Abstract
The way in which iron is handled by the duodenal mucosa, the reticuloendothelial system, the hepatic parenchymal cell, and the normoblast was investigated in copper-deficient swine.
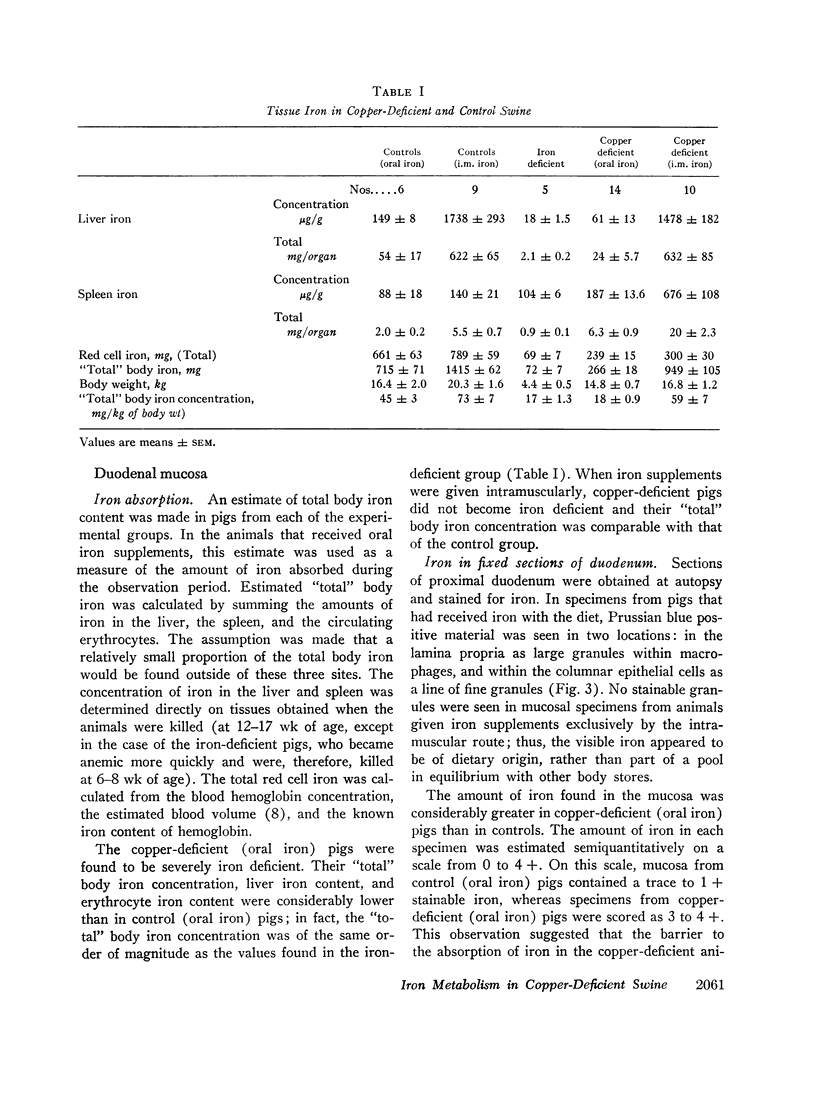
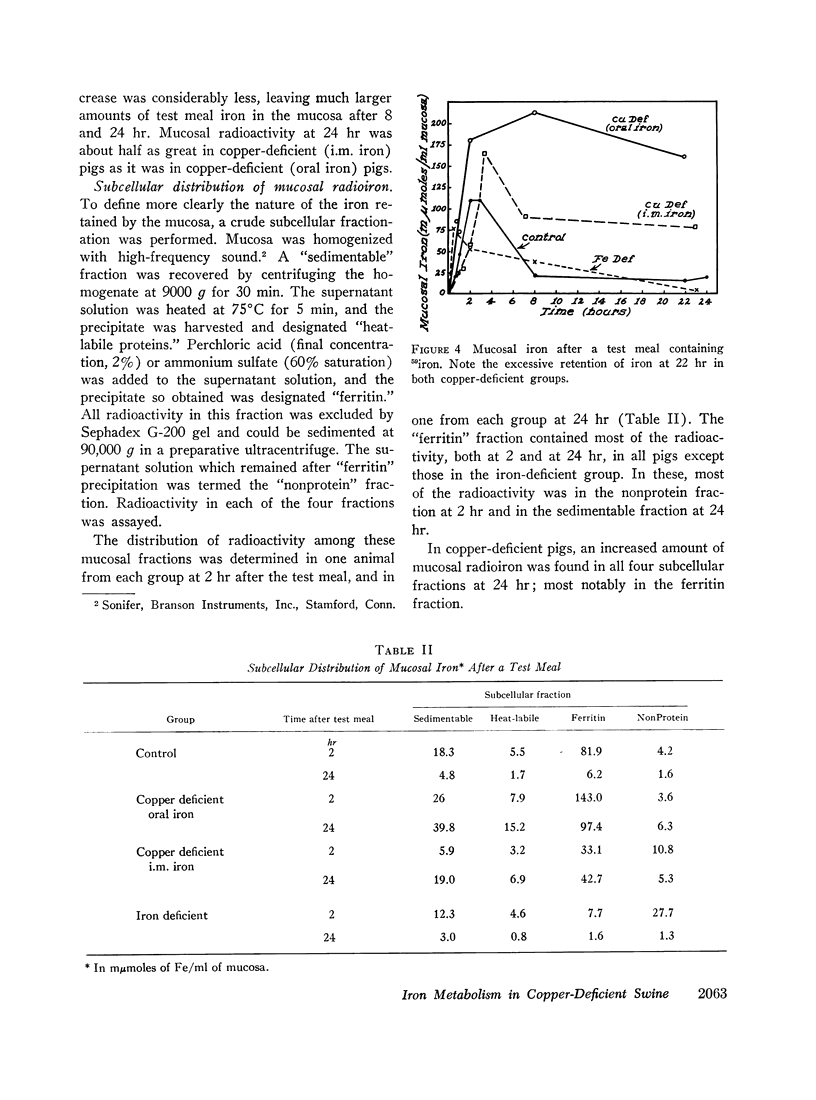
Copper-deficient swine failed to absorb dietary iron at the normal rate. Increased amounts of stainable iron were observed in fixed sections of duodenum from such animals. When 59iron was administered orally, the mucosa of copper-deficient animals extracted iron from the duodenal lumen at the normal rate, but the subsequent transfer to plasma was impaired.
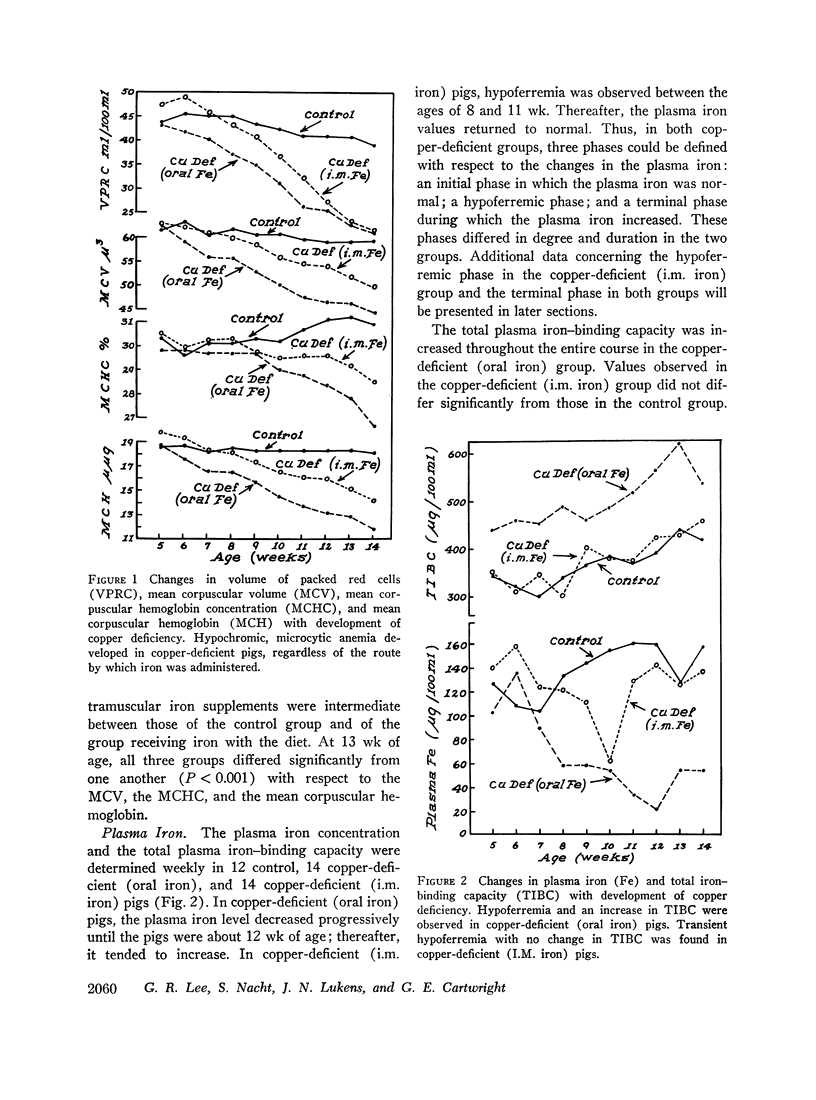
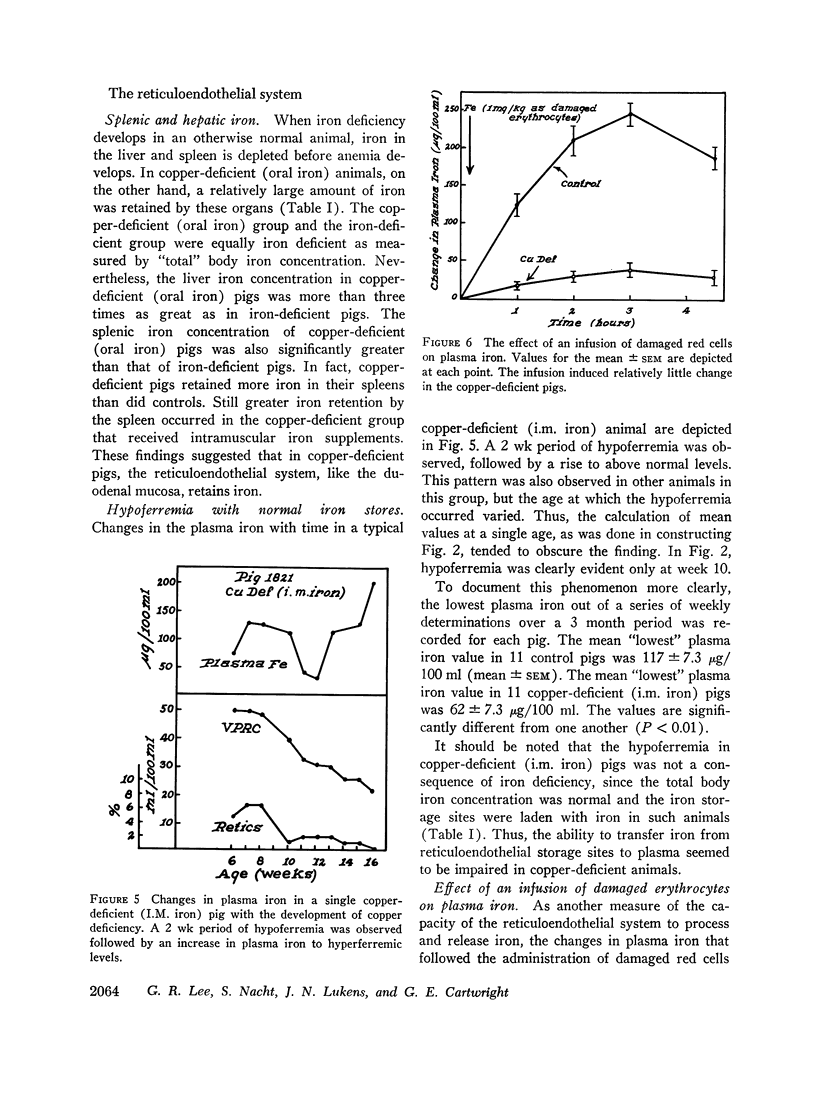
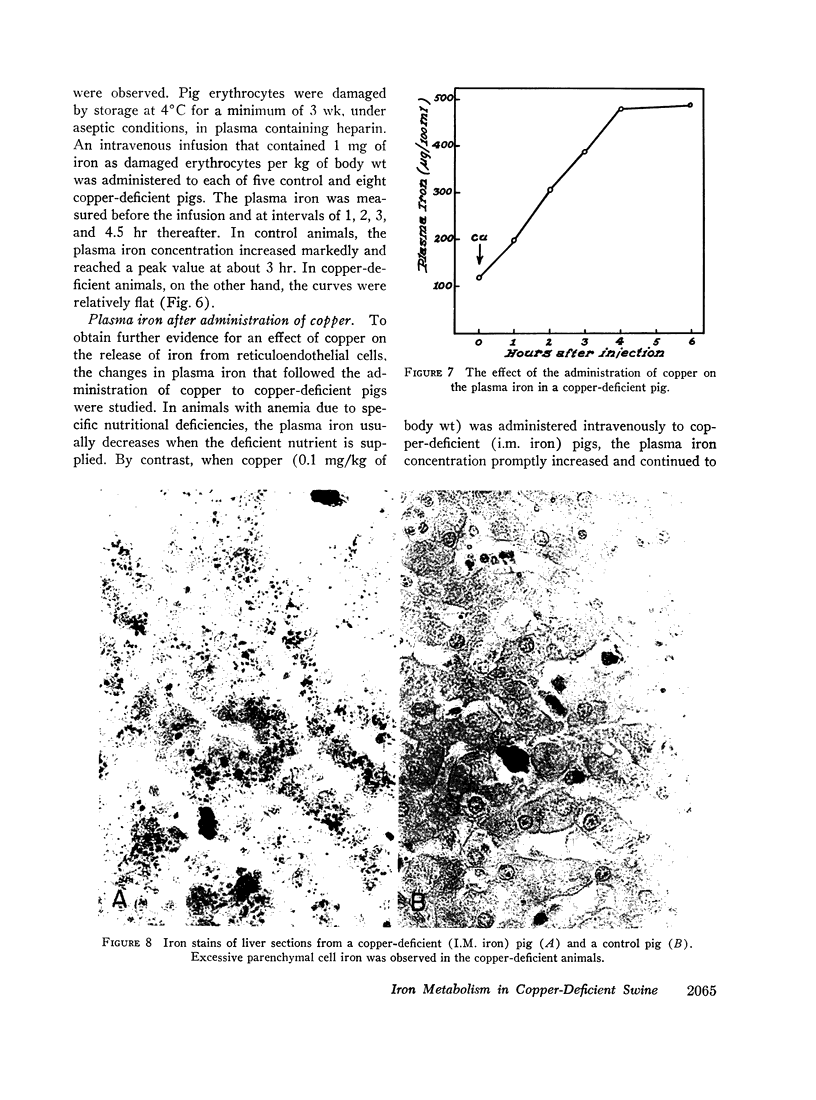
When intramuscular iron supplements were given to copper-deficient pigs, increased amounts of iron were found in the reticuloendothelial system, the hepatic parenchymal cells, and in normoblasts (sideroblasts). Hypoferremia was observed in the early stages of copper deficiency, even though iron stores were normal or increased. When red cells that were damaged by prolonged storage were administered, the reticuloendothelial system failed to extract and transfer the erythrocyte iron to the plasma at the normal rate. Administration of copper to copper-deficient animals with normal iron stores resulted in a prompt increase in the plasma iron.
The observed abnormalities in iron metabolism are best explained by an impaired ability of the duodenal mucosa, the reticuloendothelial system, and the hepatic parenchymal cell to release iron to the plasma. It is suggested that copper is essential to the normal release of iron from these tissues. This concept is compatible with the suggestion made by others that the transfer of iron from tissues to plasma requires the enzymatic oxidation of ferrous iron, and that the plasma copper protein, ceruloplasmin, is the enzyme (ferroxidase) which catalyzes the reaction.
Because excessive amounts of iron were found in normoblasts, it is suggested that an additional defect in iron metabolism affects these cells and plays a major role in the development of anemia. As a result of the proposed defect, iron cannot be incorporated into hemoglobin and, instead, accumulates as nonhemoglobin iron.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN D., SHOEMAKER W. C. METHOD FOR TISSUE HEMOGLOBIN ANALYSIS. Clin Chem. 1965 Mar;11:422–426. [PubMed] [Google Scholar]
- BAINTON D. F., FINCH C. A. THE DIAGNOSIS OF IRON DEFICIENCY ANEMIA. Am J Med. 1964 Jul;37:62–70. doi: 10.1016/0002-9343(64)90212-8. [DOI] [PubMed] [Google Scholar]
- BROWN E. B., ROTHER M. L. STUDIES OF THE MECHANISM OF IRON ABSORPTION. I. IRON UPTAKE BY THE NORMAL RAT. J Lab Clin Med. 1963 Sep;62:357–373. [PubMed] [Google Scholar]
- BUSH J. A., JENSEN W. N., ATHENS J. W., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Studies on copper metabolism. XIX. The kinetics of iron metabolism and erythrocyte life-span in copper-deficient swine. J Exp Med. 1956 May 1;103(5):701–712. doi: 10.1084/jem.103.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSH J. A., JENSEN W. N., CARTWRIGHT G. E., WINTROBE M. M. Blood volume studies in normal and anemic swine. Am J Physiol. 1955 Apr;181(1):9–14. doi: 10.1152/ajplegacy.1955.181.1.9. [DOI] [PubMed] [Google Scholar]
- CARTWRIGHT G. E., GUBLER C. J., BUSH J. A., WINTROBE M. M. Studies of copper metabolism. XVII. Further observations on the anemia of copper deficiency in swine. Blood. 1956 Feb;11(2):143–153. [PubMed] [Google Scholar]
- GUBLER C. J., LAHEY M. E., CHASE M. S., CARTWRIGHT G. E., WINTROBE M. M. Studies on copper metabolism. III. The metabolism of iron in copper deficient swine. Blood. 1952 Nov;7(11):1075–1092. [PubMed] [Google Scholar]
- HAWKINS C. F. Value of serum iron levels in assessing effect of haematinics in the macrocytic anaemias. Br Med J. 1955 Feb 12;1(4910):383–385. doi: 10.1136/bmj.1.4910.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAHEY M. E., GUBLER C. J., CHASE M. S., CARTWRIGHT G. E., WINTROBE M. M. Studies on copper metabolism. II. Hematologic manifestations of copper deficiency in swine. Blood. 1952 Nov;7(11):1053–1074. [PubMed] [Google Scholar]
- Lee G. R., Cartwright G. E., Wintrobe M. M. Heme biosynthesis in copper deficient swine. Proc Soc Exp Biol Med. 1968 Apr;127(4):977–981. doi: 10.3181/00379727-127-32848. [DOI] [PubMed] [Google Scholar]
- Marston H. R., Allen S. H. Function of copper in the metabolism of iron. Nature. 1967 Aug 5;215(5101):645–646. doi: 10.1038/215645a0. [DOI] [PubMed] [Google Scholar]
- NOYES W. D., BOTHWELL T. H., FINCH C. A. The role of the reticulo-endothelial cell in iron metabolism. Br J Haematol. 1960 Jan;6:43–55. doi: 10.1111/j.1365-2141.1960.tb06216.x. [DOI] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966 Jun 25;241(12):2746–2751. [PubMed] [Google Scholar]