Abstract
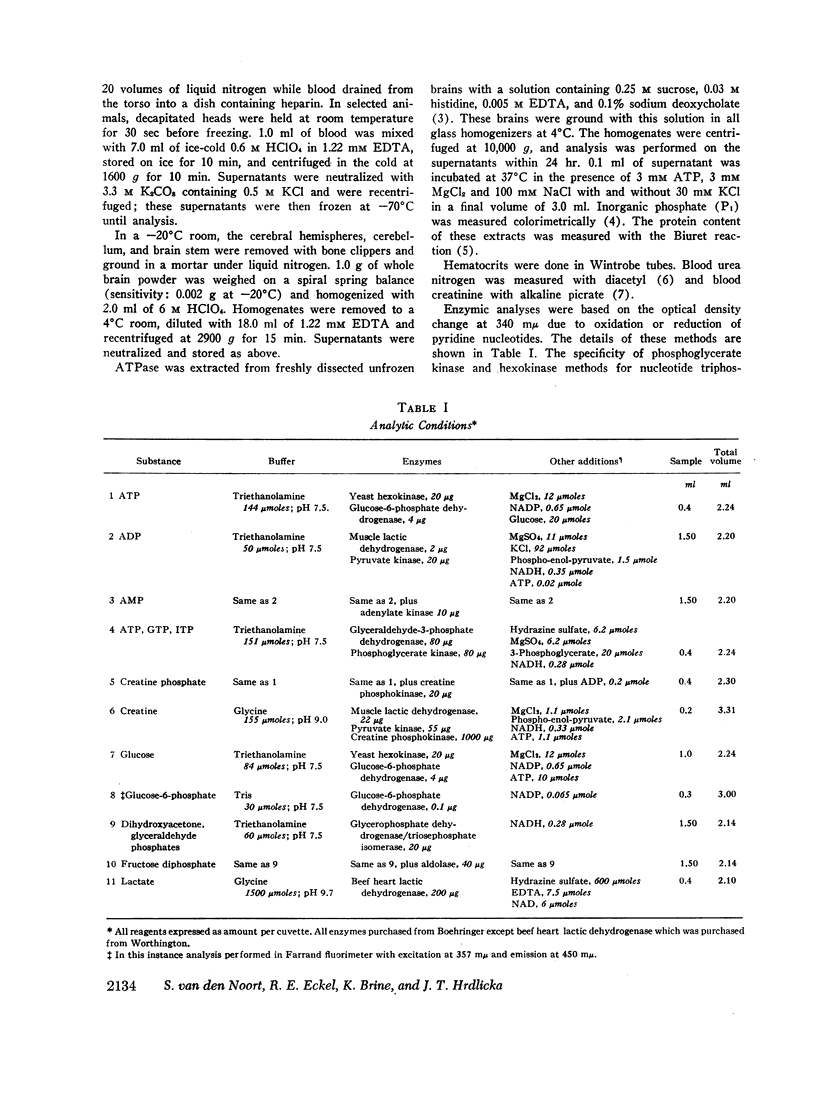
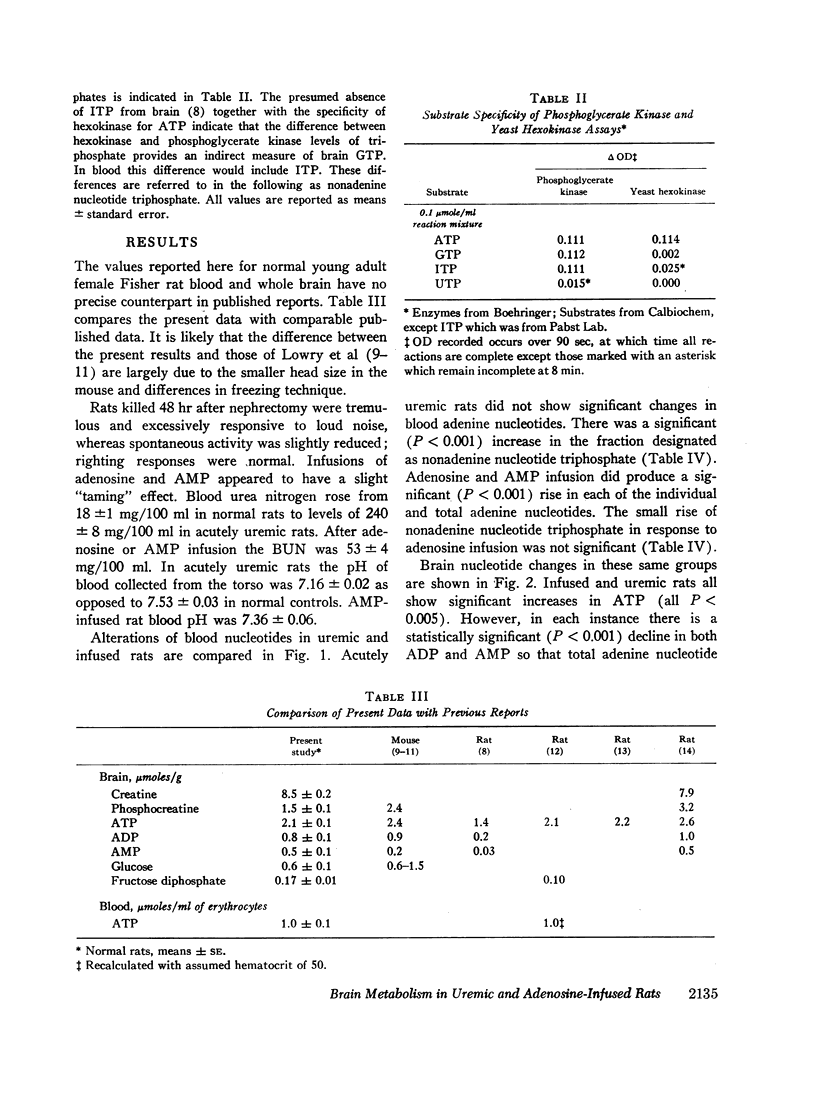
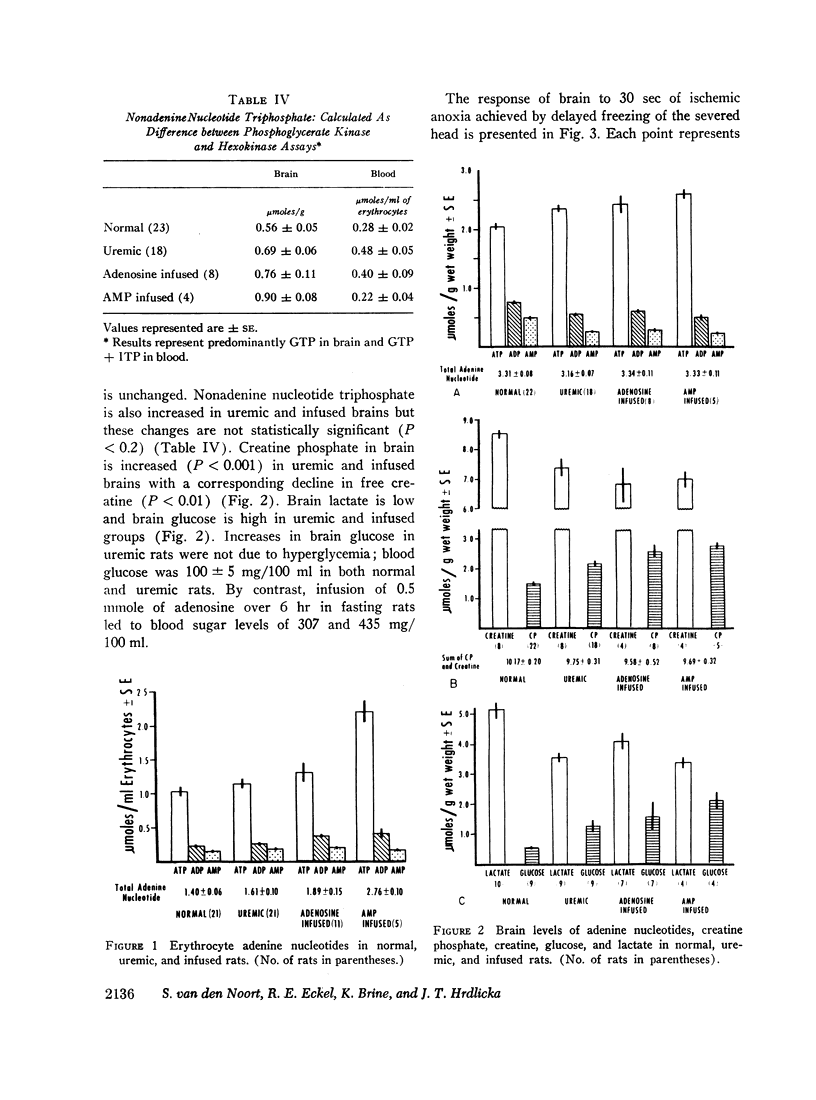
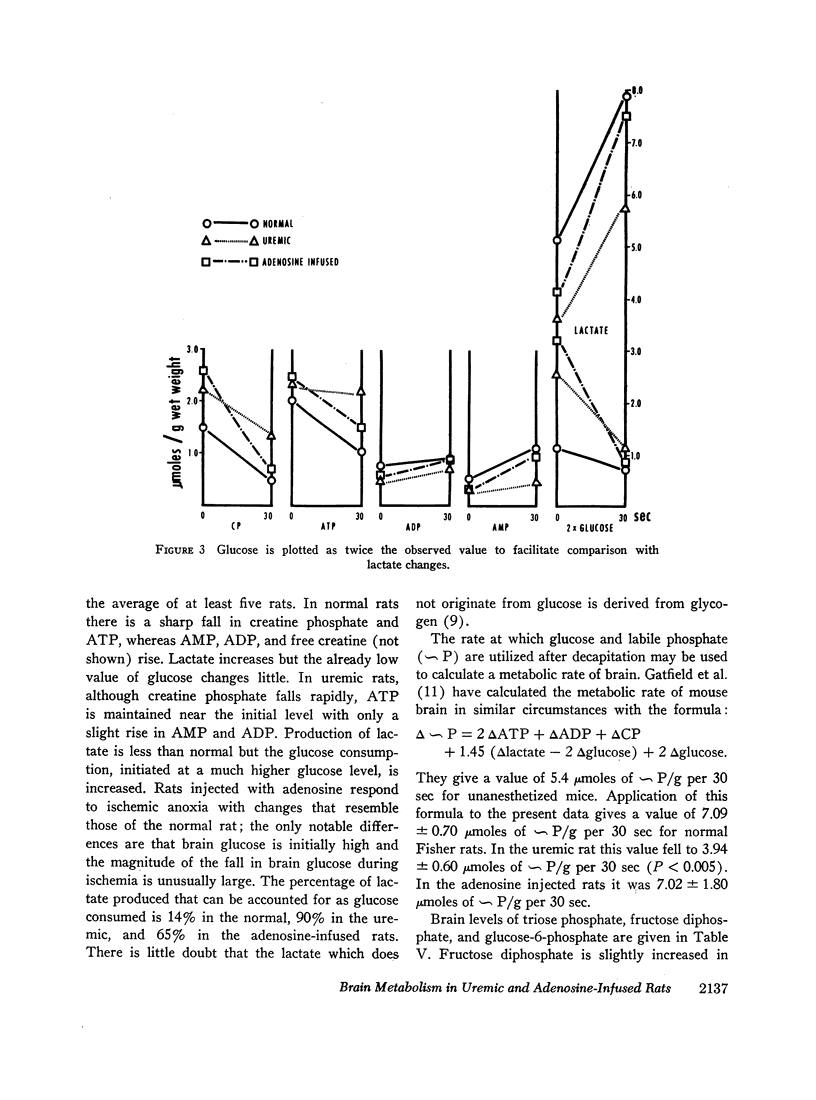
Analyses of nucleotides and glycolytic intermediates were performed on perchlorate extracts of blood and quick-frozen brain from rats nephrectomized 48 hr previously, and from rats infused for 6 hr with adenosine or AMP. Blood nucleotides of acutely uremic rats were normal. Uremic brain showed an increase of creatine phosphate (CP), ATP, and glucose with a corresponding decrease in creatine, ADP, AMP, and lactate. Other nucleotide triphosphates were increased, but total adenine nucleotide in brain was unchanged. Uremic brain failed to use ATP or produce ADP, AMP, and lactate at normal rates when subjected to the stress of ischemic anoxia. Although levels of cation responsive ATPase in extracts of uremic brain were normal, the inhibition of glycolysis in the intact brain appeared to be due to a failure of ATP hydrolysis (a diminished ATPase activity). Adenosine infusion produced mild azotemia, marked hyperglycemia, an increase in blood ATP, and an increase in total blood adenine nucleotide. Brain from rats infused with adenosine or AMP also had high levels of ATP, creatine phosphate, and glucose, whereas levels of ADP, AMP, and lactate were low. However these brains responded with normal use of ATP and normal production of lactate when stimulated by ischemic anoxia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Brod J., Sirota J. H. THE RENAL CLEARANCE OF ENDOGENOUS "CREATININE" IN MAN. J Clin Invest. 1948 Sep;27(5):645–654. doi: 10.1172/JCI102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T. C., Waddell W. J., Poole D. T. Intracellular pH based on the distribution of weak electrolytes. Fed Proc. 1967 Sep;26(5):1327–1332. [PubMed] [Google Scholar]
- GEY K. F., RUTISHAUSER M., PLETSCHER A. SUPPRESSION OF GLYCOLYSIS IN RAT BRAIN IN VIVO CHLORPROMAZINE, RESERPINE, AND PHENOBARBITAL. Biochem Pharmacol. 1965 Apr;14:507–514. doi: 10.1016/0006-2952(65)90223-6. [DOI] [PubMed] [Google Scholar]
- Gatfield P. D., Lowry O. H., Schulz D. W., Passonneau J. V. Regional energy reserves in mouse brain and changes with ischaemia and anaesthesia. J Neurochem. 1966 Mar;13(3):185–195. doi: 10.1111/j.1471-4159.1966.tb07512.x. [DOI] [PubMed] [Google Scholar]
- HEYMAN A., PATTERSON J. L., Jr, JONES R. W., Jr Cerebral circulation and metabolism in uremia. Circulation. 1951 Apr;3(4):558–563. doi: 10.1161/01.cir.3.4.558. [DOI] [PubMed] [Google Scholar]
- HURT G. A., CHANUTIN A. ORGANIC PHOSPHATE COMPOUNDS OF ERYTHROCYTES FROM INDIVIDUALS WITH UREMIA. J Lab Clin Med. 1964 Oct;64:675–684. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MANDEL P., HARTH S. Free nucleotides of the brain in various mammals. J Neurochem. 1961 Nov;8:116–125. doi: 10.1111/j.1471-4159.1961.tb13533.x. [DOI] [PubMed] [Google Scholar]
- MAYMAN C. I., GATFIELD P. D., BRECKENRIDGE B. M. THE GLUCOSE CONTENT OF BRAIN IN ANAESTHESIA. J Neurochem. 1964 Jun;11:483–487. doi: 10.1111/j.1471-4159.1964.tb11607.x. [DOI] [PubMed] [Google Scholar]
- MURPHY J. R. Erythrocyte metabolism. II. Glucose metabolism and pathways. J Lab Clin Med. 1960 Feb;55:286–302. [PubMed] [Google Scholar]
- PHILIPS F. S., THIERSCH J. B., BENDICH A. Adenine intoxication in relation to in vivo formation and deposition of 2,8-dioxyadenine in renal tubules. J Pharmacol Exp Ther. 1952 Jan;104(1):20–30. [PubMed] [Google Scholar]
- SCHENKER S., MENDELSON J. H. CEREBRAL ADENOSINE TRIPHOSPHATE IN RATS WITH AMMONIA-INDUCED COMA. Am J Physiol. 1964 May;206:1173–1176. doi: 10.1152/ajplegacy.1964.206.5.1173. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Preparation from mammallian brain and kidney of the enzyme system involved in active transport of Na ions and K ions. Biochim Biophys Acta. 1962 Apr 9;58:314–325. doi: 10.1016/0006-3002(62)91015-6. [DOI] [PubMed] [Google Scholar]
- SUGITA Y., SIMON E. R. THE MECHANISM OF ACTION OF ADENINE IN RED CELL PRESERVATION. J Clin Invest. 1965 Apr;44:629–642. doi: 10.1172/JCI105176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELT L. G., SACHS J. R., MCMANUS T. J. AN ION TRANSPORT DEFECT IN ERYTHROCYTES FROM UREMIC PATIENTS. Trans Assoc Am Physicians. 1964;77:169–181. [PubMed] [Google Scholar]


