Abstract
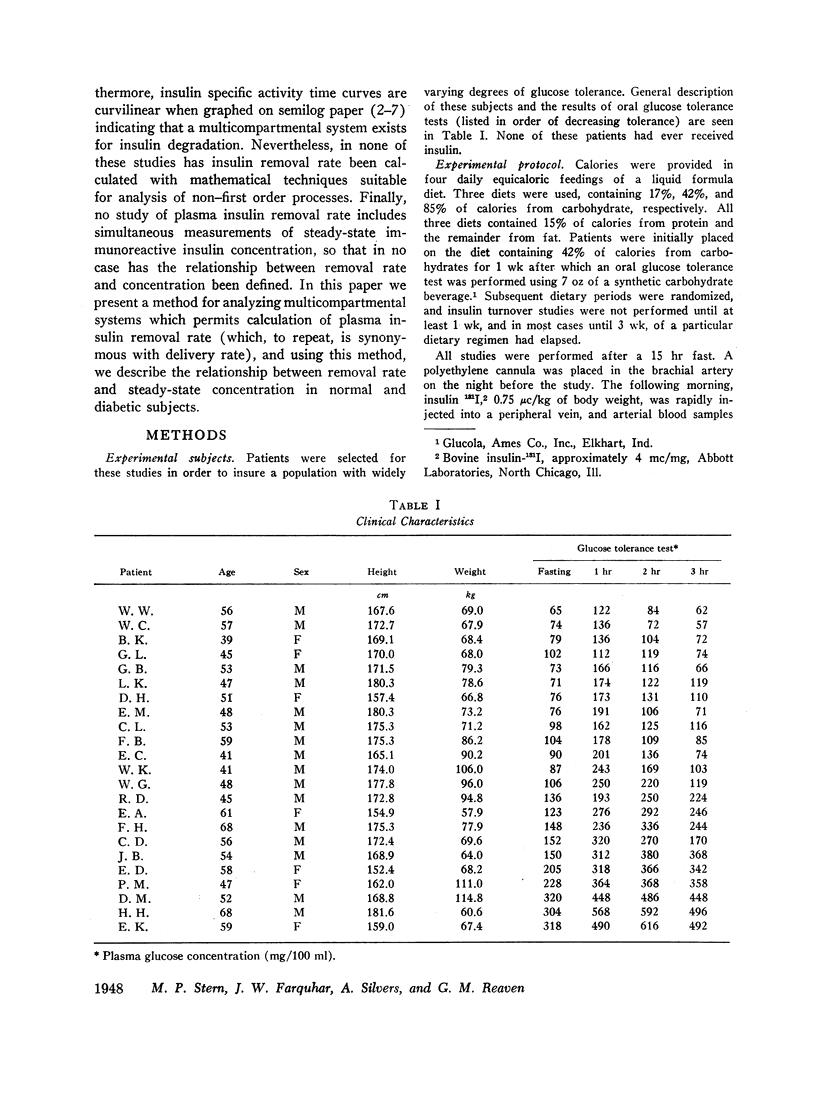
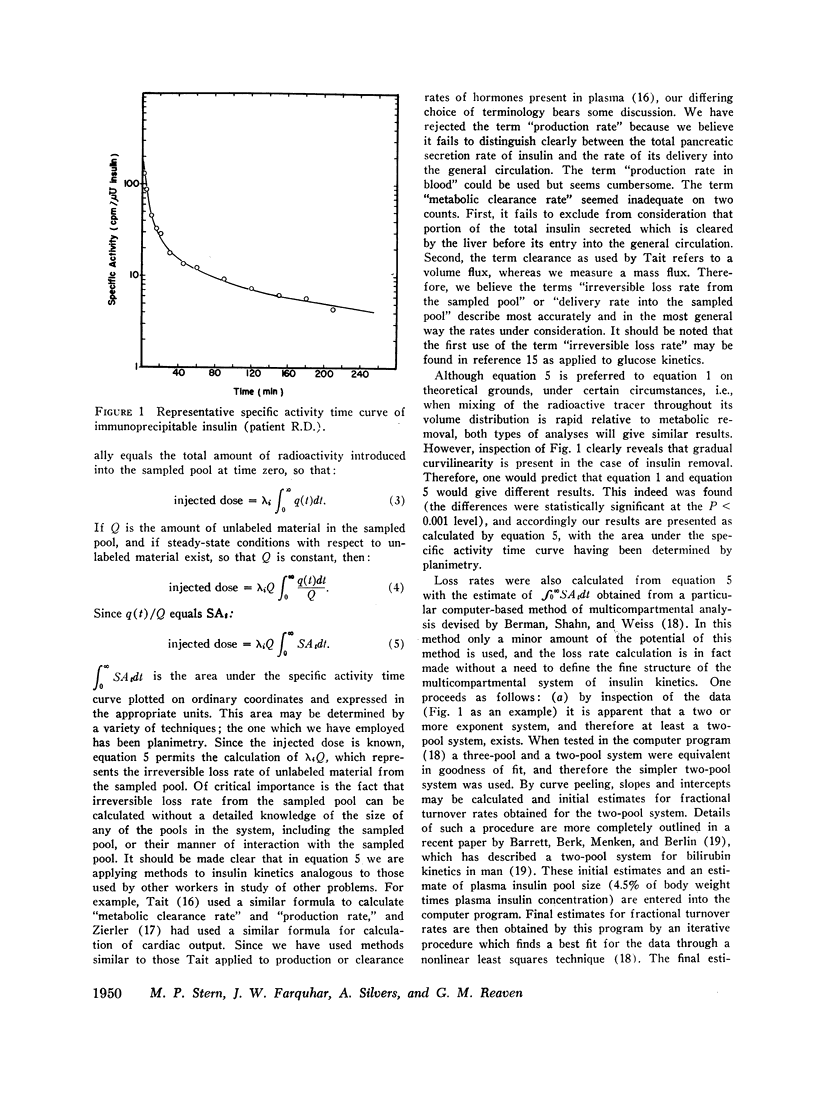
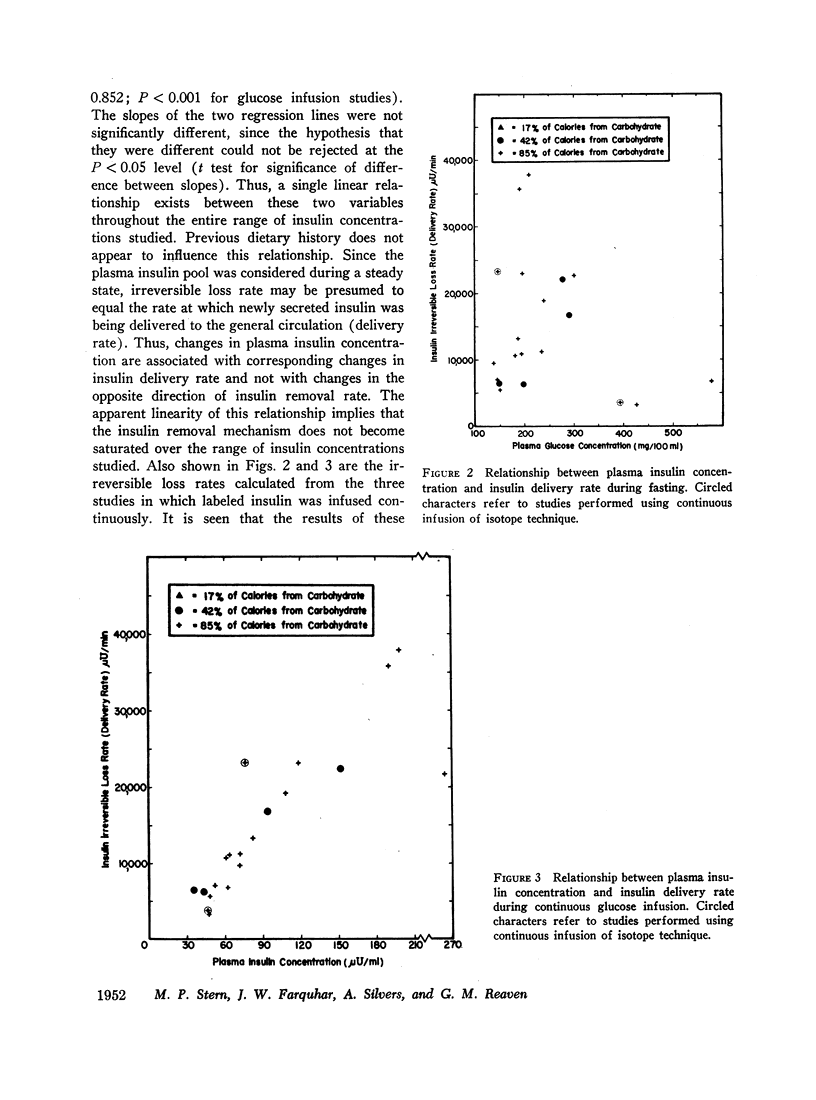
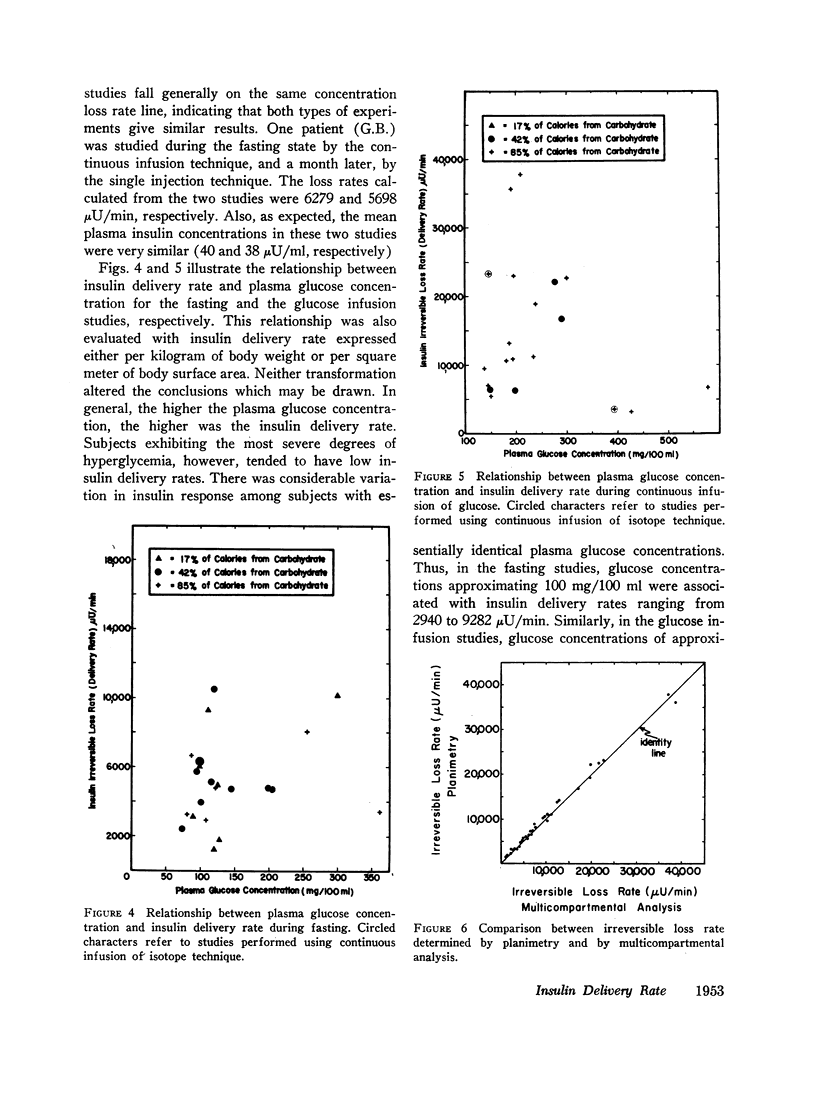
Removal of insulin-131I from plasma was studied in normal and diabetic subjects with both single injection and continuous infusion of isotope techniques. Patients were studied either in the fasting state or during steady-state hyperglycemia produced by a continuous intravenous glucose infusion. Steady-state plasma insulin concentration during these studies ranged from 10 to 264 μU/ml. Labeled insulin specific activity time curves consisted of more than one exponential, indicating that a multicompartmental system for insulin metabolism exists. A mathematical technique which is applicable to non-first order processes was used to calculate the rate at which insulin was lost irreversibly from the plasma insulin pool. A direct, linear relationship was found between insulin irreversible loss rate and plasma insulin concentration over the range of concentrations studied. This linearity implies lack of saturability of the insulin removal mechanism. Since the plasma insulin pool was in a steady state during these studies, insulin irreversible loss rate was equal to the rate at which newly secreted insulin was being delivered to the general circulation. Therefore, these results indicate that changes in plasma insulin concentration result from parallel changes in the rate of insulin delivery and not from changes in the opposite direction of the rate of insulin removal. A wide range of insulin delivery rates was found among patients with similar plasma glucose concentrations, suggesting that there exists considerable variability in responsiveness to endogenous insulin among these patients.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arquilla E. R., Ooms H., Finn J. Genetic differences of combining sites of insulin antibodies and importance of C-terminal portion of the A chain to biological and immunological activity of insulin. Diabetologia. 1966 Jun;2(1):1–13. doi: 10.1007/BF01106969. [DOI] [PubMed] [Google Scholar]
- BAKER N., SHIPLEY R. A., CLARK R. E., INCEFY G. E. C14 studies in carbohydrate metabolism: glucose pool size and rate of turnover in the normal rat. Am J Physiol. 1959 Feb;196(2):245–252. doi: 10.1152/ajplegacy.1959.196.2.245. [DOI] [PubMed] [Google Scholar]
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERSON S. A., YALOW R. S., BAUMAN A., ROTHSCHILD M. A., NEWERLY K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J Clin Invest. 1956 Feb;35(2):170–190. doi: 10.1172/JCI103262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLINGER R. E., GRADY H. J. The plasma insulin-I131 in diabetic patients. Ann Intern Med. 1958 Apr;48(4):753–764. doi: 10.7326/0003-4819-48-4-753. [DOI] [PubMed] [Google Scholar]
- BOLINGER R. E., STEPHENS R. R. COMPARISON OF DISAPPEARANCE FROM PLASMA OF INSULIN I-131 AND OF INSULIN-LIKE ACTIVITY. Proc Soc Exp Biol Med. 1964 Jul;116:812–813. doi: 10.3181/00379727-116-29381. [DOI] [PubMed] [Google Scholar]
- Barrett V., Berk P. D., Menken M., Berlin N. I. Bilirubin turnover studies in normal and pathologic states using bilirubin-14C. Ann Intern Med. 1968 Feb;68(2):355–377. doi: 10.7326/0003-4819-68-2-355. [DOI] [PubMed] [Google Scholar]
- Beck L. V., Roberts N., Blankenbaker R., King C. On insulin immunologic activity in livers and kidneys of mice injected with beef insulin. Proc Soc Exp Biol Med. 1966 Jul;122(3):768–774. doi: 10.3181/00379727-122-31248. [DOI] [PubMed] [Google Scholar]
- COX R. W., HENLEY E. D., WELSH G. W., 3rd, WILLIAMS R. H. Insulin I-131 metabolism in man; plasma-binding, distribution and degradation. Am J Med. 1956 Sep;21(3):324–338. doi: 10.1016/0002-9343(56)90034-1. [DOI] [PubMed] [Google Scholar]
- ELGEE N. J., WILLIAMS R. H. Specificity of insulin degradation reaction. Diabetes. 1955 Mar-Apr;4(2):87–91. doi: 10.2337/diab.4.2.87. [DOI] [PubMed] [Google Scholar]
- GRODSKY G. M., FORSHAM P. H. An immunochemical assay of total extractable insulin in man. J Clin Invest. 1960 Jul;39:1070–1079. doi: 10.1172/JCI104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., FORSHAM P. H. Comparative binding of beef and human insulin to insulin antibodies produced in man and guinea pigs. J Clin Invest. 1961 May;40:799–802. doi: 10.1172/JCI104313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO J. L., RONCONE A., IZZO M. J., BALE W. F. RELATIONSHIP BETWEEN DEGREE OF IODINATION OF INSULIN AND ITS BIOLOGICAL, ELECTROPHORETIC, AND IMMUNOCHEMICAL PROPERTIES. J Biol Chem. 1964 Nov;239:3749–3754. [PubMed] [Google Scholar]
- LEE N. D. Studies on insulin labeled with I 131. Ann N Y Acad Sci. 1957 Aug 30;70(1):94–108. doi: 10.1111/j.1749-6632.1957.tb35381.x. [DOI] [PubMed] [Google Scholar]
- O'Brien J. P., Sharpe A. R., Jr The influence of renal disease on the insulin I-131 disappearance curve in man. Metabolism. 1967 Jan;16(1):76–83. doi: 10.1016/0026-0495(67)90161-8. [DOI] [PubMed] [Google Scholar]
- PALMER D. L., BOLINGER R. E. Effect of nephrectomy and splanchnicectomy on plasma disappearance of labeled insulin in the rabbit. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:809–812. doi: 10.3181/00379727-107-26763. [DOI] [PubMed] [Google Scholar]
- RICKETTS H. T., WILDBERGER H. L., REGUT L. The role of the kidney in the disposal of insulin in rats. Diabetes. 1963 Mar-Apr;12:155–161. doi: 10.2337/diab.12.2.155. [DOI] [PubMed] [Google Scholar]
- SAMOLS E., RYDER J. A. Studies on tissue uptake of insulin in man using a differential immunoassay for endogenous and exogenous insulin. J Clin Invest. 1961 Nov;40:2092–2102. doi: 10.1172/JCI104435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT G. W., PROUT T. E., WEAVER J. A., ASPER S. P. A comparison of the behavior of insulin and insulin labeled with I 131 in serum. Diabetes. 1958 Jan-Feb;7(1):38–45. doi: 10.2337/diab.7.1.38. [DOI] [PubMed] [Google Scholar]
- TAIT J. F. REVIEW: THE USE OF ISOTOPIC STEROIDS FOR THE MEASUREMENT OF PRODUCTION RATES IN VIVO. J Clin Endocrinol Metab. 1963 Dec;23:1285–1297. doi: 10.1210/jcem-23-12-1285. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA H. H., NUTLEY M. L., NARAHARA H. T., WILLIAMS R. H. The mode of inactivation of insulin by rat liver extracts. J Biol Chem. 1955 May;214(1):285–294. [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharko D. S., Beck L. V., Blankenbaker R. Role of the kidney in the disposal of radioiodinated and nonradioiodinated insulin in dogs. Diabetes. 1966 Sep;15(9):680–685. doi: 10.2337/diab.15.9.680. [DOI] [PubMed] [Google Scholar]



