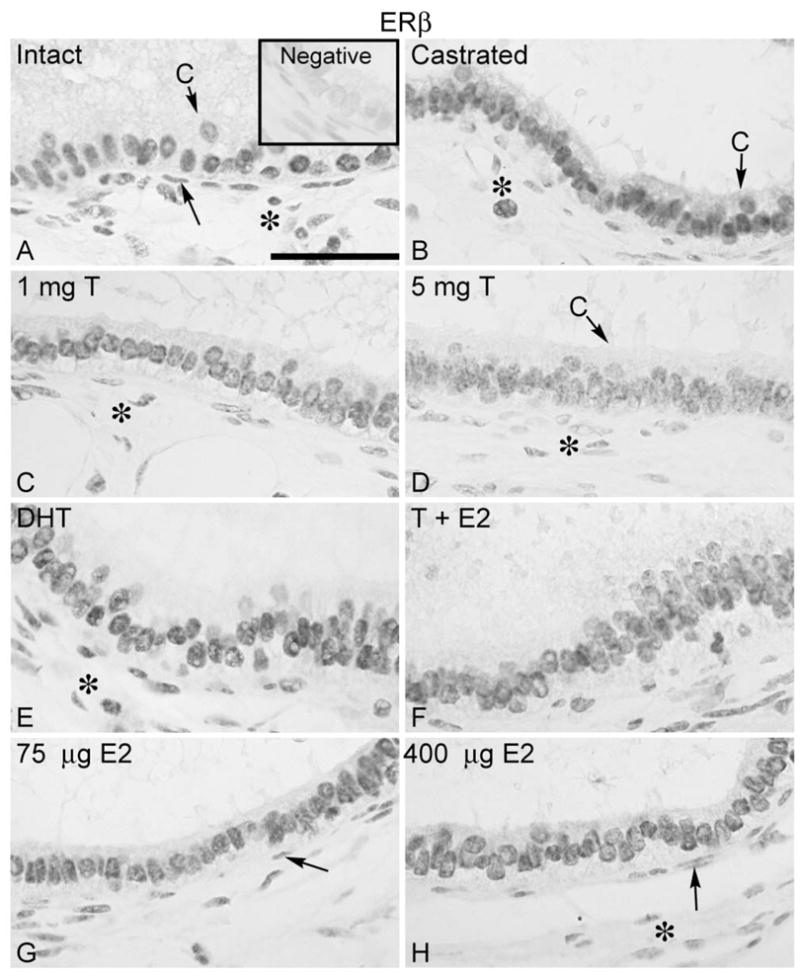
Regulation of ERβ in the efferent ductule of the rat. (A) ERβ was expressed in the nuclei of the efferent ductule epithelium, peritubular (arrow) and some stromal cells (*) in intact control rats. C, ciliated cells. Insert represents the negative control. Castration (B) or castration followed by replacement with 5 mg testosterone (T) (C, D), 5 mg DHT (E), 400 μg estradiol (E2) associated with 5 mg testosterone (F) or with estradiol alone (75 μg and 400 μg) (G, H) did not cause evident effects in ERβ expression, except for a slight decrease in staining in some areas after testosterone replacement (D). The results from efferent ductules of unilateral or bilateral ligated and unilateral castrated rats were similar to those of bilateral castrated rats (not shown). Bar = 100 μm.
Discussion
This study provided evidence that expression of ERα, ERβ and AR proteins in the rat efferent ductules is differentially regulated by androgens and estrogens. These results partially differed from those in the goat (Goyal et al. 1998), but agree with recent findings that AR and both ERs respond differently to the antiestrogen ICI 182,780, with ERα expression being dramatically down-regulated, but ERβ and AR levels remaining unchanged in the rat efferent ductule (Oliveira et al. 2003). These data suggest that there may be important interspecies differences in the regulation of ER expression in the male reproductive tract.
The effects of castration and hormone replacement on body and sex gland weight, and blood concentrations of testosterone and estradiol were similar to previously reported data for the rat (Podesta et al. 1975, Roselli & Resko 1984). The effects of androgens on sex gland weight, which was much higher than controls, was expected because a high supplemental dose of hormone was used to maintain the elevated concentrations needed to stimulate the testis-epididymal region. The rat efferent ductule epithelium regressed and the efferent ductule/epididymal weight decreased following castration, and both parameters were restored by androgens but not to control levels. Circulating estrogen alone does not appear to be sufficient to maintain the efferent ductule structure. However, concomitant administration of testosterone and estradiol was the only treatment able to restore efferent ductule/epididymal weight to the control level and induced a higher recovery of epithelial height, indicating that a cooperative action of these steroids may be necessary.
The effects of androgens on AR expression in the efferent ductule are in agreement with previous studies showing increased AR protein following testosterone or DHT treatment (Calandra et al. 1975, Sar et al. 1990, Goyal et al. 1998, Yeap et al. 1999, Zhu et al. 2000, Turner et al. 2001). Others have shown up-regulation of AR protein even in the presence of decreased mRNA (Quarmby et al. 1990, Yeap et al. 1999). These data indicate that the mechanism underlying AR regulation by androgens involves differential transcriptional and/or post-transcriptional events, which will likely be cell- and tissue-specific (Yeap et al. 1999). In this sense, recent findings in the prostate gland showed that AR is down-regulated by androgen withdrawal as well as estrogen treatment, through a post-transcriptional pathway mediated by proteasome (Woodham et al. 2003). In the present study, ligation of the efferent ductule did not affect levels of immunodetectable AR, nor did unilateral castration. Taken together, these data indicate that deprivation of circulating androgens, but not of luminal androgens or other testicular factors such as androgen binding protein (ABP), affected AR expression, suggesting that AR expression in rat efferent ductules is mainly dependent on circulating androgens. This result is in agreement with that found for efferent ductules in the goat (Goyal et al. 1998). ABP is a Sertoli cell product important for the maintenance of high levels of intratubular androgen (Danzo et al. 1977, Dohle et al. 2003). Some chemical agents that alter AR expression in the testis also alter the expression of ABP (Tirado et al. 2004). Altered expression of ABP has been associated with testis and male tract alterations similar to those caused by alterations of AR (Danzo et al. 1977, Jeyaraj et al. 2002). Therefore, it would be possible that ABP could have some effect on AR expression. We cannot exclude this possibilitiy, but ABP transgenic mice showed no significant changes in androgen receptor, which may indicate that the deleterious effects of aberrant levels of ABP may be caused by some other factor than AR modulation (Munell et al. 2002).
It was interesting to note that estrogen replacement also caused a partial recovery of AR expression in the efferent ductule following castration. Modulation of AR by estrogen has been reported in the rat testis (Turner et al. 2001) and in some areas of the rat brain (Lynch & Story 2000). In the prostate, developmental estrogenization has been shown to induce a transient up-regulation of ERα and a down-regulation of AR and ERβ levels in adult prostate epithelium (Prins & Birch 1997, Prins et al. 2001). Estrogen also appears to interfere in some physiological events usually attributed to androgens, such as modulation of prostate androgen responsive genes (prostate specific androgen (PSA), Rosner et al. 1998; C3 and sulphated glycoprotein-2 (SGP-2), Turner et al. 2001) and stimulation of spermatogenesis in hypogonadal males (Ebling et al. 2000), suggesting that the effects of estradiol are mediated via cross-reactivity with AR. Physical interactions between AR and ERα, which result in estradiol-induced modulation of AR transcriptional activity by ERα (Panet-Raymond et al. 2000) and the ability of estradiol to activate AR genes when AR complexes with the coactivator ARA70 (Yeh et al. 1998) or with sex hormone binding globulin (SHBG)-SHBG receptor (Rosner et al. 1998), have been described and they indicate alternative pathways to explain receptor interplay. It is more likely that interaction between androgen and estrogen action may exist in the male reproductive tract, similar to other tissues (Migliaccio et al. 2000, Ochiai et al. 2004).
The distribution of ERα was found to be restricted to the epithelium of the efferent ductules, consistent with findings across species investigated to date (Hess 2003). The only dramatic decrease in ERα expression in the efferent ductules was observed after estrogen replacement. ERα down-regulation by estradiol was dose dependent, confirming previous findings that ERα proteolysis is modulated by ligand concentration (Preisler-Mashek et al. 2002). Both ligation and castration were unable to disturb ERα expression in the efferent ductules. Because plasma levels of estradiol were not altered significantly by these procedures, it would appear that circulating estradiol might play a role in regulating ERα in the efferent ductule epithelium. Considering that aromatase mRNA was recently detected in the epithelium of epididymal cells in vitro (Wiszniewska 2002), the contribution of locally produced estrogen cannot be ruled out. However, it is important to point out that studies in several species have detected aromatase protein and activity in the sperm that traverse the male tract, but not in the epithelium of the ductal system (Janulis et al. 1996, 1998).
The efferent ductule is the segment of the male tract more sensitive to altered levels of estrogen or disruption of estrogen action, as shown by experiments using high doses of estrogens, antiestrogens, as well as knockout of estrogen receptors, all of them resulting in efferent ductule abnormalities, especially in the luminal diameter (Hess et al. 2002, Oliveira et al. 2001, 2002, Rivas et al. 2002, 2003, Cho et al. 2003). Also in the present study, estrogen (400 μg) was the treatment that caused greater dilation in the efferent ductules after castration. These results are in agreement with recent data showing that the primary function of estrogens/ERα in the efferent ductules is to regulate the expression of proteins involved in fluid reabsorption (Zhou et al. 2001a, Lee et al. 2001, Oliveira et al. 2002), in addition to the maintenance of the epithelial morphology (Hess 2003), although the last function appears to be more dependent on an androgen–estrogen balance (Rivas et al. 2003).
In contrast to the effects on efferent ductules, estrogen replacement induced a weak expression of ERα in the initial segment of the epididymis, a region that was negative for ERα in the control animals. Depending on the tissue and the antibodies used, estrogens have been shown to cause autologous ER up-regulation in some tissues (liver, Barton & Shapiro 1988; prostate epithelium, Prins & Birch 1997; uterus, Zou & Ing 1998; bone, Zhou et al. 2001b; thyroid, Banu et al. 2002). Considering that in most of these tissues ERα expression is usually low, it is possible that the difference between ERα expression in response to estrogen may be determined by the relative levels of expression of this receptor. In sites where ERα is abundant, such as the efferent ductule, it appears that down-regulation of ERα is the mechanism responsible for regulating the duration of physiological response to the activating ligand. On the other hand, in sites with low abundance or no ERα expression there is a likelihood that estrogens will up-regulate ERα.
Differing from AR and ERα, there was no dramatic effect on the ERβ expression in efferent ductules after any treatment presently investigated. Similar to the present result, ERβ immunoreaction was not affected by androgen/estrogen withdrawal or by steroid replacement in rat testis and prostate (Turner et al. 2001). Also, exposure of rats to the phytoestrogen genistein (Cotroneo et al. 2001) and diethylstilbestrol (Atanassova et al. 2001, McKinnell et al. 2001) caused an alteration in ERα expression, but not ERβ, in the female and male tract respectively. However, in contrast to the efferent ductules, ERβ mRNA may be regulated by androgens in the prostate. Castration down-regulates ERβ mRNA in the prostate, and testosterone restores it (Prins et al. 1998, 2001, Shughrue et al. 1998).
The antiestrogen ICI 182,780 also has no effect on ERβ, although it leads to a dramatic down-regulation of ERα in the rat efferent ductules (Oliveira et al. 2003). In agreement with these results, target disruption of ERβ has failed to cause major abnormalities in the male reproductive tract (Krege et al. 1998). Given the widespread expression of ERβ along the male reproductive tract, this lack of response to ligation, castration or hormonal modulation may be indicative that ERβ has a constitutive expression and a non-essential function in the male tract. Another possibility is that the factor that regulates ERβ is most likely of extra-testicular origin. A plausible candidate for regulation of ERβ in the efferent ductules would be luteinizing hormone (LH). It has already been shown that LH receptors (LHR) are present in the male tract (Derecka et al. 1999), and that there are correlations between ERβ and LH/LHR concentrations (Bao et al. 2000, Guo et al. 2001).
Using a similar experimental approach to the present one, Goyal et al. (1998) did not find any difference in the regulation of AR and ER in the efferent ductule of the goat. In the goat, both receptors appeared to be regulated by circulating androgens. However, it is necessary to highlight the fact that ER subtypes were not differentiated in that study, nor was the regulatory effect of estrogen and DHT investigated. In addition, other studies have shown differences in sex steroid receptor expression and steroid hormone action along the female reproductive tract between ruminants and other mammals (Miller et al. 1979, Stone et al. 1982), pointing to species differences in ER regulation. Differences in ER distribution in the male tract of rat and goat can be exemplified by the negative staining of goat efferent ductule ciliated cells and Leydig cells in the testis, both of them being positive for ERs in the rat. Confirming that diverging results may be attributed to species differences, to date the only species showing agonist uterotropic action for the antiestrogen ICI 182,780 is the ewe (al-Matubsi et al. 1998), in contrast to rat, mice, pig, monkey and human (Wakeling et al. 1991, Dukes et al. 1993, Thomas et al. 1994, Branham et al. 1996, Tarleton et al. 1999).
In conclusion, the present observations indicate that ERα, ERβ and AR are differentially regulated in the efferent ductule, where AR and ERα are selectively modulated by their own ligand, but ERβ appears to be constitutively expressed. The potential for multiple factors to be regulating the expression of several steroid hormone receptors in some cells, highlights the importance of maintaining a physiological androgen and estrogen balance to regulate the structure and function of efferent ductules in the male.
Acknowledgments
This work was supported in part by NIH Grant HD-35126 (to R A H) and by a grant from the CONRAD Program (to R A H).
References
- Agarwal VR, Sinton CM, Liang C, Fisher C, German DC, Simpson ER. Upregulation of estrogen receptors in the forebrain of aromatase knockout (ARKO) mice. Molecular and Cellular Endocrinology. 2000;162:9–16. doi: 10.1016/s0303-7207(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Williams K, Turner KJ, Fisher JS, Saunders PT, Millar MR, Sharpe RM. Age-, cell- and region-specific immunoexpression of estrogen receptor alpha (but not estrogen receptor beta) during postnatal development of the epididymis and vas deferens of the rat and disruption of this pattern by neonatal treatment with diethylstilbestrol. Endocrinology. 2001;142:874–886. doi: 10.1210/endo.142.2.7978. [DOI] [PubMed] [Google Scholar]
- Banu SK, Govindarajulu P, Aruldhas MM. Testosterone and estradiol up-regulate androgen and estrogen receptors in immature and adult rat thyroid glands in vivo. Steroids. 2002;67:1007–1014. doi: 10.1016/s0039-128x(02)00063-6. [DOI] [PubMed] [Google Scholar]
- Bao B, Kumar N, Karp RM, Garverick HA, Sundaram K. Estrogen receptor-beta expression in relation to the expression of luteinizing hormone receptor and cytochrome p450 enzymes in rat ovarian follicles. Biology of Reproduction. 2000;63:1747–1755. doi: 10.1095/biolreprod63.6.1747. [DOI] [PubMed] [Google Scholar]
- Barton MC, Shapiro DJ. Transient administration of estradiol-17 beta establishes an autoregulatory loop permanently inducing estrogen receptor mRNA. PNAS. 1988;85:7119–7123. doi: 10.1073/pnas.85.19.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branham WS, Fishman R, Streck RD, Medlock KL, De George JJ, Sheehan DM. ICI 182,780 inhibits endogenous estrogen-dependent rat uterine growth and tamoxifen-induced developmental toxicity. Biology of Reproduction. 1996;54:160–167. doi: 10.1095/biolreprod54.1.160. [DOI] [PubMed] [Google Scholar]
- Calandra RS, Blaquier JA, del Castillo EJ, Rivarola MA. Androgen dependency of the androgen receptor in rat epididymis. Biochemical and Biophysical Research Communications. 1975;67:97–102. doi: 10.1016/0006-291x(75)90288-0. [DOI] [PubMed] [Google Scholar]
- Cho HW, Nie R, Carnes K, Zhou Q, Sharief NA, Hess RA. The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reproductive Biology and Endocrinology. 2003;1:57. doi: 10.1186/1477-7827-1-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotroneo MS, Wang J, Eltoum IA, Lamartiniere CA. Sex steroid receptor regulation by genistein in the prepubertal rat uterus. Molecular and Cellular Endocrinology. 2001;173:135–145. doi: 10.1016/s0303-7207(00)00405-6. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Exploring the role of sex steroids through studies of receptor deficient mice. Journal of Molecular Medicine. 1998;76:497–511. doi: 10.1007/s001090050244. [DOI] [PubMed] [Google Scholar]
- Danzo BJ, Cooper TG, Orgebin-Crist MC. Androgen binding protein (ABP) in fluids collected from the rete testis and cauda epididymidis of sexually mature and immature rabbits and observations on morphological changes in the epididymis following ligation of the ductuli efferentes. Biology of Reproduction. 1977;17:64–77. doi: 10.1095/biolreprod17.1.64. [DOI] [PubMed] [Google Scholar]
- Derecka K, Zhang FP, Ziecik AJ, Huhtaniemi I. Ontogeny of LH receptor gene expression in the pig reproductive tract. Journal of Reproduction and Fertility. 1999;115:365–372. doi: 10.1530/jrf.0.1150365. [DOI] [PubMed] [Google Scholar]
- Dohle GR, Smit M, Weber RF. Androgens and male fertility. World Journal of Urology. 2003;21:341–345. doi: 10.1007/s00345-003-0365-9. [DOI] [PubMed] [Google Scholar]
- Dukes M, Waterton JC, Wakeling AE. Antiuterotrophic effects of the pure antioestrogen ICI 182,780 in adult female monkeys (Macaca nemestrina): quantitative magnetic resonance imaging. Journal of Endocrinology. 1993;138:203–210. doi: 10.1677/joe.0.1380203. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Brooks AN, Cronin AS, Ford H, Kerr JB. Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology. 2000;141:2861–2869. doi: 10.1210/endo.141.8.7596. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Fan X, Robaire B. Orchidectomy induces a wave of apoptotic cell death in the epididymis. Endocrinology. 1998;139:2128–2136. doi: 10.1210/endo.139.4.5888. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Hoffer AP. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biology of Reproduction. 1979;20:162–181. doi: 10.1095/biolreprod20.2.162. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. Journal of Endocrinology. 1997;153:485–495. doi: 10.1677/joe.0.1530485. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Brown D, Sharpe RM. Effect of neonatal exposure to estrogenic compounds on development of the excurrent ducts of the rat testis through puberty to adulthood. Environmental Health Perspectives. 1999;107:397–405. doi: 10.1289/ehp.99107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cadavid N, Vernet D, Fuentes Navarro A, Rodriguez JA, Swerdloff RS, Rajfer J. Up-regulation of the levels of androgen receptor and its mRNA by androgens in smooth-muscle cells from rat penis. Molecular and Cellular Endocrinology. 1993;90:219–229. doi: 10.1016/0303-7207(93)90155-d. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Hrudka F. The resorptive activity in the bull efferent ductules - a morphological and experimental study. Andrologia. 1980;12:401–404. doi: 10.1111/j.1439-0272.1980.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Williams CS. The ductuli efferentes of the goat: a morphological study. Anatomical Record. 1988;220:58–67. doi: 10.1002/ar.1092200108. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Bartol FF, Wiley AA, Neff CW. Immunolocalization of receptors for androgen and estrogen in male caprine reproductive tissues: unique distribution of estrogen receptors in efferent ductule epithelium. Biology of Reproduction. 1997;56:90–101. doi: 10.1095/biolreprod56.1.90. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Bartol FF, Wiley AA, Khalil MK, Williams CS, Vig MM. Regulation of androgen and estrogen receptors in male excurrent ducts of the goat: an immunohistochemical study. Anatomical Record. 1998;250:164–171. doi: 10.1002/(SICI)1097-0185(199802)250:2<164::AID-AR6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Guo H, Cai CQ, Schroeder RA, Kuo PC. Osteopontin is a negative feedback regulator of nitric oxide synthesis in murine macrophages. Journal of Immunology. 2001;166:1079–1086. doi: 10.4049/jimmunol.166.2.1079. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Clulow J, Jones RC. Perturbation of fluid reabsorption in the ductuli efferentes testis of the rat by testosterone propionate, 17β-oestradiol 3-benzoate, flutamide and tamoxifen. International Journal of Andrology. 1997;20:265–273. doi: 10.1046/j.1365-2605.1997.00069.x. [DOI] [PubMed] [Google Scholar]
- Hess RA. The efferent ductules: structure and functions. In: Robaire B, Hinton B, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum; 2002. pp. 49–80. [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997a;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. Journal of Andrology. 1997b;18:602–611. [PubMed] [Google Scholar]
- Hess RA, Bunick D, Bahr J. Oestrogen, its receptors and function in the male reproductive tract - a review. Molecular and Cellular Endocrinology. 2001;178:29–38. doi: 10.1016/s0303-7207(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Hess RA, Zhou Q, Nie R. The role of estrogens in the endocrine and paracrine regulation of the efferent ductules, epididymis and vas deferens. In: Robaire B, Hinton B, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum; 2002. pp. 317–338. [Google Scholar]
- Hess RA. Estrogen in the adult male reproductive tract. A review. Reproductive Biology and Endocrinology. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilio KY, Hess RA. Structure and function of the ductuli efferentes: a review. Microscopic Research and Technology. 1994;29:432–467. doi: 10.1002/jemt.1070290604. [DOI] [PubMed] [Google Scholar]
- Jackson GL, Kuehl D, Rhim TJ. Testosterone inhibits gonadotropin-releasing hormone pulse frequency in the male sheep. Biology of Reproduction. 1991;45:188–194. doi: 10.1095/biolreprod45.1.188. [DOI] [PubMed] [Google Scholar]
- Janulis L, Hess RA, Bunick D, Nitta H, Janssen S, Asawa Y, Bahr JM. Mouse epididymal sperm contain active P450 aromatase which decreases as sperm traverse the epididymis. Journal of Andrology. 1996;17:111–116. [PubMed] [Google Scholar]
- Janulis L, Bahr JM, Hess RA, Janssen S, Osawa Y, Bunick D. Rat testicular germ cells and epididymal sperm contain active P450 aromatase. Journal of Andrology. 1998;19:65–71. [PubMed] [Google Scholar]
- Jeyaraj DA, Grossman G, Weaver C, Petrusz P. Dynamics of testicular germ cell proliferation in normal mice and transgenic mice overexpressing rat androgen-binding protein: a flow cytometric evaluation. Biology of Reproduction. 2002;66:877–885. doi: 10.1095/biolreprod66.4.877. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. PNAS. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129:3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biology of Reproduction. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- Lee KH, Finnigan-Bunick C, Bahr J, Bunick D. Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ERbeta. Biology of Reproduction. 2001;65:1534–1541. doi: 10.1095/biolreprod65.5.1534. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. PNAS. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CS, Story AJ. Dihydrotestosterone and estrogen regulation of rat brain androgen-receptor immunoreactivity. Physiology and Behavior. 2000;69:445–453. doi: 10.1016/s0031-9384(99)00257-7. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Atanassova N, Williams K, Fisher JS, Walker M, Turner KJ, Saunders TK, Sharpe RM. Suppression of androgen action and the induction of gross abnormalities of the reproductive tract in male rats treated neonatally with diethylstilbestrol. Journal of Andrology. 2001;22:323–338. [PubMed] [Google Scholar]
- Mansour MM, Machen MR, Tarleton BJ, Wiley AA, Wower J, Bartol FF, Goyal HO. Expression and molecular characterization of estrogen receptor alpha messenger RNA in male reproductive organs of adult goats. Biology of Reproduction. 2001;64:1432–1438. doi: 10.1095/biolreprod64.5.1432. [DOI] [PubMed] [Google Scholar]
- al-Matubsi HY, Fairclough RJ, Jenkin G. Oestrogenic effects of ICI 182,780, a putative anti-oestrogen, on the secretion of oxytocin and prostaglandin F2 alpha during oestrous cycle in the intact ewe. Animal Reproduction Science. 1998;51:81–96. doi: 10.1016/s0378-4320(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-src complex triggers prostate cancer cell proliferation. EMBO Journal. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BG, Wild J, Stone GM. Effects of progesterone on the oestrogen-stimulated uterus: a comparative study of the mouse, guinea pig, rabbit and sheep. Australian Journal of Biological Sciences. 1979;32:549–560. doi: 10.1071/bi9790549. [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. Journal of Clinical Endocrinology and Metabolism. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- Munell F, Suarez-Quian CA, Selva DM, Tirado OM, Reventos J. Androgen-binding protein and reproduction: where do we stand? Journal of Andrology. 2002;23:598–609. [PubMed] [Google Scholar]
- Nie R, Zhou Q, Jassim E, Saunders PT, Hess RA. Differential expression of estrogen receptors alpha and beta in the reproductive tracts of adult male dogs and cats. Biology of Reproduction. 2002;66:1161–1168. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Bogh IB, Schmidt M, Greve T. Immunohistochemical localization of estrogen receptor-alpha in sex ducts and gonads of newborn piglets. Histochemistry and Cell Biology. 2001;115:521–526. doi: 10.1007/s004180100269. [DOI] [PubMed] [Google Scholar]
- Ochiai I, Matsuda KI, Nishi M, Ozawa H, Kawata M. Imaging analysis of subcellular correlation of androgen receptor and estrogen receptor alpha in single living cells using GFP color variants. Molecular Endocrinology. 2004;18:26–42. doi: 10.1210/me.2002-0262. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hess RA. Infertility and testicular atrophy in the antiestrogen-treated adult male rat. Biology of Reproduction. 2001;65:913–920. doi: 10.1095/biolreprod65.3.913. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Zhou Q, Carnes K, Nie R, Kuehl DE, Jackson GL, Franca LR, Nakai M, Hess RA. ER function in the adult male rat: short- and long-term effects of the antiestrogen ICI 182,780 on the testis and efferent ductules, without changes in testosterone. Endocrinology. 2002;143:2399–2409. doi: 10.1210/endo.143.6.8873. [DOI] [PubMed] [Google Scholar]
- Oliveira C, Nie R, Carnes K, Franca LR, Prins GS, Saunders PTK, Hess RA. The antiestrogen ICI 182,780 decreases the expression of estrogen receptor-alpha but has no effect on estrogen receptor-beta and androgen receptor in rat efferent ductules. Reproductive Biology and Endocrinology. 2003;1:75. doi: 10.1186/1477-7827-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Molecular and Cellular Endocrinology. 2000;167:139–150. doi: 10.1016/s0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- Podesta EJ, Calandra RS, Rivarola MA, Blaquier JA. The effect of castration and testosterone replacement on specific proteins and androgen levels of the rat epididymis. Endocrinology. 1975;97:399–405. doi: 10.1210/endo-97-2-399. [DOI] [PubMed] [Google Scholar]
- Preisler-Mashek MT, Solodin N, Stark BL, Tyriver MK, Alarid ET. Ligand-specific regulation of proteasome-mediated proteolysis of estrogen receptor-alpha. American Journal of Physiology, Endocrinology and Metabolism. 2002;282:E891–E898. doi: 10.1152/ajpendo.00353.2001. [DOI] [PubMed] [Google Scholar]
- Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Prins G, Birch L, Greene G. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson JA, Birch L. Estrogen receptor-beta messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Research. 2001;61:6089–6097. [PubMed] [Google Scholar]
- Quarmby VE, Yarbrough WG, Lubahn DB, French FS, Wilson EM. Autologous down-regulation of androgen receptor messenger ribonucleic acid. Molecular Endocrinology. 1990;4:22–28. doi: 10.1210/mend-4-1-22. [DOI] [PubMed] [Google Scholar]
- Rivas A, Fisher JS, Mckinnell C, Atanassova N, Sharpe RM. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol: evidence for importance of the androgen-estrogen balance. Endocrinology. 2002;143:4797–4808. doi: 10.1210/en.2002-220531. [DOI] [PubMed] [Google Scholar]
- Rivas A, Mckinnell C, Fisher JS, Atanassova N, Williams K, Sharpe RM. Neonatal co-administration of testosterone with diethylstilbestrol prevents diethylstilbestrol induction of most reproductive tract abnormalities in male rats. Journal of Andrology. 2003;24:557–567. doi: 10.1002/j.1939-4640.2003.tb02707.x. [DOI] [PubMed] [Google Scholar]
- Robaire B, Viger RS. Regulation of epididymal epithelial cell functions. Biology of Reproduction. 1995;52:226–236. doi: 10.1095/biolreprod52.2.226. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. PNAS. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Androgens regulate brain aromatase activity in adult male rats through a receptor mechanism. Endocrinology. 1984;114:2183–2189. doi: 10.1210/endo-114-6-2183. [DOI] [PubMed] [Google Scholar]
- Roselli CE, West NB, Brenner RM. Androgen receptor and 5 alpha-reductase activity in the ductuli efferentes and epididymis of adult rhesus macaques. Biology of Reproduction. 1991;44:739–745. doi: 10.1095/biolreprod44.4.739. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Ganjam VK, Taylor JA, Yuan X, Stiehr JR, Hardy MP, Lubahn DB. Transcription and translation of estrogen receptor-beta in the male reproductive tract of estrogen receptor-alpha knock-out and wild-type mice. Endocrinology. 1998;139:2982–2987. doi: 10.1210/endo.139.6.6028. [DOI] [PubMed] [Google Scholar]
- Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Androgens, estrogens, and second messengers. Steroids. 1998;63:278–281. doi: 10.1016/s0039-128x(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127:3180–3186. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Millar MR, Williams K, Macpherson S, Harkiss D, Anderson RA, Orr B, Groome NP, Scobie G, Fraser HM. Differential expression of estrogen receptor-alpha and -beta and androgen receptor in the ovaries of marmosets and humans. Biology of Reproduction. 2000;63:1098–1105. doi: 10.1095/biolreprod63.4.1098. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Sharpe RM, Williams K, Macpherson S, Urquart H, Irvine DS, Millar MR. Differential expression of oestrogen receptor alpha and beta proteins in the testes and male reproductive system of human and non-human primates. Molecular Human Reproduction. 2001;7:227–236. doi: 10.1093/molehr/7.3.227. [DOI] [PubMed] [Google Scholar]
- Schleicher G, Drews U, Stumpf WE, Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry. 1984;81:139–147. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and -beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New England Journal of Medicine. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Stone GM, McCaffery C, Miller BG. Effects of progesterone on nuclear and cytosol steroid receptor levels in the oestrogen-stimulated uterus: comparison of the sheep and mouse. Australian Journal of Biological Sciences. 1982;35:403–415. [PubMed] [Google Scholar]
- Tarleton BJ, Wiley AA, Bartol FF. Endometrial development and adenogenesis in the neonatal pig: effects of estradiol valerate and the antiestrogen ICI 182,780. Biology of Reproduction. 1999;61:253–263. doi: 10.1095/biolreprod61.1.253. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Navarro J, Pinilla L, Gonzalez LC, Huhtaniemi I, Aguilar E. Neonatal exposure to estrogen differentially alters estrogen receptor alpha and beta mRNA expression in rat testis during postnatal development. Journal of Endocrinology. 2000;165:345–357. doi: 10.1677/joe.0.1650345. [DOI] [PubMed] [Google Scholar]
- Thomas EJ, Walton PL, Thomas NM, Dowsett M. The effects of ICI 182,780, a pure anti-oestrogen, on the hypothalamic-pituitary-gonadal axis and on endometrial proliferation in pre-menopausal women. Human Reproduction. 1994;9:1991–1996. doi: 10.1093/oxfordjournals.humrep.a138380. [DOI] [PubMed] [Google Scholar]
- Tirado OM, Selva DM, Toran N, Suarez-Quian CA, Jansen M, McDonnell DP, Reventos J, Munell F. Increased expression of estrogen receptor beta in pachytene spermatocytes after short-term methoxyacetic acid administration. Journal of Andrology. 2004;25:84–94. doi: 10.1002/j.1939-4640.2004.tb02762.x. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Morley M, Atanassova N, Swanston ID, Sharpe RM. Effect of chronic administration of an aromatase inhibitor to adult male rats on pituitary and testicular function and fertility. Journal of Endocrinology. 2000;164:225–238. doi: 10.1677/joe.0.1640225. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Morley M, MacPherson S, Millar MR, Wilson JA, Sharpe RM, Saunders PT. Modulation of gene expression by androgen and oestrogens in the testis and prostate of the adult rat following androgen withdrawal. Molecular and Cellular Endocrinology. 2001;178:73–87. doi: 10.1016/s0303-7207(01)00413-0. [DOI] [PubMed] [Google Scholar]
- Vreeburg JTM. Distribution of testosterone and 5α-dihydrotestosterone in rat epididymis and their concentrations in efferent duct fluid. Journal of Endocrinology. 1975;67:203–210. doi: 10.1677/joe.0.0670203. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Bowler J, Wakeling AE, Dukes M, Bowler J. Development of novel oestrogen-receptor antagonists. A potent, specific, pure antiestrogen with clinical potential. Biochemical Society Transactions. 1991;19:899–901. doi: 10.1042/bst0190899. [DOI] [PubMed] [Google Scholar]
- Wiszniewska B. Primary culture of the rat epididymal epithelial cells as a source of oestrogen. Andrologia. 2002;34:180–187. doi: 10.1046/j.1439-0272.2002.00495.x. [DOI] [PubMed] [Google Scholar]
- Woodham C, Birch L, Prins GS. Neonatal estrogen down-regulates prostatic androgen receptor through a proteosome-mediated protein degradation pathway. Endocrinology. 2003;144:4841–4850. doi: 10.1210/en.2003-0035. [DOI] [PubMed] [Google Scholar]
- Yeap BB, Krueger RG, Leedman PJ. Differential posttranscriptional regulation of androgen receptor gene expression by androgen in prostate and breast cancer cells. Endocrinology. 1999;140:3282–3291. doi: 10.1210/endo.140.7.6769. [DOI] [PubMed] [Google Scholar]
- Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: a new pathway for sex hormones in prostate. PNAS. 1998;95:5527–5532. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. PNAS. 2001a;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Zilberman Y, Wassermann K, Bain SD, Sadovsky Y, Gazit D. Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCS) of osteoporotic mice. Journal of Cellular Biochemistry. Supplement. 2001b;36:144–155. doi: 10.1002/jcb.1096. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. Journal of Andrology. 2002;23:870–881. [PubMed] [Google Scholar]
- Zhu LJ, Hardy MP, Inigo IV, Huhtaniemi I, Bardin CW, Moo-Young AJ. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: a quantitative immunohistochemical study. Biology of Reproduction. 2000;63:368–376. doi: 10.1095/biolreprod63.2.368. [DOI] [PubMed] [Google Scholar]
- Zou K, Ing NH. Oestradiol up-regulates oestrogen receptor, cyclophilin, and glyceraldehyde phosphate dehydrogenase mRNA concentrations in endometrium, but down-regulates them in liver. Journal of Steroid Biochemistry and Molecular Biology. 1998;64:231–237. doi: 10.1016/s0960-0760(97)00194-5. [DOI] [PubMed] [Google Scholar]