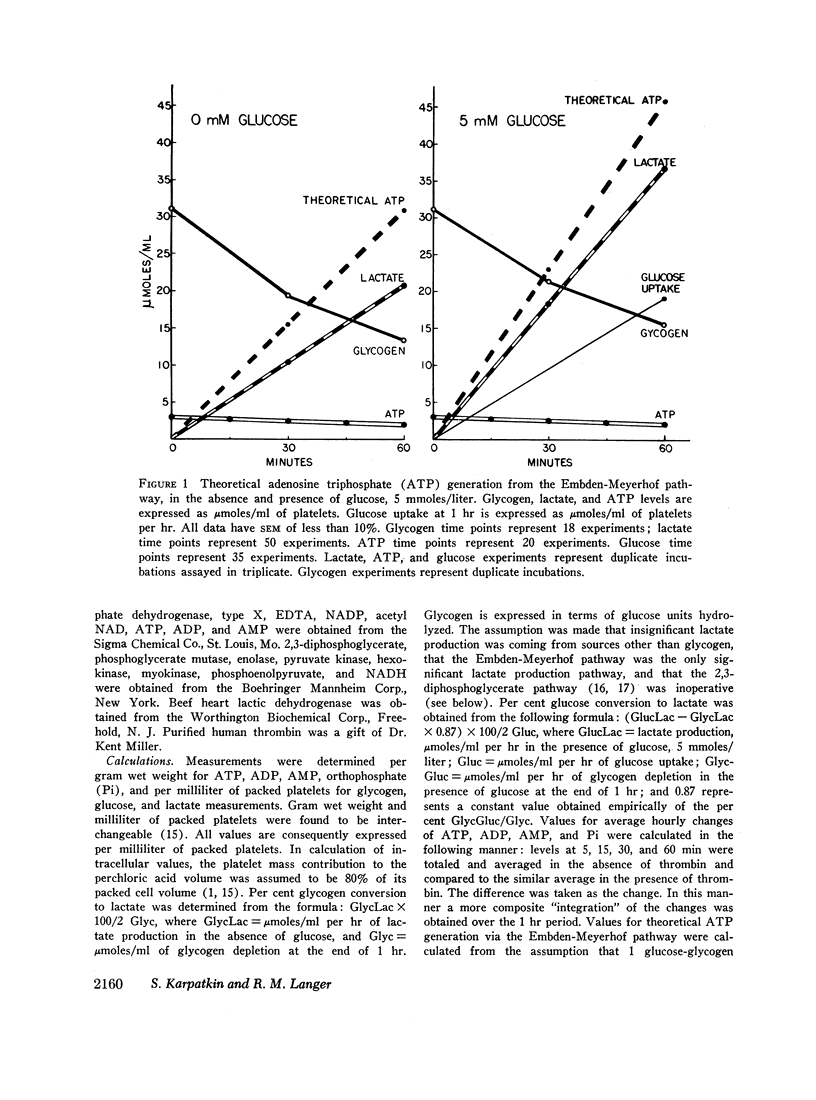
Abstract
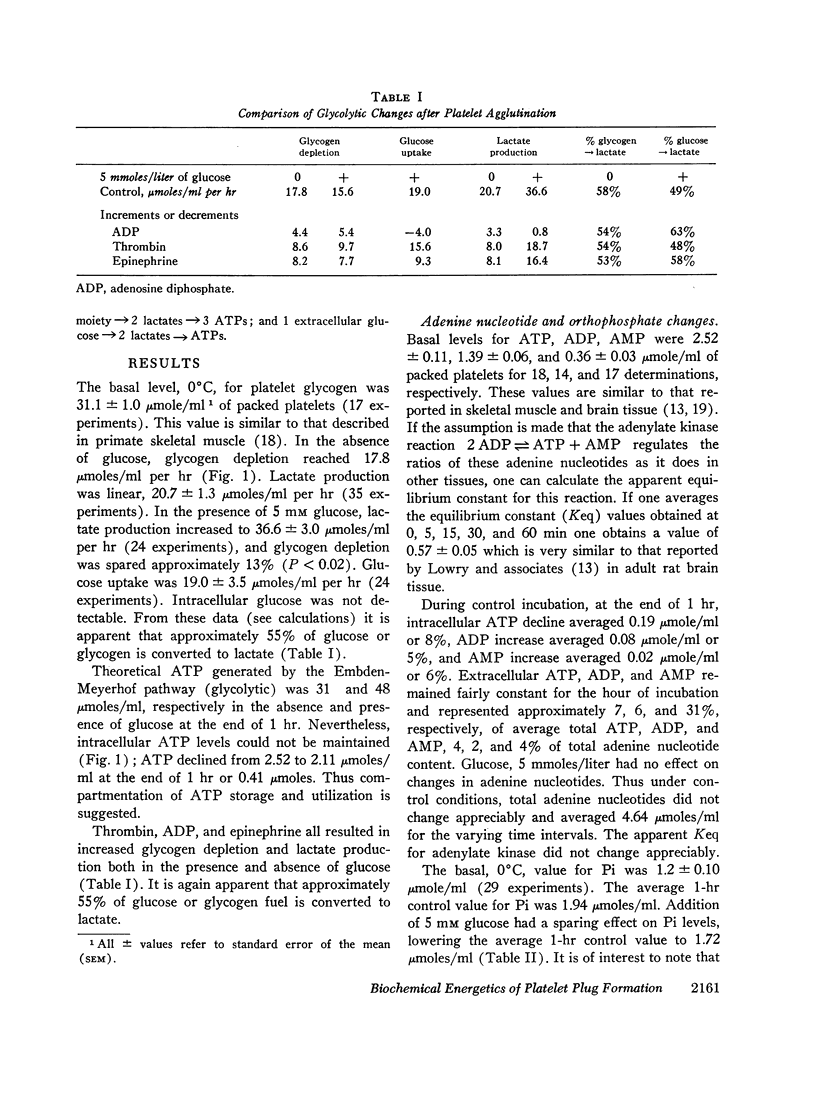
Washed human platelets were incubated in a modified Ringer's solution, pH 7.1, at 37°C for 1 hr. Intracellular basal levels for glycogen, adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), and orthophosphate were 31.1, 2.52, 1.39, 0.36, and 1.2 μmoles/ml of platelets, respectively. Extracellular ATP, ADP, and AMP remained fairly constant and represented 4, 2, and 4% of total adenine nucleotide content. Total adenine nucleotide content remained unchanged during the period of control incubation. Glycogen depletion was 17.8 μmoles/ml at the end of 1 hr; lactate production was 20.7 μmoles/ml per hr. In the presence of glucose, lactate production increased 100%, and glycogen depletion was spared 13%. Approximately 55% of glucose or glycogen fuel was converted to lactate.
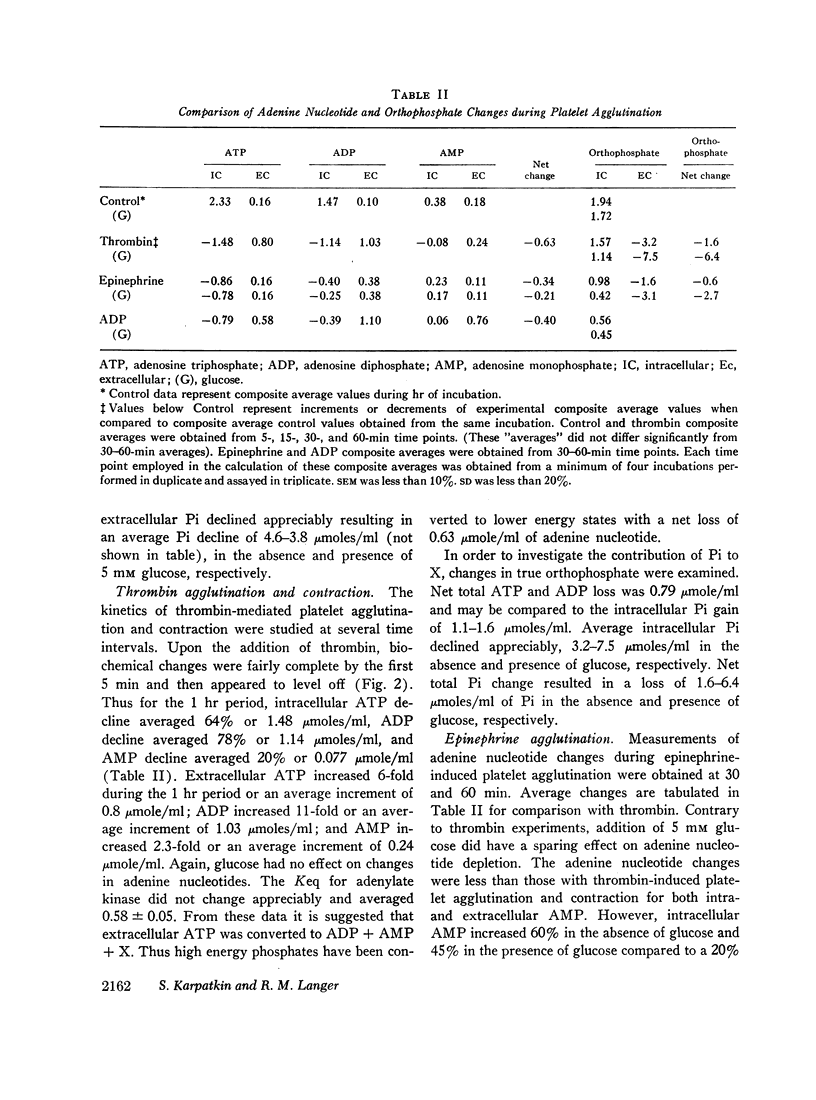
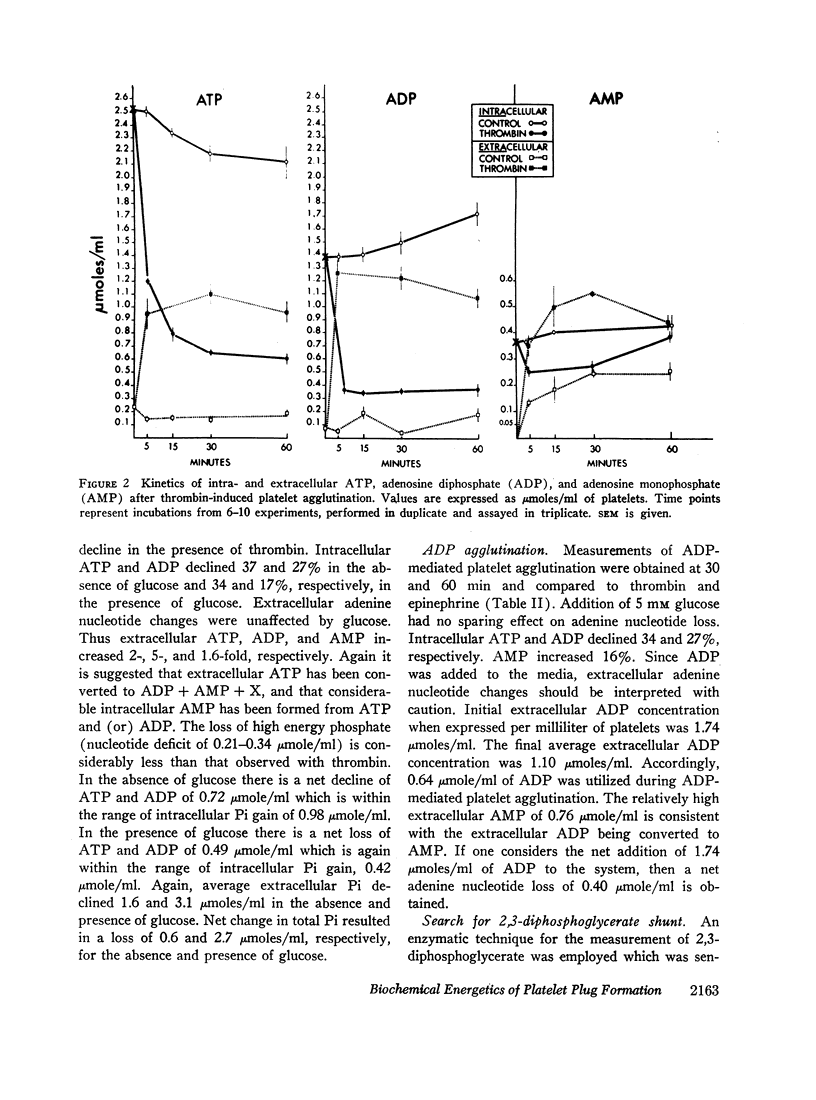
The agglutinating agents, thrombin, ADP, and epinephrine, resulted in increased glycogen depletion and lactate production both in the presence and absence of glucose. The effect of thrombin was greater than epinephrine. The effect of epinephrine was greater than ADP. All three agglutinating agents resulted in loss of high energy phosphates (net decline in adenine nucleotides) with release of adenine nucleotides into the extracellular environment. The effect of thrombin was greater than ADP. The effect of ADP was greater than epinephrine. In experiments with ADP addition, significant quantities of ADP were converted to AMP extracellularly. In experiments with thrombin and epinephrine appreciable quantities of extracellular orthophosphate were taken up by plateletes and could not be accounted for by changes in intracellular orthophosphate or adenine nucleotide. Sufficient ADP was released during exposure to thrombin and epinephrine to account for platelet agglutination. Changes in intracellular adenine nucleotides and orthophosphate could be correlated with the activation of regulator glycogenolytic and glycolytic enzymes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETTEX-GALLAND M., LUSCHER E. F. Studies on the metabolism of human blood platelets in relation to clot retraction. Thromb Diath Haemorrh. 1960 Mar 1;4:178–195. [PubMed] [Google Scholar]
- BORN G. V. Changes in the distribution of phosphorus in platelet-rich plasma during clotting. Biochem J. 1958 Apr;68(4):695–704. doi: 10.1042/bj0680695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty C. H., Peterson R. D., Basinger G. M., Bocek R. M. Major metabolic pathways for carbohydrate metabolism of voluntary skeletal muscle. Am J Physiol. 1966 Feb;210(2):404–410. doi: 10.1152/ajplegacy.1966.210.2.404. [DOI] [PubMed] [Google Scholar]
- Bettex-Galland M., Lüscher E. F. Thrombosthenin, the contractile protein from blood platelets and its relation to other contractile proteins. Adv Protein Chem. 1965;20:1–35. doi: 10.1016/s0065-3233(08)60387-3. [DOI] [PubMed] [Google Scholar]
- Born G. V. Effects of adenosine diphosphate (ADP) and related substances on the adhesiveness of platelets in vitro and in vivo. Br J Haematol. 1966 Jan;12(1):37–38. doi: 10.1111/j.1365-2141.1966.tb00123.x. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The non-competitive inhibition of brain hexokinase by glucose-6-phosphate and related compounds. J Biol Chem. 1954 Oct;210(2):597–606. [PubMed] [Google Scholar]
- Chambers D. A., Salzman E. W., Neri L. L. Characterization of "ecto-ATPase" of human blood platelets. Arch Biochem Biophys. 1967 Mar;119(1):173–178. doi: 10.1016/0003-9861(67)90444-4. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E., CORICF The effect of contraction and of epinephrine on the phosphorylase activity of frog sartorius muscle. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1191–1199. doi: 10.1073/pnas.48.7.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorstein F., Carroll H. J., Puszkin E. Electrolyte concentrations, potassium flux kinetics, and the metabolic dependence of potassium transport in human platelets. J Lab Clin Med. 1967 Dec;70(6):938–950. [PubMed] [Google Scholar]
- HASLAM R. J. ROLE OF ADENOSINE DIPHOSPHATE IN THE AGGREGATION OF HUMAN BLOOD-PLATELETS BY THROMBIN AND BY FATTY ACIDS. Nature. 1964 May 23;202:765–768. doi: 10.1038/202765a0. [DOI] [PubMed] [Google Scholar]
- HOVIG T. The ultrastructure of rabbit blood platelet aggregates. Thromb Diath Haemorrh. 1962 Dec 20;8:455–471. [PubMed] [Google Scholar]
- Hanson T. L., Fromm H. J. Rat skeletal muscle hexokinase. II. Kinetic evidence for a second hexokinase in muscle tissue. J Biol Chem. 1967 Feb 10;242(3):501–508. [PubMed] [Google Scholar]
- KARPATKIN S., HELMREICH E., CORI C. F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3139–3145. [PubMed] [Google Scholar]
- Karpatkin S. Studies on human platelet glycolysis. Effect of glucose, cyanide, insulin, citrate, and agglutination and contraction on platelet glycolysis. J Clin Invest. 1967 Mar;46(3):409–417. doi: 10.1172/JCI105542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitt A. S. Pyruvate kinase deficiency and related disorders of red cell glycolysis. Am J Med. 1966 Nov;41(5):762–785. doi: 10.1016/0002-9343(66)90036-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lowry O. H., Passonneau J. V. Kinetic evidence for multiple binding sites on phosphofructokinase. J Biol Chem. 1966 May 25;241(10):2268–2279. [PubMed] [Google Scholar]
- MITCHELL J. R., SHARP A. A. PLATELET CLUMPING IN VITRO. Br J Haematol. 1964 Jan;10:78–93. doi: 10.1111/j.1365-2141.1964.tb00681.x. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., PARMEGGIANI A. REGULATION OF GLYCOGENOLYSIS IN MUSCLE. 3. CONTROL OF MUSCLE GLYCOGEN PHOSPHORYLASE ACTIVITY. J Biol Chem. 1964 Aug;239:2440–2445. [PubMed] [Google Scholar]
- Nachman R. L., Marcus A. J., Safier L. B. Platelet thrombosthenin: subcellular localization and function. J Clin Invest. 1967 Aug;46(8):1380–1389. doi: 10.1172/JCI105630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPOPORT S., LUEBERING J. Glycerate-2,3-diphosphatase. J Biol Chem. 1951 Apr;189(2):683–694. [PubMed] [Google Scholar]
- Robinson C. W., Jr, Kress S. C., Wagner R. H., Brinkhous K. M. Platelet agglutination and deagglutination with a sulfhydryl inhibitor, methyl mercuric nitrate: relationships to platelet ATPase. Exp Mol Pathol. 1965 Oct;4(5):457–464. doi: 10.1016/0014-4800(65)90010-9. [DOI] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V., O'Connell E. L. Role of inorganic phosphate in stimulating the glucose utilization of human red blood cells. Biochem Biophys Res Commun. 1964 Feb 18;15(1):33–37. doi: 10.1016/0006-291x(64)90098-1. [DOI] [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A., GUILLORY R. J. An estimation of the true inorganic phosphate content of frog sartorius muscle. J Biol Chem. 1961 Jul;236:2071–2075. [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. The inhibition of brain hexokinase by adenosinediphosphate and sulfhydryl reagents. J Biol Chem. 1954 Feb;206(2):925–936. [PubMed] [Google Scholar]
- Salzman E. W., Chambers D. A., Neri L. L. Possible mechanism of aggregation of blood platelets by adenosine diphosphate. Nature. 1966 Apr 9;210(5032):167–169. doi: 10.1038/210167a0. [DOI] [PubMed] [Google Scholar]
- Spaet T. H., Lejnieks I. Studies on the mechanism whereby platelets are clumped by adenosine diphosphate. Thromb Diath Haemorrh. 1966 Jan 31;15(1):36–51. [PubMed] [Google Scholar]
- White J. G., Krivit W. Fine structural localization of adenosine triphosphatase in human platelets and other blood cells. Blood. 1965 Nov;26(5):554–568. [PubMed] [Google Scholar]


